Abstract
Smart self-healing coatings have been attracting tremendous interest due to their capability for preventing crack propagation in the protective coatings by releasing active agents like isocyanate molecules from micro/nanocapsules. The quality of healed area and subsequent use of the healed coated module are directly related to the chemical composition of healing agent. Faster curing rate and more appropriate physical properties were anticipated for moisture curing of bulky isocyanate molecules than the low molecular weight monomeric analogous. For practical utilization of these advantages, encapsulation of such bulky isocyanate molecules was considered in this work. To this end, optimized preparation and characterization of novel single-layer polyurethane-type microcapsules, richly and efficiently loaded with bulky isocyanate molecules is described. This healing agent was prepared through the reaction of excess amount of isophorone diisocyanate (IPDI) with 2-ethyl-2-hydroxymethyl-1,3-propanediol (TMP). The healing agent was then encapsulated with a polyurethane shell via an oil-in-water (O/W) emulsion polymerization technique. The mixing rate and surfactant concentration were altered to optimize the size and shell thickness of the microcapsules. The prepared microcapsules were very stable after 10 months, and they just lost less than 7 wt% of their loaded isocyanate molecules. The microcapsules were loaded into an epoxy-based coating and the crack healing efficiency of incorporated healing agent was clearly recorded. Microcapsules containing monomeric IPDI were also prepared and crack healing efficiency of these two healing agents regarding crack healing was compared.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Self-healing materials have been receiving considerable attentions in coating industries due to their promising potential to diminish deterioration and reducing the overall maintenance cost of coatings [1–4]. The self-healing capability can be achieved through either intrinsic material properties (reversible covalent or non-covalent crosslinking) or extrinsic phases containing healing agents [4, 5]. In case of extrinsic self-healing materials, a small volume fraction of a healing agent is added to the base formulation of the coating material. Usually, healing agents are loaded into a carrier and then added to coating formulation. Application of different systems such as hollow glass fibers [6–8], microvascular networks [9, 10], electro-spun hollow fibers [11], and polymeric microcapsules [12–17] was reported in the literature as carriers for healing materials. Among different possible healing agents, application of those one-component materials, which needs no catalyst for their reactions, attracted more interest. Possible toxicity and high cost of catalysts as well as simplicity of systems containing fewer ingredients were main motivations for more concentration on catalyst-free systems. In this regard, synthesis and application of one-component and moisture cure system consisting of encapsulated isocyanate monomers was highlighted in recent open and patent literatures [18–25]. In practice, these materials can easily transform to a polyurea protective layer via activation and reaction with moisture without needing any other chemicals [26]. Also, by using isocyanates as healing agent, it is possible to prepare autonomous self-healing system in aqueous or humid environments.
Recently, the reports based on encapsulation of isocyanates have been limited to the blocked form of isocyanates [27, 28] and there are a few reports about encapsulation of liquid reactive isocyanates. For the first time, Sottos et al. [18] described the synthesis of polyurethane (PU)-based microcapsules filled with IPDI monomer. This monomer was microencapsulated into PU shell composed of a toluene diisocyanate-based prepolymer and 1,4-butandiol (BD) via an interfacial polymerization technique. Yang et al. [19] also reported microencapsulation of a more reactive aliphatic diisocyanate, hexamethylene diisocyanate (HDI), into PU shell. The shell was formed by in situ reaction of a commercially available methylene diphenyl diisocyanate (MDI)-based prepolymer and BD. The self-healing property of an epoxy resin-based coating containing 10 wt% of these microcapsules was evaluated through examination of healed area using scanning electron microscopy (SEM). The results showed healing of scratched region after 48 h immersion of coated sample in NaCl solution. In order to improve the stability and shelf life of encapsulated isocyanate monomer, Di Credico et al. [22] reported the preparation of IPDI monomer encapsulated into a double-layer shell composed of PU and urea-formaldehyde resin (PUF). The scratched region in the epoxy coating containing 15 wt% of above-mentioned microcapsules healed after 48 h immersion in NaCl solution. To improve micromechanical property of microcapsules shell, IPDI was encapsulated into the PUF shell reinforced with carbon nanotubes [23].
The self-healing property of protective alkyd varnish coatings (AVCs) containing IPDI-filled PUF microcapsules was studied by Wang et al. [24]. Recently, Nguyen et al. [25] reported encapsulation of isocyanurate trimer of HDI into a polyurea shell using an interfacial oil-in-water polymerization reaction. They found that the stability of microcapsules was improved by functionalization of shell’s outer surface with hydrophobic moieties. Reduction of water molecules affinity toward the hydrophobic shell and consequently lower diffusion through modified shell was the reason for observing such a behavior.
