Abstract
A novel strategy was designed for the preparation of a long-chain branched polypropylene (PP) with improved foamability via reactive extrusion in the presence of supercritical CO2 (scCO2). Benzoyl peroxide was used as a radical initiator and trimethylolpropane triacrylate (TMPTA) was applied as a polyfunctional reactive monomer during extrusion. Fourier transform infrared spectroscopy and high temperature GPC confirmed that TMPTA was grafted onto PP chains, and the presence of scCO2 promoted the grafting and branching reactions, and hindered polymer degradation. A possible mechanism was proposed to explain the effect of scCO2 on the branching reactions. In addition, rheological behavior of pure PP and modified PP samples was studied to investigate the effect of long chain branching of PP on the melt viscosity and strength, and foaming behavior was studied to confirm the subsequent effect on its foamability. It was found that the long chain branching increased the melt viscosity and strength of modified PP samples, which favored the foamability, and that the foaming windows were expanded in the presence of scCO2. Thus, it provided an advanced foaming approach via preparation of long-chain branched PP through reactive extrusion with scCO2 both working as the reactive medium and the foaming agent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypropylene (PP) has many desirable properties, such as good thermal stability, good mechanical properties, excellent chemical resistance, and easy recycling. These merits have made PP widely applied in packaging, automotive electronic, electrical equipment, etc. [1, 2]. However, conventional PP has low melt strength due to its linear molecular structure, which severely limits its wide use in the applications, such as foaming, thermoforming, and blow molding [3–5]. Therefore, it is technically required to prepare high melt strength PP to enhance its end properties and thus to better serve the foaming applications.
Long chain branching on PP backbones has been proved to be an effective approach to obtain high melt strength PP. Up to now, several approaches have been developed to prepare long-chain branched PP, including in situ polymerization [6–9], high-energy electron beam irradiation [10–13], and reactive extrusion [14–17]. Among these methods, reactive extrusion was the most promising one due to its merits such as low cost, easy operation, and high productivity. During the conventional reactive extrusion process, PP, peroxides, and polyfunctional monomers are fed into an extruder, where the grafting reaction occurs in a temperature range of 30–50 °C higher than the melting point of PP. The reaction temperature is relatively high, which can cause PP to undergo severe thermal degradation [18]. Indeed, such a degradation was reported to alter its molecular weight and distribution, and deteriorate mechanical performance of the final products [19].
In recent years, several approaches have been used to suppress the degradation and to improve the grafting degree of PP in reactive extrusion process. For example, Graebling [14] prepared long-chain branched PP via the reactive extrusion in the presence of thiuram disulfide, which effectively suppressed β-scission and increased the branching efficiency. Shi et al. [20, 21] found that pre-confinement of dicumyl peroxide in the lamellar structures of organically modified montmorillonite slowed down the release of primary radicals and significantly improved the selectivity of the grafting of maleic anhydride onto PP and decreased the probability of chain scission of PP. Cartier et al. [22] reported that the addition of styrene as a second monomer increased both the rate and yield of the grafting reaction of glycidyl methacrylate (GMA) and reduced PP chain scission. However, the above-mentioned three strategies require the addition of extra chemical agents to the extruder, which resulted in byproducts in the final products.
Recently, supercritical carbon dioxide (scCO2) has been applied to reduce PP chain scission in both solid-state [23–26] and molten [27, 28] grafting processes. The scCO2 is usually used as a green solvent due to its excellent physical properties, such as a moderate supercritical condition for easy processing, non-toxicity, and low cost [29]. The excellent swelling property of scCO2 promotes the diffusion of both the monomers and the radicals during extrusion. Additionally, scCO2 can significantly reduce the viscosity of polymer melt due to an increase in the free volume. As a result, the reactive temperature can be significantly decreased, and hence the degradation of PP can be prominently reduced. For example, Cao et al. [28] prepared maleic anhydride-grafted PP (PP-g-MAH) via reactive extrusion in the presence of scCO2, and it was found that the grafting was significantly promoted. However, till now, no systematic work has been reported about the preparation of long-chain branched PP via reactive extrusion in the presence of scCO2 for an advanced foaming technology. In this perspective, scCO2 both works as a foaming agent and a reactive extrusion medium. Polymer degradation during reactive extrusion would be hindered, while long chain branching of PP would render higher melt strength. Therefore, it is highly expected that the advances of the new strategy would be in favor of an expansion of the foaming window and an improvement of the foam structure.
