Abstract
The unique chemical properties of nanostructured materials are dependent on the surface thermodynamic properties which depend on the size of nanoparticles. But the quantitative influences of particle size on the surface thermodynamic properties of nanoparticles are not clear completely. In this paper, the relations between surface thermodynamics properties and particle size of nanoparticles have been deduced, and the method of obtaining the surface thermodynamic properties by electrochemistry has also been proposed. Experimentally, the electrode potentials and the electrode temperature coefficients of nano-Ag2O electrodes with different particle sizes were determined at different temperatures. The results indicate that with the size of Ag2O nanoparticles decreasing, the molar surface Gibbs energy, the molar surface entropy and the molar surface enthalpy correspondingly increase. Moreover, when the particle radius exceeds 10 nm, these physical quantities are all linearly related with the reciprocal of average particle radius, and the experimental results are consistent with the theoretical formulas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanothermodynamics have been developed by Hill in the early 1960s, and he nicely deduced the basic thermodynamic equations for nanoscale materials [1–4]. Currently, the thermodynamic properties of nanoparticles made of both the bulk phase and the surface phase are well known [5–7]. Almost all of the particular properties of nanostructured materials are derived from the effect of surface thermodynamics [8]. Understanding and predicting the thermodynamic properties of nanoparticles are essential for the practical applications of nanostructured materials [9–11]. What is more, a few studies of surface thermodynamic properties of nanoparticles have been reported. Navrotsky et al. [12–14] obtained the surface enthalpies of nano-Akaganeite, nanophase titanium dioxide and nanophase zinc oxide for the first time, using a series of calorimetric measurements. Furthermore, the surface Gibbs energy, surface enthalpy and surface entropy of nano-ferrihydrite and nano-calcium molybdate were also successfully acquired by some researchers [15, 16].
All of the works mentioned above show that the effect of particle size on the surface thermodynamic properties remains unclear, which is due to the difficulties in determining the parameters of surface thermodynamic properties [6, 17]. To date, how to acquire surface thermodynamic properties of nanoparticles is still an intractable problem. So, it is essential to establish a new method of determining the surface thermodynamic properties of nanoparticles.
In this paper, the relations between surface thermodynamic properties and nanoparticle sizes were deduced. The method of obtaining the surface thermodynamic properties by electrochemistry was proposed and the surface thermodynamic properties of nano-Ag2O with different particle sizes were obtained using the electrochemical method. Meanwhile, the influences of particle sizes on surface thermodynamic properties were also discussed.
Theoretical relations of surface thermodynamic properties with nanoparticle size
Under constant temperature and pressure, the surface Gibbs energy of spherical nanoparticles can be expressed as
where the superscript s denotes the surface quantities; σ, A, r, N are the surface tension, interfacial area, particle radius and the numbers of nanoparticles, respectively.
The amount of substance of nanoparticle is
where M and ρ are the molar mass and the density of the particle, respectively.
Then, molar surface Gibbs energy can be obtained by combining Eqs. (1) with (2),
It can be seen from the above formula that the surface tension σ is related to particle radius r, but the effect of particle size on surface tension will be neglected when the radius of the nanoparticles exceeds 10 nm [18, 19]. So, the surface Gibbs energy increases with the decreasing particle radius.
Surface enthalpy H s m of nanoparticles can be derived by Gibbs–Helmholtz equation,
where subscript p denotes the pressure, α is the volume expansion coefficient, namely α = 1/V m (∂V m /∂T) p and V m denotes the molar volume of nanoparticles.
As is observed from Eq. (4), (∂σ/∂T) p is usually a negative value, and the order of magnitude of volume expansion coefficient α for nanocrystals is usually 10−5 [20, 21], and therefore, {(∂σ/∂T) p + 2σα/3} is a negative value. So, the molar surface enthalpy increases with the decreasing nanoparticle radius.
Similarly, the molar surface entropy S s m can be expressed as
So, in conclusion, it is obvious that there is a linear relationship between the surface thermodynamic properties (the molar surface Gibbs energy, the molar surface enthalpy and the molar surface entropy) and the reciprocal of particle radius when the radius exceeds 10 nm.
A determination method of surface thermodynamic properties of nanoparticles by electrochemistry
The electrode reaction of nano-Ag2O is expressed as follows:
For chemical reaction in nanosystem, the change in molar Gibbs energy function can be expressed as Eq. (7) if both the bulk (Δ r G b m ) and the surface (Δ r G s m ) were taken into account [5]:
Under constant temperature and pressure, Eq. (7) can be expressed as follows:
where ν B denotes the stoichiometric number of component B; μ b B and μ s B denote the chemical potential of component B in bulk phase and surface phase, respectively.