Close inspection of works done regarding isocyanate monomers encapsulation and preparation of corresponding self-healing coatings revealed that the rate of healing reactions and crack filling time were not satisfactory. In our opinion, this was the reason for evaluating the healing phenomena by immersion of scratched coating samples in aqueous media for long time. Another concern in this area is the quality of the healed area on the coating. It is clear that polyurea formed upon moisture curing of low molecular weight monomeric isocyanates cannot have enough mechanical strength due to high crosslink density or/and low molecular weight between crosslinked points. To overcome these limitations, replacing monomeric isocyanate by a higher molecular weight bulky isocyanate molecule in the form of isocyanate-terminated urethane prepolymer was considered in this study. The bulky isocyanate compound was prepared through NCO functionalization of a triol with controlled structure, and subsequently encapsulated into a polyurethane shell. The encapsulation condition was tuned to make microcapsules with desired morphology and acceptable healing agent loading. The stability of prepared microcapsules was evaluated by determining the healing agent content after 10 months storage under controlled conditions. The healing performance of a model epoxy-based coating containing the prepared microcapsules was studied and compared with similar system containing the same microcapsules composed of monomeric analogous healing agent. Visual inspection of healed scratched areas confirmed superior behavior of novel microcapsules developed in this study.
Experimental
Materials
Toluene diisocyanate (TDI), isophorone diisocyanate (IPDI), 2-ethyl-2-hydroxy methyl-1,3-propanediol (TMP), chlorobenzene (CB), 1,4-butandiol (BD), dibutyltin dilaurate (DBTL), N-methyl-2-pyrrolidone (NMP), and Gum arabic (GA) were purchased from Merck. Epoxy resin, KER 828, based on diglycidyl ether of bisphenol A (DGEBA) and its amine containing hardener (CRAYAMID 140C) was obtained from HEXION Specialty Chemicals and Cray Valley, respectively. TDI and IPDI were purified by vacuum distillation. TMP was freed from moisture in a vacuum oven at 60 °C overnight. CB was pre-dried by keeping on CaCl2 and then fully dried by distillation under reduced pressure over P2O5. Other chemicals were used as-received without further purification.
Synthesis of isocyanate-terminated prepolymer based on IPDI as healing agent (BIH)
Into a 500-mL three-necked round-bottomed flask equipped with N2 inlet/outlet, a dropping funnel, a condenser, a heating mantel, and a magnetic stirrer, IPDI (49.7 g, 0.22 mol) and CB solvent (30 mL) were added. The dropping funnel was charged with TMP (10 g, 0.07 mol) dissolved into 20 mL of CB solvent. The temperature was controlled at 50 °C and under magnetic stirring, TMP was dropped into the flask during 30 min. After adding one drop of DBTDL to the flask, the reaction temperature was increased to 75 °C. The reaction was continued until the measured NCO content of product reached the calculated theoretical value (15.7 %.). The final solid content of product was fixed on 40 wt% by adding extra CB solvent. This solution was kept refrigerated until subsequent use.
Synthesis of isocyanate-terminated prepolymer based on TDI as shell constituent (BIS)
Into a 500-mL three-necked round-bottomed flask equipped with N2 inlet/outlet, a dropping funnel, a condenser, a heating mantel, and a magnetic stirrer, TDI (38.28 g, 0.22 mol) and CB solvent (30 mL) were added. The dropping funnel was charged with TMP (10 g, 0.07 mol) dissolved into 20 mL of CB solvent. The temperature was controlled at 40 °C and under magnetic stirring, TMP solution was dropped into the flask during 45 min. One drop of DBTDL was added to the flask and then the reaction temperature was increased to 60 °C. The reaction continued until the measured NCO content of the product reached the calculated theoretical value (19.2 %.). Excess CB solvent was used to obtain the product with 40 wt% solid content. This solution was kept refrigerated until subsequent use.
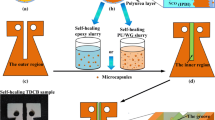
Synthesis of microcapsules containing BIH as core material (MBIH)
GA (13.2 g) and deionized water (120 mL) were placed into a three-necked round-bottomed polymerization kettle equipped with a mechanical stirrer (three-bladed propeller), a thermometer, a gas outlet, and an oil bath. The mixture was stirred at ambient temperature for 3 h. In a separate beaker was placed a mixture composed of BIH solution (30.43 g) and BIS (2.5 g). The beaker content was stirred magnetically and after complete homogenization (about 5 min), the resulting solution was transferred to dropping funnel. The content of funnel was added dropwise to the polymerization kettle during 15 min. During this step the speed of the mechanical stirrer was fixed at 500 rpm. The temperature of stably formed emulsion was increased to 50 ± 2 °C. Then the reactor was charged with BD (1.5 g) as chain extender and the temperature was gradually increased to about 70 ± 2 °C. Stirring of the reaction mixture was continued for about 1 h, then the resulting suspension was cool down to ambient temperature and stirring was continued overnight. A sinter glass filter was utilized for separation of the prepared microcapsules. They washed with distilled water for several times, and then left to dry at room temperature for 48 h.