The main objective of this study was to prepare long-chain branched PP via reactive extrusion with supercritical CO2 working both as the reactive extrusion medium and the foaming agent, and thus to improve the foaming behavior and cell structure of PP. In the reactive extrusion, benzoyl peroxide (BPO) was used as the radical initiator, and trimethylolpropane triacrylate (TMPTA) was applied as the polyfunctional monomer. Fourier transform infrared spectroscopy (FTIR) was used to confirm whether the monomer was grafted on modified PP chains after reactive extrusion and further to study the effect of scCO2 on the grafting degree and the grafting efficiency. High temperature GPC (HT-GPC) was applied to study the molecular weight change. A simple schematic was present to show how grafting and branching reactions might happen. A possible mechanism was proposed to explain the effect of scCO2 on those reactions. Rheological behavior of the pure PP and modified PP samples was studied to investigate the effect of branching of PP on the melt viscosity and strength. Foaming behavior of the pure PP and modified PP samples was studied to investigate how the long chain branching affected the foamability of the modified PP samples, and how the foaming window for modified PP samples was changed in the presence of different contents of scCO2. It was found that the presence of scCO2 effectively reduced polymer degradation and enhanced the branching reactions in preparation of long-chain branched PP compared to the traditional reactive extrusion without scCO2. Therefore, this study provided an effective and inexpensive means to obtain PP foams with a well-defined cell structure and a high expansion ratio, contributed from the long-chain branched PP in situ formed in the reactive extrusion with scCO2 both working as the reactive extrusion medium and the foaming agent.
Experimental
Materials
Linear commercial homopolymer PP, PPH-T03 with MFI of 2.8 g/10 min at 230 °C/2.16 kg, was provided by Sinopec Zhenhai Refining & Chemical Company (China). The initiator benzoyl peroxide (BPO) was provided by Taian Xingguo Industrial & Trade Co., Ltd. The polyfunctional monomer (trimethylolpropane triacrylate, TMPTA) was purchased from DSM-AGI Corporation. The antioxidant Irganox 1010 was supplied by BASF Corporation (China). The physical blowing agent, CO2 (99 % purity), was obtained from Ningbo Wanli Gas Corporation.
Equipment and sample preparation
The tandem extrusion system used for reactive extrusion is shown in Fig. 1. It consists of two extruders, an ISCO syringe pump to inject scCO2 into the polymer melts, a heat exchanger with homogenizing static mixers, a gear pump (Zenith, PEP-II), and an extrusion rheological device. The first extruder with a screw diameter of 45 mm was driven by a 25 hp drive motor (SIMO, Z4-132-3), and the second extruder with a screw diameter of 65 mm was driven by a 40 hp drive motor (SIMO, Z4-160-31). Other details of the experimental apparatus can be found in the reported literature [30].
For better mixing of BPO and TMPTA in PP pellets, they were dissolved in acetone, and then the mixture was blended with PP in a high-speed mixer. The mixture was stirred for 10 min and then placed in a ventilating cabinet till the acetone was volatilized. Finally, the modification of PP was carried out in a continuous extrusion process. Formulations of the pure PP and modified PP samples are shown in Table 1. The scCO2 was injected into the extruder at the three quarter points of the first extruder, and the compressed pressure was maintained at 18 MPa using a syringe pump and pressure regulators. The flow rate was adjusted to keep the scCO2 concentration at 3.0, 5.0, and 7.0 wt% of that of the mixture. The temperature was set at 190 °C for the first extruder, while for the second extruder, it was set at 190, 170, 165, and 160 °C for the samples prepared with 0, 3.0, 5.0, and 7.0 wt% scCO2, respectively. The temperature of the die was set up the same as that of the second extruder. Foamed pure PP and modified PP samples were collected for purification.
The collected samples were dissolved in xylene at 145 °C for 1 h, and then the solutions were transferred into acetone with electromagnetic stirring for 6 h. The unreacted TMPTA monomers and copolymerized TMPTA remained soluble, while PP and modified PP were precipitated. After that, the solutions were filtered by Buchner funnel and the solid was collected. The process (dissolving, precipitation and filtration) was repeated three times to ensure the purity of the samples. After the samples were dried at 80 °C for 24 h, they were compressed into films at 190 °C for characterizations.