Since there is only one dispersed phase (nano-Ag2O) in the electrode reaction, we can drive the change in molar surface Gibbs energy:
In the above-mentioned electrode reaction, the number of nanoparticles is invariable, but the particle radius has changed. So, the partial derivative of G s against n can be obtained by simultaneous Eqs. (1) and (2), and the partial molar surface Gibbs energy \( G_{{{\text{Ag}}_{ 2} {\text{O}}}}^{s} \) is given by
Similarly, the partial molar surface enthalpy and the partial molar surface entropy of nanoparticles can be deduced as follows:
So, comparing Eqs. (3), (4), (5) to Eqs. (10), (11),(12), respectively, we can draw a conclusion that the molar surface quantities to partial molar surface quantities ratio is 3:2.
When the electrode reaction occurs reversibly, the change in molar Gibbs energy is equal to the reversible electric work, as follows:
where z is charge transfer number, E is the electrode potential in the electrode reaction and F is the Faraday constant.
According to Eq. (7), the electrode potential of a nanoelectrode can be expressed as follows:
where E b is the electrode potential of ordinary electrode and E s is the surface electrode potential.
The change in molar surface Gibbs energy can be expressed as follows:
Applying Eqs. (10) and (15), the molar surface Gibbs energy of nano-Ag2O can be obtained as follows:
Similarly, the molar surface entropy and the molar surface enthalpy of nano-Ag2O can be derived as follows:
The relation between electrode potential of nano-Ag2O electrode and particle radius can be expressed according to the theory of electrochemical thermodynamics for nanoelectrodes deduced by our research group [22, 23]:
where the \( \nu_{{{\text{Ag}}_{ 2} {\text{O}}}} \) denotes the stoichiometric number in the electrode reaction, \( {\text{M}}_{{_{{{\text{Ag}}_{ 2} {\text{O}}}} }} \),\( \rho_{{{\text{Ag}}_{ 2} {\text{O}}}} \),\( \sigma_{{_{{{\text{Ag}}_{ 2} {\text{O}}}} }} \) and \( r_{{{\text{Ag}}_{ 2} {\text{O}}}} \) are the molar mass, density, surface tension and particle radius of nano-Ag2O, respectively.
It is well known that the surface tension σ is related to particle radius r, but when the particle size of dispersed phase becomes larger (r > 10 nm), the effect is not significant, and the σ can be regarded as a constant approximately [17, 18]. Then, it can be seen from Eq. (19) that there is a linear relation between the electrode potential and the reciprocal of radius of nano-Ag2O.
When the particle radius tends to infinity, the electrode potential of ordinary electrode E b can be obtained. Furthermore, the surface electrode potential E s can also be obtained. Meanwhile, the surface temperature coefficient is obtained by linear fitting the surface electrode potentials at different temperatures, and the molar surface Gibbs energy, molar surface entropy and molar surface enthalpy of nano-Ag2O with different particle radii can be calculated using Eqs. (16), (17), (18), respectively.
Experimental procedure
Preparation method and process
Complex precipitation method was used to synthesize nano-Ag2O, using silver nitrate as raw materials and sodium hydroxide as precipitating agent. After the reaction was completed, the nano-Ag2O particles were washed with distilled water and anhydrous ethanol, and then dried in the vacuum drying box for 30 h at 333 K. Nano-Ag2O particles with different sizes were obtained by changing the reaction concentration, adding order, stirring method, dispersing agent and the reaction temperature, which are shown in Table 1.
Characterization of nano-Ag2O
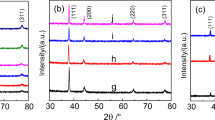
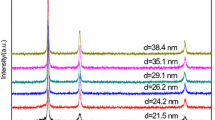
The nano-Ag2O structure was characterized using a Germany Bluker D8 Advance Powder diffractometer (Cu Kα, λ = 0.154178 nm), and the XRD spectrum is shown in Fig. 1. The average crystallite radius r − of nano-Ag2O is the average value of different diffraction directions of the crystallite. The crystallite size was calculated by Scherrer formula based on the half peak width of a characteristic diffraction peak. The crystallite sizes used in this experiment are 10.4, 12.9, 15.3, 18.1 and 30.5 nm, respectively.