The same procedure was repeated under different mixing speeds of 600, 750, and 850 rpm, and the effect of mixing speed on characteristics of resulting microcapsules was studied. Also, the effect of GA concentration on size and morphology of microcapsules was evaluated. For this purpose, different concentrations (wt/wt%) of GA (11, 22, and 33) were examined at fixed mixing speed of 750 rpm.
Synthesis of microcapsules containing IPDI as core material (MIPDI)
Microcapsules filled with IPDI monomer were synthesized by procedure described in “Synthesis of microcapsules containing BIH as core material (MBIH)” section. BIS (2.5 g) and IPDI (6.67 g) were mixed together and added to aqueous solution of GA (11 wt%). After the addition of BD (1.5 g), the stirring (600 rpm) of dispersion was continued at 50 ± 2 °C.
Preparation of self-healing epoxy coatings
10 wt% of microcapsules prepared at modified conditions (750 rpm and 22 wt% surfactant concentration) was gently added into the epoxy resin via magnetic stirring for 10 min. The mixture was placed in a low pressure oven for 30 min to remove bubbles and trapped air. A stoichiometric amount of the hardener, with a resin:hardener weight ratio of 2:1, was added to the mixture. Neat epoxy coating was also prepared as a control sample. The coating sample with a wet film thickness of 500 ± 20 μm was applied using film applicator (Zehntner ZUA2000 universal applicator 0–3000 µm) on the degreased PDFT sheet. The specimens were left for about 30 min at ambient temperature, postcured for 30 min at 80 °C, and then again left for 48 h at room temperature. Dry film thickness of the sample was measured as 325 ± 25 μm using Mega check 20-ST (Fe-NFe).
Measurements
The NCO content of prepolymers was determined according to the back titration procedure described in ASTM D 2572 using Eq. (1):
where B and V (mL) designate the volume of consumed HCl in titration of the blank and the sample, respectively. N represents normality of HCl, and W is the weight (g) of sample [18].
A Bruker EQUINOX 55 FTIR spectrophotometer (Ettlingen, Germany) was used for characterization of prepolymers and microcapsules. For this purpose, small amounts of microcapsules were crushed under cryogenic conditions. The extracted core and residual shell materials were then characterized separately.
The weight ratio of microcapsules to the initial weight of substances was considered as the reaction yield %, and calculated according to Eq. (2) [19]:
W Mic, W Pre-pol, W Diol, and W IPDI stand for weight of microcapsules, prepolymer, BD, and IPDI, respectively.
The core and shell contents were determined using solvent extraction method. To this end, a desired amount of microcapsules was crushed under cryogenic condition and the core materials were extracted from the shell using dried NMP solvent. The shell particles were then filtered and washed several times and each of them was examined separately.
For determination of encapsulated solvent content, small amount of microcapsules was placed in a low pressure oven (150 mmHg) at 100 °C and the weight changes were measured till the weight became constant. The weight of trapped solvent was calculated by subtraction of the initial and final weights of microcapsules.
The shape, morphology, and size of the synthesized microcapsules were analyzed using an optical microscopy (OM) (Olympus CX21FS1) equipped with a Canon Powershot SX40 digital camera and a scanning electron microscope (SEM) (Inca 250, Oxford) operated in the secondary electron mode at 20 kV. The mean diameter of the microcapsules was determined through at least 100 measurements in SEM micrographs and by analyzing the data in Image J software.
The quality of healed region in epoxy coatings containing either MBIH or MIPDI was examined by salt spray method. Anticorrosion self-healing coatings were prepared by dispersing 10 wt% of microcapsules into the epoxy resin/hardener (2/1 wt/wt) mixture. The mixture was degassed under low pressure by keeping in an oven for 30 min. Neat epoxy coating was also prepared as a control sample. A steel panel was surface treated by being polished with sandpaper NO. 400, degreased by acetone, and finally washed with water. After drying, a coating layer with a wet thickness of 500 μm and final thickness of 300–350 μm was applied on steel panel. After completion of curing reaction under previous described conditions, cross scratches were applied on the surface of panels by razor blade according to the procedure described in ASTM D 1653 test practice. All edges and backside of the coated substrates were covered with hot melt mixture of beeswax and colophony resin. 24 h after scratching the samples (the appropriate required time for completion of healing process), the coated specimens were placed in a salt spray chamber, following the procedure of ASTM B 117, and employing 5 wt% (50 g L−1) NaCl solution at 35 ± 1 °C. The surface image of samples was recorded at pre-determined time intervals to follow the progression of corrosion on coated samples with and without added microcapsules.
