Abstract
In this study, the antifungal effects of copper and silver nanoparticles against two wood-rotting fungi were investigated. European beech (Fagus sylvatica L.) and Scots pine (Pinus sylvestris L.) sapwood specimens of dimensions 50 × 25 × 15 mm3 were vacuum impregnated using dispersions of copper and silver nanoparticles within two concentrations, i.e. 1 and 3 g/l. Beech wood specimens were tested against white-rot fungus (Trametes versicolor) and pine wood against brown-rot fungus (Poria placenta) according to EN113. Furthermore, leachability, retention and protection efficiency (mass loss due to decay) were analysed afterwards. The highest value of retention was observed for pine sapwood (~2 kg/m3) for both nanoparticle solutions. The amount of nanoparticles in the wood did not increase proportionally with an increasing concentration, but only 1.5–2 times increase was reached. An average leaching of 15–35% was observed for copper nanoparticles, depending on used wood species and concentration. Significantly, lower leaching (max. 15%) was observed for pine sapwood impregnated by silver nanoparticles with a concentration of 3 g/l. The highest antifungal effect [under 5% of mass loss (ML)] against both tested fungi was found for nano-copper treatment at the concentration of 3 g/l. However, this effect of treatment seems to be almost negligible after the leaching test. Therefore, this study aims to present fundamental material properties of wood treated with copper and silver nanoparticles, and provide groundwork for further research (e.g. fixation of substances in the wood structure, etc.).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interaction of nanoparticles with biomolecules and microorganisms is an expanding field of research. It has been suggested that nanometre-sized metal particles have different physical and chemical properties from their macroscale counterparts that alter their interaction with biological structures and physiological processes [1]. Civardi et al. [2] evaluated the new generation of preservatives with Cu-based nanoparticles to be more efficient against wood-rotting fungi than conventional formulations. There are also some other applications like prevention of leaching in otherwise soluble metal oxides [3] or altering treatability properties such as penetration and biocide distribution [4, 5]. Nevertheless, potential environmental and health risks and the risk governance recommendations have to be taken into account [6]. Cu-based nanoparticles and/or their derivatives may during decomposition of treated wood accumulate in the mycelia of Cu-tolerant fungi and end up in their spores, which are dispersed into the environment. Choi and Hu [7] reported the toxicity of silver nanoparticles against nitrifying bacteria through the generation of oxygen radicals. The production of lignocellulose degrading enzymes was decreased by iron and copper aggregated nanoparticles in the white-rot fungus Trametes versicolor [8]. Such unknown and unpredictable influences on decomposition processes in the environment should be considered especially concerning treated wood with the ground contact.
Copper remains the primary biocide component used to protect wood. There are several brown-rot fungi (BRF) species which are copper tolerant [9], and BRF metabolite oxalic acid is a major factor leading to copper tolerance [10–12]. Oxalic acid is accumulated at greater concentrations in liquid or semisolid culture, whereas white-rot fungi decompose metabolized oxalic acid by oxalate-decomposing enzyme systems [13–15]. The high affinity with heavy metals and ability of their accumulation is known for white-rot fungi [16, 17]. The preservative formulation affects copper tolerance of fungi [12] and inhibition often depends upon the co-biocidal components [18]. The different properties of nanoparticles as higher surface area or reactivity could positively influence the resistance of brown-rot fungi.
Kartal et al. [3] reported that treatment with nano-copper (leached and unleached) significantly improved decay resistance (ML less than 10%) to Gloeophyllum trabeum (brown-rot fungus) and T. versicolor (white-rot fungus) but failed after exposure to Antrodia spp. Chang et al. [19] published that Cu nanoparticles are more toxic than micro ones with the same composition. Results confirmed that size and surface characteristics of Cu nanoparticles influenced its toxicity. Matsunaga et al. [5] stated that nano-Cu preservatives are able to deliver bioactive components into wood cell walls and thus improve leach resistance as well as bio-durability. Mass loss of Paulownia treated with nano-copper aqueous dispersion (400 ppm) was less than 3% and accompanied by the structural changes observed using SEM images of the undecayed and decayed treated wood [20].