Characterizations
Fourier transform infrared spectroscopy (FTIR) spectra of samples were obtained using an IR spectrometer (Nicolet 6700, Thermo Scientific, Inc) with Omnic software for data collection and analysis.
Molecular weight and distribution were investigated using a high-temperature gel permeation chromatography (HT-GPC, PL-GPC220) with 1, 2, 4-trichlorobenzene as solvent at a concentration of 0.1 mg/ml. Measurements were carried out at 150 °C with a flow rate of 1 ml/min. Based on a previous study [31], for samples with low contents of long chain branches, the classical GPC (without multi-sensor) can provide some qualitative information on the molecular weight. Therefore, the classical GPC was applied in this study to measure the molecular weight change. The GPC was calibrated with polystyrene (PS) standards with narrow molecular weight distribution, covering the molecular weight range from 103 to 107 g/mol. Then, the calibration curve of PS was converted to PP through calculation with the Mark–Houwink constants.
Rheological measurements were conducted for oscillatory shear and uniaxial extensional flow on an ARES rotational rheometer (Physica MCR-301). Shear measurements were carried out in parallel plate geometries (25, 1 mm gap) in a frequency range of 0.01–100 rad/s at 190 °C. The extensional experiments were performed at 190 °C using the extensional viscosity test method (SER mode) with an extension rate at 0.5 s−1.
The foamed samples were immersed in liquid nitrogen to freeze fracture the samples for scanning electron microscopic (SEM) examination. A TM-1000 SEM was used to investigate the morphology of the fractured surface at an acceleration voltage of 4.0 kV after a layer of gold was sputtered. The mass densities of the samples before (ρ) and after (ρ f) foaming were measured by the water displacement method based on ISO 1183-1987. Cell size and density of the foamed samples were determined from the SEM micrographs. The cell diameter was the averaged size of at least 100 cells on the SEM micrographs. The cell density (N 0), defined as the number of cells per cubic centimeter of unfoamed polymer, was determined using Eq. 1 as follows:
where n is the number of cells in the SEM micrograph, M is the magnification factor, and A is the area of the micrograph (cm2). ϕ is the volume expansion ratio of the polymer foam, which can be calculated through Eq. 2:
Results and discussion
Effect of scCO2 on grafting degree
FTIR spectra of the purified PP samples are shown in Fig. 2a. Compared to the pure PP, a new absorption band at 1735 cm−1 was observed in the samples modified with TMPTA, which was assigned to the absorption of the carbonyl groups (–C=O) derived from TMPTA molecules. This observation indicated that TMPTA was grafted onto the PP chains. The absorption at 841 cm−1 was assigned to the absorption of the CH3 groups in the PP backbone [32]. Therefore, the carbonyl index (CI), which was described as the absorbance ratio of the areas of the bands at 1735 and 841 cm−1 (CI = A 1735/A 841), could be used to investigate the extent of the grafting reaction [33]. As shown in Fig. 2b, the CI values increased with increased scCO2 concentration, indicating that the presence of scCO2 enhanced the grafting reaction.
In order to quantitatively investigate the effect of scCO2 on the grafting reaction, the grafting degree and grafting efficiency are needed to be calculated using the CI values. In this study, the grafting degree was defined as the weight fraction of TMPTA in the modified PP samples, and the grafting efficiency referred to the percentage of the TMPTA grafted onto the PP backbones. To calculate the grafting degree and efficiency, a calibrated CI curve was needed to build the relationship between the CI value and the content of TMPTA in the modified PP samples. The blends of PP with 0.5, 1.0, 2.0, and 4.0 wt% TMPTA were prepared by continuous extrusion, respectively. Subsequently, the blends were compressed into films for FTIR characterization without purification. The CI values for these samples were obtained in the same way as explained in Fig. 2. Thereby, a calibrated CI curve is obtained in Fig. 3a based on the dependence of CI values on the TMPTA contents in the PP compounds. The equation was obtained as “Y = −0.01 + 1.296X,” in which Y and X represent CI value and TMPTA contents, respectively. When the CI values obtained in Fig. 2b were put into the equation, the contents of TMPTA in the grafted PP samples were obtained, which was defined as the grafting degree. As the TMPTA content in the formulation of PP samples for reactive extrusion was set (Table 1), the grafting efficiency was obtained as the value of the grafting degree divided by that value. Figure 3b shows the grafting degree and grafting efficiency for the modified PP samples. It was found that with scCO2 content increasing from 0 to 7.0 wt%, the grafting degree increased from 0.95 to 1.38 wt%, and the grafting efficiency increased from 23 to 35 %. It indicated that scCO2 played an important role in favoring the grafting reaction.