Stability of synthesized microcapsules was evaluated by visual inspection of SEM micrographs recorded for samples aged for 10 months under ambient conditions. The variation of microcapsules’ size and shape was considered as an indication of microcapsules stability.
Results and discussion
Synthesis and characterization of prepolymers and microcapsule
The synthetic routes followed for the preparation of prepolymers (BIH and BIS) needed as healing agent and shell constituent are depicted in Scheme 1. Both of these materials were prepared through the reaction of TMP with excess amounts of either IPDI or TDI.
It is clear that BIH with preformed urethane linkages made through the reaction of IPDI and TMP (an aliphatic tri-functional alcohol) has higher molecular weight than parent IPDI monomer. In fact, the reactive isocyanate groups of BIH are decorated over a bigger molecule; therefore, fewer numbers of reactions are needed for the production of network with enough dimensional stability and consequently better ability to cover underlying surface. In addition, BIH eventually can produce a polyurethane-urea network upon exposure to moisture. In comparison, for IPDI as healing agent the formed network mainly consists of urea linkages. For BIH-derived network, the chain segments between crosslinked points consist of flexible aliphatic groups of parent TMP; therefore, higher chance for molecular weight build-up of formed network to be preserved before reaching the verification point. However, for IPDI-derived system, the low molecular weight of segments between crosslinked points restricts the progress of network formation, and network with inferior dimensional stability forms in this case. [29, 30]. BIH was prepared through the reaction of TMP with IPDI. This reaction leads to decrease the NCO content and also increase the molecular weight of the basic reactants. Smaller amount of these bigger reactants are needed to achieve the network with sufficient dimensional stability. Therefore, in comparison with IPDI, BIH has higher rate of moisture curing.
The synthetic route followed for the preparation of isocyanate-filled microcapsules was adapted from the procedure reported by Di Credico et al. [22], which used polyisocyanate-based TDI (Desmodur L-75) and BD as main shell constituents as well as ethyl acetate solvent [22]. CB was used as solvent in whole process instead of ethyl acetate in the present work. CB is more hydrophobic than ethyl acetate and therefore, it can protect better the encapsulated isocyanate from the penetrated moisture. Also, its higher boiling point than ethyl acetate can lead to lower evaporation and more chance for remaining encapsulated during storage of microcapsules under ambient conditions.
Scheme 2 shows the reaction sequences for preparation of polyurethane shell. The water-soluble diol (BD) was used as chain extender for polyurethane shell formation because the relative rate for reaction of this diol with NCO group is first order of magnitude higher than the reaction of water with NCO group [18, 31]. Additionally, the diol could favorably diffuse into the organic phase and promote surface reactions prior to significant hydrolysis of isocyanate groups with water. The large difference in reactivity of IPDI and TDI [31, 32] provided the necessary condition for the formation of stable shell wall and the formation of microcapsules with a high BIH core content.
For chemical characterization of prepolymers and polyurethane shell, FTIR spectroscopy was utilized (Fig. 1). The FTIR spectra of the prepolymers showed sharp and intense peak at 2256 cm−1 related to the free NCO groups. However, there was no related peak at FTIR spectrum of neat shell compound that indicated complete reaction of BIS and BD. FTIR spectrum of the whole microcapsules showed characteristic bands at 3371 cm−1 associated to the stretching vibration of urethane N–H groups. Peak corresponding to urethane C=O stretching vibration was observed at 1704 cm−1. The combination of urethane N–H out of plane bending and urethane C–N stretching vibrations appeared as a peak at 1523 cm−1. Also, the presence of NCO group peak at FTIR spectrum of this compound indicated that the reactive isocyanate groups of BIS was successfully encapsulated. Therefore, the adapted method was effective for preserving isocyanate groups of core materials during polymerization procedure.
Among different parameters affecting the morphology, size, and size distribution of the microcapsules, variations of mixing rate and surfactant concentration were chosen in the present work. As rule of thumb, the optimum overall thickness of coatings should be about three times larger than the largest microcapsule [33]. Therefore, it was tried to control the microcapsules’ size under 100–120 µm, since the desired intended thickness of final coating was considered at about 300–350 µm.