Recent studies have reported the effectiveness of nano-sized silver particles as an antimicrobial agent [21, 22], and several mechanisms have been proposed to explain the inhibitory effect of silver nanoparticles on bacteria, such as high affinity with sulphur and phosphorus [23, 24]. The antifungal properties of silver are less known, because most of the studies have been focused on bacterial and viral pathogens in animals. According to Kim et al. [25], silver nanoparticles exhibited excellent antifungal activity on Candida albicans by disrupting the cell membranes and inhibiting the normal budding process due to the destruction of the membrane integrity. Authors also demonstrated the damage of fungal hyphae by silver nanoparticles but also the inhibition of conidial germination in phytopathogenic fungi Raffaelea spp. Furthermore, Pulit et al. [26] showed growth inhibition of Cladosporium cladosporioides and Aspergillus niger strains even at low concentrations. Dorau et al. [24] explained the theoretical mechanism of fungi-silver relation and reported the low to moderate wood resistance against BRF when treated by silver salts. Unsuitable preservation efficiency against white-rot fungus T. versicolor was observed for wood treated by silver nanoparticles at different concentrations, i.e. 200 and 400 ppm [27]. However, Akhtari and Arefkhani [20] stated that the micronized metals including silver were very effective in weight loss after 4 months and prevention of decay by T. versicolor.
Silver nanoparticles can be used for some other applications. Taghiyari et al. [28] studied the effect of silver nanoparticles on the rate of heat transferred to the core section of medium density fibreboard (MDF). The silver nanoparticles size ranged from 30 to 80 nm. Results showed that the treatment with silver nanoparticles significantly contributed to the faster transfer of heat to the core section of MDF panels.
The list of active biocidal substances, approved by European Chemicals Agency (ECHA), does not contain silver nanoparticles. It is not assumed that ECHA would authorize the use of silver or other heavy metals for wood protection. Therefore, the practical application for these substances is uncertain [29].
In the present study, the biological durability and leachability of European beech (Fagus sylvatica L.) and Scots pine (Pinus sylvestris L.) wood treated with dispersions of copper and silver nanoparticles were investigated in order to understand performance of such material and its potential application/utilization.
Materials and methods
Test materials
European beech (Fagus sylvatica L.) and Scots pine (Pinus sylvestris L.) sapwood non-defects specimens of dimensions 50 × 25 × 15 mm3 were used according to the EN 113 standard. Specimens were sorted into 9 groups and each group consists 10 specimens. All together there were 90 specimens.
Chemical preparation of nanoparticles
The dispersion of copper nanoparticles with a concentration of 1 g/l of Cu was prepared by reduction of copper sulphate pentahydrate (Sigma-Aldrich, p.a.) solution by sodium borohydride (Sigma-Aldrich, p.a.) in a presence of sodium salt of polyacrylic acid with molecular weight ~1200 (Sigma-Aldrich, 45% aqueous solution). The 3.929 g of copper sulphate pentahydrate was dissolved in 895 ml of deionized water followed by addition of 5 ml of 45% sodium salt polyacrylic acid solution. Afterwards, 100 ml of sodium borohydride solution (100 mg in 100 ml of deionized water) was added to the reaction mixture [30].
The dispersion of silver nanoparticles with a concentration of 1 g/l of Ag was prepared by reduction of silver nitrate (Sigma-Aldrich, p.a) solution by sodium borohydride in a presence of sodium salt of polyacrylic acid with molecular weight ~1200. The 1.574 g of silver nitrate was dissolved in 890 ml of deionized water followed by addition of 5 ml of ammonia solution and 5 ml of 45% sodium salt polyacrylic acid solution. Afterwards, 100 ml of sodium borohydride solution (100 mg in 100 ml of deionized water) was added to the reaction mixture. Immediately after addition of sodium borohydride, the dispersion turned dark-brown colour indicating formation of silver nanoparticles [31].
The prepared nanoparticles were characterized by dynamic light scattering measurements (ZetaSizer NanoZS, Malvern, UK) and transmission electron microscopy (Jeol 2010, Japan).