Preparation of nano-Ag2O electrode
The tapered glass tube was made by blowtorch and a piece of silver rod was inserted into the tube. The blocky nanoelectrode was fabricated by pouring the nanoparticles into the tube, and the schematic of nano-Ag2O electrode is shown in Fig. 2.
Measurement of electrode potential
A 1.00 mol/L sodium hydroxide solution was prepared as electrolyte solution, and primary cell was composed of the saturated calomel electrode and nano-Ag2O electrode. Finally, the electromotive force of the cell was determined by compensation method using potentiometer. Then, nano-Ag2O electrode potential is equal to the electromotive force plus the saturated calomel electrode potential.
Experimental results and discussion
Electrode potential and surface electrode potential
The electromotive forces of the primary cell and the electrode potentials of nanoelectrodes with different particle radii of Ag2O particles at different temperatures are shown in Table 2.
The relation between the electrode potentials of nano-Ag2O and particle sizes is shown in Fig. 3. It can be seen clearly that the electrode potentials of different temperatures were affected by particle sizes, which increase with the decreasing particle radius, and there are better linear relationships.
According to Eq. (19), the value of the electrode potential of ordinary electrode E b was deduced from Fig. 3 when the particle radius tended to infinity. The surface electrode potential with different average particle radii of nano-Ag2O particles were calculated at different temperatures by combining the values of E b and Eq. (19). The results are shown in Table 3.
The relations between the surface electrode potential of nano-Ag2O and temperature are shown in Fig. 4. It can be seen that the surface electrode potentials with different particle sizes were affected by temperature. The surface electrode potentials decrease with the increasing temperature, and there are better linear relationships. Therefore, the surface electrode potential and temperature coefficients of nano-Ag2O particles were evaluated from the slopes provided in Fig. 4 and are listed in Table 4.
Molar surface Gibbs energy of nano-Ag2O
The molar surface Gibbs energy of nano-Ag2O can be directly determined from Eq. (16), and the values are given in Table 5.
It is estimated that for the general metal nanoparticles, the order of magnitudes of σ, M and ρ are 100, 10−2–10−1 and 103–104, respectively. So, the Gibbs energy of metal spherical nanoparticles with different particle sizes can be calculated using Eq. (3). Therefore, r = 10−6 m, the order of magnitude of G s m is 10−3–10−1 kJ/mol. r = 10−9 m, and the order of magnitude of G s m is 100–102 kJ/mol. The order of magnitude is in good accordance with the electrochemical experimental value of Table 5.
The plot of G s m versus r −1 is shown in Fig. 5.
It can be seen from Fig. 5 that the molar surface Gibbs energy increases with the decreasing particle size, which is in agreement with the theoretical analysis of Eq. (3). The results indicate clearly that under constant pressure and temperature conditions, the smaller the particle sizes are, the higher the chemical activity and more unstable the nanoparticles will be.
Molar surface entropy and enthalpy of nano-Ag2O
According to the surface electrode potentials and the surface temperature coefficients, the molar surface entropy and enthalpy of nano-Ag2O are calculated using Eqs. (17) and (18); the results are shown in Table 6.
The relations between the molar surface entropy, the molar surface enthalpy, respectively, and the reciprocal of average particle radius can be obtained from the data of Table 6 and are shown in Figs. 6 and 7.
As illustrated in Figs. 6 and 7, it is obvious that when the radius of nanoparticles decreases, the molar surface entropy and the molar surface enthalpy increase, respectively, which are in agreement with the above-mentioned theoretical analysis of Eqs. (17) and (18). It also can be seen that when the particle radius tends to infinity, the molar surface entropy and the molar surface enthalpy can be viewed as zero.
Conclusions
The above results show that the surface thermodynamic properties of nanoparticles are strongly affected by their size. With the decreasing particle radius of nano-Ag2O, the molar surface Gibbs energy, the molar surface entropy and the molar surface enthalpy increase, and there are better linear relations between them and the reciprocal of particle size. The experimental results are also in good agreement with the theoretical equations.
The theoretical equations and the influence regularities of particle size on the surface thermodynamic properties can provide both tools to guide experiments and a basis for testing the foundations. In this paper, the electrochemical determination of the surface thermodynamic properties of nanoparticles is reliable and accurate. Although the method is applied to the study on the surface thermodynamic properties of nano-Ag2O, there is no reason to believe that its success is restricted to this nanomaterial.