To this end, the preparation procedure of microcapsules was repeated at different mixing rates of 500, 600, 750, and 850 rpm, while the surfactant concentration was fixed at constant amount of 11 wt%. In the second set of experiments, the constant mixing speed of 750 rpm was chosen and the effect of surfactant concentration at two levels of 22 and 33 wt% was studied. The optical and SEM images of synthesized microcapsules prepared at different conditions are shown in Fig. 2. Spherical microcapsules were obtained in all conditions except those microcapsules obtained at low mixing rate or high surfactant concentration. This finding was in accordance to the results reported by Yang and his co-workers regarding IPDI-filled PU microcapsules [18]. Under all of studied conditions, microcapsules with smooth outer surface with some wrinkles were produced. Inhomogeneous reaction kinetics, fluid-induced shear forces, and shell-determined elastic forces were considered as main determining factors for observed morphology of microcapsules [34].
Morphology of microcapsules (OM and SEM images in left and right columns) and shell wall profile (SEM image in right column) in different conditions a–d Surfactant concentration of 11 wt/wt% and mixing rate of 500, 600, 750, and 850 rpm respectively, and e, f Mixing rate of 750 rpm and surfactant concentration of 22 and 33 wt/wt%, respectively
The shell thickness of the microcapsules is a key parameter that affects its functionality. When the shell is too thick, the microcapsule will not rupture under applied forces, and therefore the core material cannot release and consequently self-healing is not reachable. In contrast, if the applied preparation procedure results in microcapsules with a thin-shell thickness, the rupture of shell and release of core material into polymeric matrix can occur during the mixing of these microcapsules into the matrix [28, 35]. Therefore, it is crucial to adjust shell thickness of microcapsules and tune their stiffness based on the specific properties of host coating matrix such as viscosity and surface-free energy. Figure 3 shows SEM micrographs of the ruptured microcapsules prepared at different conditions. The SEM analysis showed that the wall thickness for microcapsules varied in the range of 1.88–4.10 µm depending on the mixing rate (500–850 rpm) and surfactant concentration (11 to 33 wt%).
The recorded results showed that with increasing mixing speed, the particle size of microcapsule was decreased and the size distribution became narrower. The same results were also reported during microencapsulation of dicyclopentadiene in shell composed of urea–formaldehyde resin and for encapsulation of IPDI into a polyurethane shell material [18, 36]. Variation of mixing rate controls the equilibrium between shear forces and interfacial tensions [37]. At low mixing rate, interfacial tension dominates and droplets remain large but at higher mixing rate, droplets experience strong shear forces and as a result, larger droplets are broken into the smaller ones. The size distribution of the prepared microcapsules plays an important role on practical performance of the modified coatings. Therefore, the mixing rate of 850 rpm was not selected as the optimum condition, since at this rate a wide distribution of microcapsules size was attained. Based on the recorded data, the mixing rate of 750 rpm was selected and the surfactant concentration was adjusted for this mixing speed. The effect of variation of mixing speed and wt% of surfactant on the characteristic properties of microcapsules is tabulated in Table 1. By increasing the mixing rate, the average size and shell thickness of the microcapsules were decreased. In constant wt% ratio of core/shell, with increasing mixing speed, the ratio of volume/surface area is decreased, therefore, the thickness of microcapsules’ shell is decreased and less polymerized materials are formed around the core droplets [18].
However, by increasing the surfactant wt%, the size and shell thickness of the microcapsules were firstly decreased and then increased. The exact mechanism by which surfactant concentration influences the microcapsule formation is not still clear [19], but it is accepted that the surfactant concentration influences the interfacial tension of emulsion media before the critical micelle concentration (CMC) is reached [38, 39]. It is believed that higher surfactant concentration yields smaller oil droplets in oil-in-water emulsion system and as a result, microcapsules with smaller diameter were formed [40]. Yang et al. [19] reported that beyond CMC, no considerable change in diameter of microcapsules can occur. However, in the present study, the diameter of the microcapsules became bigger by increasing the surfactant wt% from 22 to 33. This observation indicated that the CMC of the employed GA was in the range of 22–33 wt%, and beyond the CMC the interfacial tension might be a dominating factor and oil droplets might be coagulated. Therefore, the ellipsoidal microcapsules with bigger diameter were produced. Based on the obtained results, the optimum condition for microencapsulation was selected as 750 rpm for mixing rate and 22 wt% for surfactant concentration.
For microcapsules prepared at different conditions, yield of synthesis, the fraction of encapsulated BIH, and the amount of trapped solvent were evaluated (Table 1). The yield was calculated at about 42 ± 2.5 wt% under different conditions. The yield value has not been significantly affected by variation of mixing rate and surfactant concentration. Similar results were also reported by Huang and Yang for polyurethane-based microcapsules filled with HDI [19].