Preservative treatment
The impregnation was carried out using the laboratory vacuum-pressure impregnation plant (JHP-1-0072). Specimens were impregnated by copper and silver nanoparticles solution with 1 and 3 g/l concentrations. Concentrations of nanoparticles were chosen according to preliminary tests. All specimens were impregnated within single batch at vacuum of 80 kPa for 120 min.
Leaching test (EN 84)
Treated specimens were conditioned at 20 °C and 65% of RH for 4 weeks until the equilibrium moisture content was reached. Subsequently, specimens were submerged in deionized water (20 °C, pH 5.5), impregnated by vacuum at 80 kPa for 20 min and kept in water for 2 h after impregnation. Following 14 days of leaching, water was changed nine times. The leached water for the chemical analysis of the active ingredient amount was collected in certain periods, i.e. after the vacuum impregnation, after the 1st day of leaching and subsequently as a mixture of water collected during the period from 2nd to 4th and from 5th to 14th days.
Leachate and retention analysis
The amount of leached elements was analysed from the collected leaching water. Additionally, two impregnated specimens for each treatment group were split and ground to wood powder for retention analysis. Concentrations of copper and silver were determined by the AAS technique using flame ionization employing ContrAA 300 (Analytik Jena AG, Germany), equipped with a high-resolution Echelle double monochromator (spectral bandwidth of 2 pm at 200 nm) and a continuum radiation source (xenon lamp). The absorption lines used for the analyses were 324.754 for copper and 328.068 for silver.
Decay test
Decay test was performed according to EN 113 standard. Two fungi were used for the determination of nanoparticles protection efficiency—white-rot fungus (T. versicolor) for treated beech and brown-rot fungus (Poria placenta) for treated pine sapwood specimens. Two nanoparticle solutions (Cu, Ag) and two concentrations (1 and 3 g/l) with ten replicates were tested. Treated and untreated specimens were steam sterilized and placed in Kolle flask on the sterile culture medium MEA previously exposed to fungi for 2 weeks. Kolle flasks were incubated for 16 weeks at 22 °C and 75% of RH. At the end of the exposure time, the mycelium was carefully removed from the surface of specimens. Specimens were then oven-dried at 103 ± 2 °C to constant weight and weighed afterwards. The fungicidal efficiency of the treatment was determined by the ML due to fungi degradation.
Results and discussion
Retention of nanoparticles
An average value of chemical retention based on water solution retention and active component concentration for the studied wood species are shown in Table 1. Slightly higher retention was observed for Scots pine sapwood, which is in agreement with other authors [32, 33]. Humar et al. [34] stated that the influence of species on the retention is less significant for vacuum impregnation; however, pine sapwood showed the highest retention level when compared to beech or spruce wood. This can be explained by the difference in the anatomical structure and porosity of mentioned species. A higher concentration of the active component did not influence the water solution retention, but increased the biocide amount in wood. The results showed that the amount of nanoparticles within wood did not increase proportionally with an increasing concentration, but only 1.5–2 times increase was reached. Unfortunately, the lower number of used specimens might influence the value, which is obviously too low for beech wood impregnated with a higher (3 g/l) concentration of Cu nanoparticles.
Absorption of preservatives and colour changes within the whole specimen’s volume was confirmed by its splitting. Specimen’s surfaces were intensively coloured to brown with the exception of pine treated by copper nanoparticles, which got a green tone (Fig. 2). Matsunaga et al. [5] found that copper nanoparticles can pass through Southern pine pores in bordered pit membranes (an average diameter of 300–4000 nm) and even penetrate the cell walls, but most of them are too large to enter the cell wall’s nano-capillary network. The partial conversion of copper from nanoparticles to Cu2+ ions, which subsequently diffuse in the cell walls of tracheids, is another alternative [35]. Hardwood species are penetrated mainly through vessels, whose length as flow paths is much longer than the length of softwood tracheids [36]. However, the pore size in hardwood homogenous membranes is smaller than those in the margo area of softwood pits and varies from 5 to 420 nm, depending on species [37]. This can cause a higher conducting of nanoparticles on vessel pit membranes and therefore a reduced protection of other tissue elements, e.g. libriform fibres. For instance, Cronshow [38] found that colloidal gold and carbon particles (diameters of 64 nm) are not able to pass through the pit membranes of Eucalyptus regnans wood. Dimensions of nanoparticles used in the present study should be small enough to spread homogenously in wood conductive elements (tracheids or vessels) and pass through their pit membrane pores.