References
Hill TL (1963) Thermodynamics of small systems. W. A. Benjamin, New York
Hill TL (2001) Perspective: nanothermodynamics. Nano Lett 1:111–112
Hill TL (2001) Extension of nanothermodynamics to include a one-dimensional surface excess. Nano Lett 1:159–160
Hill TL (2001) A different approach to nanothermodynamics. Nano Lett 1:273–275
Xue YQ, Gao BJ, Gao JF (1997) The theory of thermodynamics for chemical reactions in dispersed heterogeneous systems. J Colloid Interface Sci 191(1):81–85
Luo WH, Hu WY, Xiao ShF (2008) Size effect on the thermodynamic properties of silver nanoparticles. J Phys Chem C 112(7):2359–2369
Hu WY, Xiao SF, Deng HQ, Luo WH, Deng L (2010) Thermodynamic properties of nano-silver and alloy particles. Silver nanoparticles p. 6587–6593
Ball P, Garwin L (1992) Science at the atomic scale. Nature 355:761–766
Gleiter H (2000) Nanostructured materials: basic concepts and microstructure. Acta Mater 48:1–29
Li ZH, Truhlar DG (2014) Nanothermodynamics of metal nanoparticles. Chem Sci 5:2605–2624
Rajagopal AK, Pande CS, Abe S (2004) Nanothermodynamics: a generic approach to material properties at nanoscale. arXiv:cond-mat/0403738.
Mazeina L, Deore S, Navrotsky A (2006) Energetics of bulk and nano-akaganeite, β-FeOOH: enthalpy of formation, surface enthalpy, and enthalpy of water adsorption. Chem Mater 18:1830–1838
Zhang P, Xu F, Navrotsky A, Lee JS, Kim S, Liu J (2007) Surface enthalpies of nanophase ZnO with different morphologies. Chem Mater 19(23):5687–5693
Park TJ, Levchenko AA, Zhou HJ, Wong SS, Navrotsky A (2010) Shape-dependent surface energetics of nanocrystalline TiO2. J Mater Chem 20:8639–8645
Hiemstra T (2015) Formation, stability, and solubility of metal oxide nanoparticles: surface entropy, enthalpy, and free energy of ferrihydrite. J. Geochim ET Cosmochim Acta 158:179–198
Li XX, Fan GC, Huang ZY (2015) Synthesis and surface thermodynamic functions of CaMoO4 nanocakes. J Entropy 17:2741–2748
Wu Q, Liu M, Wu ZJ, Li YL, Piao LY (2012) Is Photooxidation Activity of 001 Facets Truly Lower Than That of 101 Facets for Anatase TiO2 Crystals? J Phys Chem C 116:26800–26804
Cui ZX, Zhao MZ, Lai WP, Xue YQ (2011) Thermodynamics of size effect on phase transition temperatures of dispersed phases. J Phys Chem C 115:22796–22803
Samsonov VM, Shcherbakov LM, Novoselov AR, Lebedevet AV (1999) Investigation of the microdrop surface tension and the linear tension of the wetting perimeter on the basis of similarity concepts and the thermodynamic perturbation theory. Colloids Surf A 160:117–121
Yang CC, Xiao MX, Li W, Jing Q (2006) Size effects on Debye temperature, Einstein temperature, and volume thermal expansion coefficient of nanocrystals. Solid State Commun 139:148–152
Li WJ, Xue YQ, Cui ZX (2016) Size dependence of surface thermodynamic properties of nanoparticles and its determination method by reaction rate constant. J Physica 495:98–105
Xue YQ, Luan CH, Fan JC (1999) The electrochemical thermodynamics for chemical reactions in dispersed cells. J Colloid Interface Sci 217:107–110
Yang YF, Xue YQ, Cui ZX, Zhao MZ (2014) Effect of particle size on electrode potential and thermodynamics of nanoparticles electrode in theory and experiment. Electrochimica Acta 136:565–571
Dean JA (1999) Lange’s Handbook of Chemistry. 15th edn. McGrawe-Hill Book Co, New York, p 8.113
Acknowledgements
The project is supported by the National Natural Science Foundation of China (Nos. 21373147 and 21573157).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., zhu, J., Xue, Y. et al. Size-dependent surface thermodynamic properties of silver oxide nanoparticles studied by electrochemical method. J Mater Sci 52, 1039–1046 (2017). https://doi.org/10.1007/s10853-016-0399-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0399-1