The characteristic properties of encapsulated BIH fraction prepared at various conditions are tabulated in Table 1. The fraction of encapsulated BIH was slightly decreased by either increasing the mixing speed or surfactant wt%. Similar result was also reported for PU microcapsules filled with IPDI [18] and HDI monomers [19]. This phenomenon was attributed to the easier diffusion of the chain extender (BD) and water throughout the thinner microcapsule shells produced at higher mixing rates. Consequently, more BIH molecules may be deactivated via reaction with either water or BD [19]. Correspondingly, the solvent content was increased with increasing mixing rate. However, the maximum solvent content was observed for microcapsules prepared at 22 wt% surfactant, indicating the dependency of solvent content on microcapsules’ shell thickness. For microcapsules with thinner shell thickness, the solvent can diffuse easily across the shell, and accordingly more trapped solvent was expected for microcapsules with thinner shell. Comparable results were reported by Sottos et al. [18] for microcapsules containing IPDI.
Self-healing epoxy-based coatings containing MBIH
The healing performance of the coating containing microcapsules strongly depends on the size of microcapsules and amount of encapsulated healing agent [41, 42]. Better filling of defect can be achieved by much more amount of healing agent within the cracked area. Healing efficiency of an epoxy-based coating containing 10 wt% MBIH microcapsules was evaluated. An artificial crack was created on the coated panel using a scalpel blade No 11. The same crack was created on a neat epoxy coating. The scratched area was then inspected, by evaluating the images obtained via scanning electron microscopic analysis (Fig. 4). It was clearly observed that the crack created in neat epoxy coating remained unfilled, but the crack on the coating containing MBIH was successfully filled with released healing agent from the ruptured microcapsules.
Same method was followed for evaluation of healing performance of coatings containing encapsulated HDI [19] and IPDI monomers [22]. In these works, the scratched coatings were immersed in salt water solution for 24 h, however, in the current study, the coating was kept under ambient conditions. In fact, due to higher molecular weight of BIH in comparison to HDI or IPDI, the molecular weight build-up and production of cured material could occur faster and more efficiently.
For further examination of this observation, BIH and IPDI were applied directly on the glass substrate with a wet film thickness of 5 μm using Doctor Blade film applicator. Drying rate of the films was then evaluated according to procedure described in ASTM D 1640 standard test practice. While a tacking and a curing time of about 15 min and 10 h was attained for BIH, the IPDI film remained at liquid form even after 3 days exposure at ambient conditions. The faster curing of BIH in comparison to IPDI was evidenced by result of this experiment.
Additional information regarding higher ability of BIH for formation of film with better quality than IPDI was obtained by SEM examination of healed area of cracked epoxy coatings loaded by equal amounts of microcapsules containing BIH or IPDI. Figure 5 shows the SEM micrographs of the fracture surface of epoxy coatings containing 10 wt% of two different categories of the microcapsules after conditioning under ambient atmosphere for 24 h. The micrographs show that the crack was healed by released BIH (Fig. 5a), while the crack was still empty for sample loaded with IPDI containing microcapsules (Fig. 5b).
To find out better perspective regarding improved healing performance of coatings containing MBIH microcapsules over corresponding ones containing monomeric isocyanate, MIPDI, the scratched coated panels were subjected to salt spray experiment. The visual observations of the samples during 1 week exposure to natural salt spray chamber are shown in Fig. 6. It was clearly observed that the corrosion process in the scotched area began after 48 h for the sample containing 10 wt% MIPDI, however, the corrosion process was not started till 1 week passed from exposing of panel to corrosive environment. This result was further evidence for the superior performance of BIH compared with IPDI as healing agent. Better coverage of damaged area upon moisture curing of released BIH was the reason for the observed behavior.
Shelf life of microcapsules
To find out some information regarding stability and shelf life of BIH-loaded microcapsules prepared under different conditions, the weight of extractable BIH was determined after storing the microcapsules under ambient conditions for 10 months. The results were then compared with measured weight of BIH extracted from fresh microcapsules [18, 19, 22]. The results (Table 1) show that the microcapsules prepared at higher mixing rate lost higher amount of their encapsulated BIH upon aging. Also, by changing the surfactant amount at constant mixing rate, the highest stability (the lowest loss of encapsulated healing agent) was obtained for microcapsules prepared with highest amount of surfactant. Keeping the previous results in mind, it was concluded that the stability of microcapsules and preservation of encapsulated healing agent directly related to shell thickness of microcapsules. The microcapsules with thicker shell acted as better barrier against penetration of moisture. Consequently for such microcapsules, lower amounts of encapsulated BIH molecules were deactivated via reaction with penetrated moisture [19]. Yang et al. [18] also reached to the similar result in the case of polyurethane microcapsules filled with IPDI as healing agent.
For better elucidation of the stability of microcapsules, the morphology of aged microcapsules was examined by SEM images and compared with fresh microcapsules (Fig. 7). These images confirmed that the microcapsules kept their spherical shape after aging for ten months. The same observation was reported by Di Credico et al. [22] for polyurethane microcapsule filled with IPDI after 6 months of aging.