Figure 1 shows average diameters of copper and silver nanoparticles, which were 15.6 and 7.8 nm, respectively.
Leachability of nanoparticles
Leaching rates for all analysed groups at different stages of the process are shown in Figs. 2, 3. The highest leaching rate was noticed after initial leaching periods, i.e. vacuum and first day of leaching. The amount of leached substances gradually decreased with time. Similar leaching progress was presented by Thaler and Humar [39] for copper-based preservatives. The accessibility of copper deposited in a higher concentration in the wood surface layer is considered as the main reason.
The percentage loss of nanoparticles impregnated within wood during leaching is depicted in Fig. 4. Nano-metals were leached more intensively from specimens impregnated with higher concentration (3 g/l) and from pine sapwood, when wood species are compared. Copper nanoparticles leaching ranged from 15 to 35% of its original retention, and depends on species and concentration. Furthermore, silver leaching was significantly lower and reached maximally 15% for pine sapwood with a concentration of 3 g/l. Available literature sources about nanoparticle leaching are very limited and the results presented are rather contradictory. Copper compounds without proper fixation are very easily leached from wood [39, 40]. Regarding nanoparticles, some authors anticipated its higher fixation rather than soluble forms of wood preservatives, for instance due to changes in charge properties and Van der Waals forces [3, 6]. Moreover, Dorau et al. [24] stated that size of silver nanoparticles is too large to interact with a functional group in wood constituents unlike silver ions, which, in addition, penetrate wood more rapidly and effectively. Lesar et al. [41] showed 29 and 57% leaching of copper sulphate impregnated in spruce wood at Cu concentrations of 0.1 and 0.5%, respectively (EN 84). Leaching of copper sulphate (1%) from southern yellow pine was 24% after 14 days, compared to nanoparticles, where leaching was almost negligible [3]. Ding et al. [42] determined a higher rate of leaching for smaller nanoparticles (10 vs. 50 nm), which ranged between 12 and 16% depends on usage and type of polymer stabilizers. The results indicated that nanoparticles should be less susceptible to leaching, but a lot of factors, including size, type of stabilizers or treated species, could influence final behaviour. In service, leaching and mobility of metal components are, apart from exposure time, dependent on factors such as water volume and flow rate, exposed surface area or leachant pH and temperature [43]. Acidic as well alkaline environments increase the leachibility of heavy metals to a large extent compared with water with a neutral pH [44].
Lower leachability of silver nanoparticles could be explained by their smaller diameter when compared to copper particles (see Fig. 1). Smaller nanoparticles better penetrate deeper into wood structure and they are not deposited and aggregated on pit membranes passing from one cell to another. Ding et al. [42] showed by contrast that smaller nanoparticles are more susceptible to be leached because of their higher mobility in the liquid phase. However, the particle size differences are not so obvious in the present study.
A higher amount of copper and silver in leachates was found for pine wood, which can be explained by its higher retention level (Table 1). The differences within pit pore size may influence the mobility of particles, which are not driven by vacuum during leaching process and thus can be easily retained by the homogenous membrane of beech rather than more porous margo of pine wood. Various leaching rates for both species might be explained by the diverse chemical structure together with a related amount of reaction sites [45, 46].
Specimens treated with copper (3 g/l), unlike silver, showed lower leaching of the active ingredient compared to the lower concentration. It can be explained by a distinctively higher retention determined from the water solution uptake than the actual nanoparticle retention in wood (Fig. 5). The higher concentration of preservatives is usually associated with higher leaching, because of the limited amount of functional groups in wood, which could react with impregnated chemical compounds [34].
Treatment efficiency
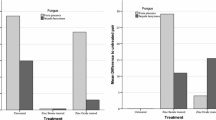
Sixteen weeks of exposition to white-rot fungus, T. versicolor and brown-rot fungus Poria placenta resulted in sufficient ML of control (untreated) specimens (beech and pine sapwood), which indicates both fungi activities according to the EN 113 standard (Fig. 6).
Mass loss due to the exposure of leached and unleached specimens to white-rot fungus (T. versicolor) and brown-rot fungus (P. placenta) are presented in Fig. 4. The ML caused by fungi degradation was significantly reduced for treated unleached wood, independently of concentration or fungus. The adequate performance (ML < 3%) of nanoparticle-impregnated specimens against BRF was achieved only when copper with concentration of 3 g/l was used, while all the applied treatments provide similar level of protection of wood against white-rot fungus. The silver nanoparticles showed the lowest protection effect with the highest variability when the treated wood was exposed to P. placenta.
Copper is biocidally inactive in low quantities, but acts as an essential micronutrient. However, at higher levels, it is a toxic heavy metal for most of the living cells. Copper tolerance is usually associated with brown-rot fungi, which was shown in many reports also for P. placenta [9, 11]; besides that P. placenta is also known to be tolerant to zinc compounds [47]. Pohleven et al. [48] determined the minimum inhibitory level for related Antrodia vaillantii on 1600 ppm in nutrient medium, whereas for T. versicolor, it was found to be ten times lower [49]. The effect of copper on growth of different fungi was evaluated by Guillén and Machuca [50]. The increase of copper concentration to 3 mM led to complete growth inhibition in the majority of the WRF (white-rot fungi), and T. versicolor was strongly suppressed. In contrast, two of three BRF presented a higher growth rate. The results of the present study are in agreement with previous findings by other authors, and the copper concentration of 3 g/l was found to protect wood even against brown-rot fungus P. placenta. The resistance of fungi to copper-based preservatives is influenced by pH of substrate, which BRF are able to significantly reduce by oxalic acid production, even in first non-enzymatic stage of decay [51, 52]. The production of intracellular and extracellular chelating compounds such as oxalic acid reduces toxicity of copper or other metals by the formation of insoluble, and therefore bio-unavailable, inert forms of oxalate [53, 54]. Guillén and Machuca [50] showed only minor acidification of malt extract agar (MEA) medium by T. versicolor after copper addition, unlike copper-tolerant brown-rot fungi. The white-rot fungi capability of adsorption and accumulation of metals together with low concentration of metabolized oxalic acid leads to high efficiency of used treatments.
Silver is well known for its great antimicrobial and antifungal properties. Moreover, it is an element with the highest toxicity for microorganisms followed by other heavy metals like copper, lead, or tin, and lowest toxicity for animal cells at the same time [21, 26, 55]. Rezaei et al. [27] stated that nano-silver in low concentration (400 ppm) is not able to inhibit T. versicolor activity within poplar wood. However, Paulownia fortunei wood impregnated with 400 ppm aqueous suspension of silver, copper, and zinc oxide nanoparticles demonstrated resistance to decay caused by T. versicolor [20]. Silver and copper nanoparticles suspension used in the particleboard increased resistance against T. versicolor, with higher efficiency of copper [28]. The ionic silver-based biocides (1% Ag solution) were tested by Dorau et al. [24] and did not provide southern yellow pine wood with sufficient resistance to brown-rot fungi. In the present study, silver nanoparticles inhibited the tested white-rot fungus, whereas BRF were only suppressed which resulted in lower ML in comparison to control specimens. Highley [56] tested different compounds that inhibit the action of extracellular celluloses. It showed that enzymes responsible for insoluble cellulose degradation, typical of white-rot fungi, were inhibited by the metals mercury, silver, copper, and manganese. Only mercury and silver were able to inhibit enzymes decomposing soluble cellulose, which are produced by BRF as well. Possible mechanisms by which microorganisms avoid the toxical effect of silver ions are summarized by Guggenbichler et al. [57], i.e. biomethylation; complex formation with metal ions; development of efflux pumps; binding of metal ions to cell surfaces or the removal of metal ions by precipitating. Analogous mechanisms as immobilizing copper by precipitating copper oxalate are anticipated in case of brown-rot fungi, which eliminate silver higher efficiency in celluloses inhibition.