The practical importance of microcapsules stability was further evidenced by evaluating the gap-filling ability of aged microcapsules loaded into an epoxy coating. For this purpose, an epoxy coating containing 10 wt% of microcapsules synthesized 10 months ago was prepared. A crack was created on the coated panel using scalpel blade. The SEM analysis of scratched region (Fig. 8) showed that the crack was completely filled by released core materials. This result confirmed the suitability of reaction condition applied for preparation of intended microcapsules.
To get more information regarding the quality of prepared coating throughout the healed area, the coatings were subjected to electrochemical impedance spectroscopy assay, and the tensile strength of intact, scratched, and healed coating samples was measured. Some more comprehensive salt spray evaluation was also considered. The results of these studies will be presented in Part II of this study.
Conclusion
A bulky polyurethane prepolymer based on IPDI, BIH, as a reactive moisture curable healing agent was prepared and encapsulated into polyurethane-based shell through an oil-in-water interfacial polymerization technique. Two main parameters including mixing rate and surfactant concentration were optimized to reach to the spherical microcapsules with proper mean diameter (45 ± 1.8 µm) and shell thickness (1.88 µm). The mean diameters of the microcapsules and their shell thickness were decreased by increasing the mixing rate. By increasing mean diameter of microcapsules, the healing agent fraction was decreased and the solvent content was increased. The yield of synthesized microcapsules was about 42 %. SEM analysis approved the stability of microcapsules aged for 10 months under ambient condition. And most importantly, both fresh and aged microcapsules containing BIH loaded into an epoxy coating could fill the cracked area and heal the damaged surface successfully and much better than corresponding system made from IPDI as healing agent. The result of salt spray experiment also confirmed better performance of MBIH-containing coating for healing and coverage of damaged area over monomeric isocyanate analogous system (MIPDI).
References
Trask R, Williams H, Bond I (2007) Self-healing polymer composites: mimicking nature to enhance performance. Bioinspir Biomim 2:1–12
Wu DY, Meure S, Solomon D (2008) Self-healing polymeric materials—a review of recent developments. Prog Polym Sci 33(5):479–522
Ghosh S (2009) Self-healing materials: fundamentals, design strategies, and applications. Wiley, Weinheim
Blaiszik BJ, Kramer SLB, Olugebefola SC, Moore JS, Sottos NR, White SR (2010) Self-healing polymers and composites. Annu Rev Mater Res 40:179–211
Ji Xiaofan, Huang Feihe (2015) A rapidly self-healing supramolecular polymer hydrogel. J Mater Sci 58(3):436–437. doi:10.1007/s11426-015-5338-5
Pang JWC, Bond IP (2005) A hollow fiber reinforced polymer composite encompassing self-healing and enhanced damage visibility. Compos Sci Technol 65(11–12):1791–1799
Trask R, Bond I (2006) Biomimetic self-healing of advanced composite structures using hollow glass fiber Smart. Mater Struct 15:704–710
Williams H, Trask R, Bond I (2007) Self-healing composite sandwich structures. Smart Mater Struct 16(4):1198–1207
Bejan A, Lorente S, Wang KM (2006) Networks of channels for self-healing composite materials. J Appl Phys 100(033528):1–6
Toohey K, Sottos N, Lewis J, Moore J, White S (2007) Self-healing materials with microvascular networks. Nat Mater 6:581–585
Park JH, Braun PV (2010) Coaxial electrospinning of self-healing coatings. Adv Mater 22:496–499
White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S (2001) Autonomic healing of polymer composites. Nature 409:794–797
Lewis G, Wellborn B, Jones L, Biggs P (2009) A room-temperature autonomically-healing PMMA bone cement: influence of composition on fatigue crack propagation rate. J Appl Biomater 7:90–96
Jackson AC, Bartelt JA, Marczewski K, Sottos NR, Braun PV (2011) Silica-protected micron and sub-micron capsules and particles for self-healing at the microscale. Macromol Rapid Commun 32:82–87
Coope TS, Mayer UFJ, Wass DF, Trask RS, Bond IP (2011) Self-healing of an epoxy resin using scandium (III) triflate as a catalytic curing agent. Adv Funct Mater 21:4624–4631
Lee J, Bhattacharyya D, Zhang MQ, Yuan YC (2011) Fracture behaviour of a self-healing microcapsule-loaded epoxy system. Express Polym Lett 5(3):246–253