As expected, higher ML usually occurred in leached wood specimens when compared to unleached ones (Fig. 4). Whereas in unleached copper treated specimens both concentration sufficiently blocked decay activity of fungi (ML < 5%), after leaching the efficiency of treatment was significantly reduced. Especially, the ML caused by brown-rot fungus was as high as those in the control specimens. Low fixation of copper nanoparticles led to remove of their substantial part from wood. Therefore, remaining amount is lower than required minimum inhibitory level. Copper acts as an essential trace mineral—e.g. is a cofactor in the catalytic centre of laccase which is one of ligninolytic enzymes [58]. At low concentration, copper is biocidally inactive [16, 59], but can stimulate the growth of fungi [60]. When the efficacy of copper and silver nanoparticles is compared, it can be seen that the latter is more effective than copper, when the wood is subjected to leaching. Higher retention of silver nanoparticles together with better fixation in the wood enables to leave sufficient level of active substances for reduction of fungi activity (brown-rot fungi) or even inhibition (white-rot fungus).
Conclusions
This work deals with the antifungal effects of copper and silver nanoparticles against two wood-rotting fungi. Pine sapwood showed the highest retention for both concentrations. An average leaching of copper nanoparticles (15–35%) was higher compared to leaching of silver (up to 15%). This research has shown that copper nanoparticles at the concentration of 3 g/l are effective against both tested fungi (under 5% of ML) but it seems to be ineffective after leaching. Nano-silver treatment shows very low ML (under 1%) and high efficiency against T. versicolor fungi for leached and unleached specimens, but very low efficiency against Poria placenta decaying. Further steps for research in this area should be to improve fixation and determinate the influence of nanoparticles form (preparation method/technique).
References
Nel A, Xia T, Mädler L, Li N (2003) Toxic potential of materials at the nanolevel. Science 311:622–627
Civardi C, Schwarze FWMR, Wick P (2015) Micronized copper wood preservatives: an efficiency and potential health risk assessment for copper-based nano-particles. Environ Pollut 200:126–132
Kartal SN, Green IIIF, Clausen CA (2009) Do the unique properties of nanometals affect leachibility or efficiacy against fungi and termites? Int Biodeterior Biodegrad 63:490–495
Stirling R, Drummond J, Zhang J, Ziobro RJ (2008) Microdistribution of micronized copper in southern pine. International Research Group on Wood Protection, IRG/WP 08–30479
Matsunaga H, Kiguchi M, Evans PD (2009) Microdistribution of copper-carbonate and iron oxide nano-particles in treated wood. J Nanopart Res 11:1087–1098
Clausen CA (2007) Nanotechnology: implications for the wood preservation industry. International Research Group on Wood Protection. Stockholm, Sweden. IRG/WP/07-30415, pp 15
Choi OK, Hu ZQ (2009) Nitrification inhibition by silver nano-particles. Water Sci Technol 59:1699–1702
Shah V, Dobiášová P, Baldrian P, Nerud F, Kumar A, Seal S (2010) Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus Trametes versicolor. J Hazard Mater 178:1141–1145
Young GY (1961) Copper tolerance of some wood-rotting fungi, Forest Products Laboratory Report 2223, pp 10
Clausen CA, Green IIIF, Woodward BM, Evans J, De Groot RC (2000) Correlation between oxalic acid production and copper tolerance in Wolfiporia cocos. Int Biodeterior Biodegrad 46:69–76
Green IIIF, Clausen CA (2005) Copper tolerance of brown-rot fungi: oxalic acid production in southern pine treated with arsenic-free preservatives. Int Biodeterior Biodegrad 56:75–79
Köse C, Kartal SN (2010) Tolerance of brown-rot and dry-rot fungi to CCA and ACQ wood preservatives. Turk J Agric For 34:181–190
Takao S (1965) Organic acid production by basidiomycetes. Appl Microbiol 13:732–737
Espejo E, Agosin E (1991) Production and degradation of oxalic acid by brown rot fungi. Appl Environ Microb 57:1980–1986
Mäkelä M, Galkin S, Hatakka A, Lundell T (2002) Production of organic acids and oxalate decarboxylase in lignin-degrading white rot fungi. Enzyme Microb Technol 30:542–549
Baldrian P (2003) Interactions of heavy metals with white rot fungi. Enzyme Microb Technol 32:78–91
Bayramoğlu G, Bektaş S, Arıca MY (2003) Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J Hazard Mater 101:285–300
Illman BI, Yang VW, Ferge L (2000) Bioprocessing preservative-treated waste wood. International Research Group on Wood Preservation, IRG/WP 00-50145, pp 11
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nano-particles. Materials 5:2850–2871
Akhtari M, Arefkhani M (2013) Study of microscopy properties of wood impregnated with nano-particles during exposed to white-rot fungus. Agric Sci Dev 2(11):116–119
Rai M, Yadav A, Gade A (2009) Silver nano-particles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Yacaman MJ (2005) The bactericidal effect of silver nano-particles. Nanotechnology 16:2346–2353
Ravishankar RV, Jamuna BA (2011) Nano-particles and their potential application as antimicrobials. In: Méndez-Vilas A (ed) Science against microbial pathogens: communicating current research and technological advances. Formatex, Badajoz
Dorau B, Arango R, Green IIIF (2004) An investigation into the potential of ionic silver as a wood preservative. In: Proceedings of the 2nd Wood-frame housing durability and disaster issues conference. Forest Products Society, Las Vegas, pp 133–145
Kim KJ, Sung WS, Suh BK, Moon SK, Choi JS, Kim JG (2009) Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals 22:235–242
Pulit J, Banach M, Szczygłowska R, Bryk M (2013) Nanosilver against fungi. Silver nano-particles as an effective biocidal factor. Acta Biochim Pol 60:795–798
Rezaei VT, Usefi A, Soltani M (2011) Wood protection by nano silver against white rot. In: Proceedings of 5th Symposium on Advanceds in science and technology, Mashhad
Taghiyari HR, Schmidt O, Bari E, Tahir MDP, Karimi A, Nouri P, Jahangiri A (2014) Effect of silver nano-particles on the rate of heat transfer to the core of the medium-density fiberboard mat, IRG/WP 14-40653
Goodell B, Nicholas DD, Schultz TP (2003) Wood deterioration and preservation: advances in our changing world. American Chemical Society, Washington. ISBN 0841237972
Prucek R, Kvítek L, Panáček A, Vančurová L, Soukupová J, Jančík D, Zbořil R (2009) Polyacrylate-assisted synthesis of stable copper nano-particles and copper(I) oxide nanocubes with high catalytic efficiency. J Mater Chem 19(44):8463–8469
Kvítek L, Prucek R, Panáček A, Sivera M, Měřínská D, Tesaříková D (2015) Aqueous dispersion of silver nano-particles. Patent number 28867, Industrial Property Office (CZ)
Tascioglu C, Yalcin M, Troya TD, Sivrikaya H (2012) Termiticidal properties of some wood and bark extracts used as wood preservatives. BioResources 7(3):2960–2969
Sen S, Tascioglu C, Tirk K (2009) Fixation, leachability, and decay resistance of wood treated with some commercial extracts and wood preservative salts. Int Biodeterior Biodegrad 63:135–141
Humar M, Žlindra D, Pohleven F (2007) Influence of wood species, treatment method and biocides concentration on leaching of copper–ethanolamine preservatives. Build Environ 42:578–583
Matsunaga H, Kataoka Y, Kiguchi M, Evans P (2010) Copper nano-particles in southern pine wood treated with a micronised preservative: can nano-particles penetrate the cell walls of tracheids and ray parenchyma? IRG/WP 10–30547:14
Cooper PA, Churma R (1990) Estimating diffusion path length in treated wood. For Prod J 40:61–63
Choat B, Cobb RA, Jansen S (2007) Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol 177:608–626. doi:10.1111/j.1469-8137.2007.02317.x
Cronshow J (1960) The fine structure of the pits of (Eucalyptus regnans F.Muell.) and their relation to the movement of liquids into the wood. Aust J Bot 8:53–57