Cho SH, White SR, Braun PV (2009) Self-healing polymer coatings. Adv Mater 21(6):645–649
Yang J, Keller MW, Moore JS, White SR, Sottos NR (2008) Microencapsulation of isocyanates for self-healing polymers. Macromolecules 41:9650–9655
Huang M, Yang J (2011) Facile microencapsulation of HDI for self-healing anticorrosion coatings. J Mater Chem 21:11123–11130
Huang M, Yang J (2014) Salt spray and EIS studies on HDI microcapsule-based self-healing anticorrosive coatings. Prog Org Coat 77:168–175
Khun NW, Sun DW, Huang MX, Yang GL, Yue CY (2014) Wear resistant epoxy composites with diisocyanate-based self-healing functionality. Wear 313:19–28
Credico BD, Levi M, Turri S (2013) An efficient method for the output of new self-repairing materials through a reactive isocyanate encapsulation. Eur Polym J 49:2467–2476
Wang W, Xu L, Liu F, Li X, Xing L (2013) Synthesis of isocyanate microcapsules and micromechanical behavior improvement of microcapsule shells by oxygen plasma treated carbon nanotubes. J Mater Chem A 1:776–782
Wang W, Xu L, Li X, Yang Y, An E (2014) Self-healing properties of protective coatings containing isophorone diisocyanate microcapsules on carbon steel surfaces. Corros Sci 80:528–535
Nguyen LT, Hillewaere XKD, Teixeira RFA, Berg OVD, Prez FED (2015) Efficient microencapsulation of a liquid isocyanate with in situ shell functionalization. Polym Chem 6:1159–1170
Biliet S, Hillewaere XKD, Teixeira RFA, Du Prez FE (2013) Chemistry of crosslinking processes for self-healing polymers. Macromol Rapid Commun 34:290–309
Cheong IW, Kim JK (2004) Synthesis of core–shell polyurethane–urea nanoparticles containing 4,40-methylenedi-p-phenyl diisocyanate and isophorone diisocyanate by self-assembled neutralization emulsification. Chem Commun 21:2484–2485
Kessler MR, Mauldin TC, Hondred PR, Ding R (2011) Biorenewable polymers and composites with self-healing functionality. 18th International conference on composite materials
Mishra AK, Narayan R, Raju KVSN (2012) Structure-property correlation study of hyper branched polyurethane-urea (HBPU) coatings. Prog Org Coat 74(3):491–501
Kantheti S, Sarath PS, Narayan R, Raju KVSN (2013) Synthesis and characterization of triazole rich polyether polyols using click chemistry for highly branched polyurethanes. React Funct Polym 73:1597–1605
Shaffer MW. In: ICE 2004 technology conference, Chicago, Oct 25–29, 2004
Ni P, Zhang M, Yan N (1995) Effect of operating variables and monomers on the formation of polyurea microcapsules. J Membr Sci 103(1–2):51–55
Kumar A, Stephanson L, Murry J (2006) Self healing coatings for steel. Prog Org Coat 55(3):244–253
Finken R, Seifert U (2006) Wrinkling of microcapsules in shear flow. J Phys 18:185–191
Murphy EB, Wudl F (2010) The world of smart healable materials. Prog Polym Sci 35:223–251
Brown EN, Kessler MR, Sottos NR, White SR (2003) In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. J Microencapsul 20:719–730
Rallison JM (1984) The deformation of small viscous drops and bubbles in shear flow. Annu Rev Fluid Mech 16:45–66
Kuo YM, Wu CT, Wu WH, Chao DY (1994) Effect of surfactants on the particle sizes of red 170 polyurea microcapsules. J Appl Polym Sci 52:1165–1173
Yoshizawa H, Kamio E, Hirabayashi N, Jacobson J, Kitamura Y (2004) Membrane formation mechanism of cross-linked polyurea microcapsules by phase separation method. J Microencapsul 21:241–249
Tcholakova S, Denkov ND, Danner T (2004) Role of surfactant type and concentration for the mean drop size during emulsification in turbulent flow. Langmuir 20:7444–7458
Behzadnasab M, Esfandeh SM, Mirabedini MJ, Zohuriaan-Mehr RR (2014) Farnood, preparation and characterization of linseed oil-filled urea-formaldehyde microcapsules and their effect on mechanical properties of an epoxy-based coating. Colloids Surf A 457:16–26
Huang M, Yang J (2011) Facile microencapsulation of HDI for self-healing anticorrosion coatings. J Mater Chem 21:11123–11130
Acknowledgement
The authors would like to acknowledge Iran Polymer and Petrochemical Institute for the financial support during the course of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haghayegh, M., Mirabedini, S.M. & Yeganeh, H. Microcapsules containing multi-functional reactive isocyanate-terminated polyurethane prepolymer as a healing agent. Part 1: synthesis and optimization of reaction conditions. J Mater Sci 51, 3056–3068 (2016). https://doi.org/10.1007/s10853-015-9616-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9616-6