Thaler N, Humar M (2014) Copper leaching from copper-ethanolamin treated wood: comparison of field test studies and laboratory standard procedures. Bioresources 9(2):3038–3051
Richardson BA (1978) Wood preservation. E & FN SPON, London, p 1993
Lesar B, Kralj P, Žlindra D, Kancilija Humar M (2008) Comparison of standard procedures for estimation of biocides leaching from impregnated wood. Zb Gozd Lesar 86:59–64
Ding X, Meneses MB, Albukhari SM, Richter DL, Matuana LM, Heiden PA (2013) Comparing leaching of different copper oxide nanoparticles and ammoniacal copper salt from wood. Macromol Mater Eng 298:1335–1343. doi:10.1002/mame.201200439
Lebow S (1996) Leaching of wood preservative components and their mobility in the environment: summary of pertinent literature. US Department of Agriculture, Forest Service, Forest Products Laboratory, Madison, p 36
Moghaddam AH, Mulligan CN (2008) Leaching of heavy metals from chromated copper arsenate (CCA) treated wood after disposal. Waste Manag 28:628–637
Radivojevic S, Cooper PA (2010) The effects of wood species and treatment retention on kinetics of CCA-C fixation reactions. Wood Sci Technol 44:269–282
Temiz A, Alfredsen G, Yildiz UC, Gezer ED, Köse G, Akbas S, Yildiz S (2014) Leaching and decay resistance of alder and pine wood treated with copper based wood preservatives. Maderas-Ciencia y tecnologia 16:63–76
Clausen CA, Yang VW, Arango RA, Green IIIF (2009) Feasibility of nanozinc oxide as a wood preservative. Proc Am Wood Protect Assoc 105:255–260
Pohleven J, Brzin J, Vrabec L, Leonardi A, Čokl A, Štrukelj B, Kos J, Sabotič J (2011) Basidiomycete Clitocybe nebularis is rich in lectins with insecticidal activities. Appl Microbiol Biotechnol 91(4):1141–1148
Humar M, Lesar B (2008) Fungicidal properties of individual components of copper–ethanolamine-based wood preservatives. Int Biodeterior Biodegrad 62:46–50
Guillén Y, Machuca A (2008) The effect of copper on the growth of wood-rotting fungi and a blue-stain fungus. World J Microbiol Biotechnol 24:31–37
Humar M, Petrič M, Pohleven F (2001) Changes of the pH value of impregnated wood during exposure to wood-rotting fungi. Eur J Wood Wood Prod 59:288–293
Humar M, Šentjurc M, Amartey SA, Pohleven F (2005) Influence of acidification of CCB (Cu/Cr/B) impregnated wood on fungal copper tolerance. Chemosphere 58:743–749
Hastrup ACS, Green IIIF, Clausen CA, Jensen B (2005) Tolerance of Serpula lacrymans to copper-based wood preservatives. Int Biodeterior Biodegrad 56:173–177
Gadd G (1999) Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. Adv Microbial Physiol 11:47–91
Golubovich VN, Rabotnova IL (1974) Kinetics of growth inhibition by silver ions. Microbiology 43:948–950
Highley TL (1975) Inhibition of cellulases of wood-decay fungi. US Department of Agriculture, Forest Service, Forest Service Laboratory, Madison, p 8
Guggenbichler JP, Böswald M, Lugauer S, Krall T (1999) A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection 27:s16–s23
Vršanská M, Burešová A, Damborský P, Adam V (2015) Influence of different inducers on ligninolytic enzyme activities. J Metallomics Nanotechnol 3:64–70
Hrastnik D, Budija F, Humar M, Petrič M (2013) Influence of liquefied and CCB containing liquefied wood on growth of wood decay fungi. Maderas-Cienc Tecnol 15:105–118
Wazny J, Thornton JD (1986) Comparative laboratory testing of strains of the dry rot fungus Serpula lacrymans. Holzforschung 40:383–388
Acknowledgements
This work was carried out at Research Center Josef Ressel in Brno-Útěchov. It was financially supported by the Internal Grant Agency (IGA) of the Faculty of Forestry and Wood Technology, Mendel University in Brno, (LDF_PSV_2016015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pařil, P., Baar, J., Čermák, P. et al. Antifungal effects of copper and silver nanoparticles against white and brown-rot fungi. J Mater Sci 52, 2720–2729 (2017). https://doi.org/10.1007/s10853-016-0565-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0565-5