Effect of scCO2 on molecular weight change
It was well accepted that in reactive extrusion process, a higher temperature would cause severe polymer degradation, whereas a lower temperature could hinder the degradation. In this study, the processing temperature in reactive extrusion was dramatically decreased in the presence of scCO2, so it is highly expected that the degradation would be reduced. HT-GPC results for the pure PP and modified PP samples are shown in Fig. 4. When only peroxide was loaded, the molecular weight of PP1 was significantly decreased, as severe chain scission occurred via β-scission of tertiary macroradicals, formed after hydrogen abstraction by the primary radicals decomposed by the peroxide. When both the peroxide and the monomer (TPMTA in this study) were loaded (PP2), the molecular weight of the modified PP was increased compared to that modified only with radical initiator (PP1). Based on the literatures [32, 33], the addition of a polyfunctional monomer stabilized the macroradicals through converting some of the tertiary ones into the more stable secondary ones, which tended to undergo recombination (branching reactions). Through the branching reactions, the molecular weight was increased. Further, in the presence of 7.0 wt% scCO2 as the reactive medium, PP5 showed a higher molecular weight than PP2, which was prepared without scCO2. It indicated that the presence of scCO2 further suppressed the chain scission and promoted the branching reactions. However, it should be pointed out here that the weigh average molecular weight of all the modified PP samples was lower than that of pure PP. It meant that even with scCO2 as the reactive medium, polymer degradation still happened to the modified samples, probably through β-scission, but the presence of scCO2 did restrict the polymer degradation to some extent.
A schematic about grafting and branching reactions
To give a clear picture how the grafting and branching reactions took place in the reactive extrusion, a schematic of reactions is presented in Fig. 5. Possible reactions that might occur in the extrusion include (not limited to) the following: The radical initiator (BPO) was decomposed into primary radicals (RO·) (reaction (1)), which could react with the monomers (TMPTA) to form TMPTA copolymers (reaction (2)), or could react with PP to form macroradicals (reaction (3)). The tertiary macroradicals, e.g., (a) could undergo β-scission to form (b) and (c) (reaction (4)), or it could be stabilized by reacting with the monomers to form secondary radicals like (d) (reaction (5)). The macroradical (d) could subsequently abstract hydrogen radical to be stabilized, forming short chain-branched PP, or it could react with (a), (b), (c), or (d) to eventually form long-chain branched PP. It could be easy to understand from Fig. 5 that the reactions, like (5) were grafting reactions, while those like (6), (7), (8), and (9) were branching reactions, and that grafting should occur before the relative branching. It might be worth mentioning that a successful grafting of the monomers onto PP chains would not necessarily mean that a long branched chain (mainly PP) would be produced; however, a short branched chain (polymerized TMPTA) could also be possible. In other words, not every grafting point would necessarily produce a long branched chain. Anyway, the schematic was present only to clearly and simply give a picture how grafting and branching reactions might happen. Many other reactions could also take place in the reactive extrusion, such as multiple chain scission reactions occurring on one PP chain, attacked by primary radicals at different sites, or branching reactions through recombination of different kinds of macroradicals, etc. In addition, further chain scission on grafted or branched PP samples could also happen, which ultimately result in lower molecular weight of the modified PP relative to the pure PP. However, the detailed discussion of additional reactions is beyond the scope of the present work. In the rest of the study, the effect of scCO2 on preparation of long-chain branched PP and the consequent foamability will be specially and intensively discussed.
Mechanism of enhanced branching reactions with scCO2
Based on the results above, scCO2 played an important role in suppressing the degradation of PP and enhancing the grafting and branching reactions. Figure 6 shows a possible mechanism for the effect of scCO2 on the grafting and branching reactions. It was supposed that all the species, including primary radicals (formed by decomposition of BPO), monomers (TMPTA), the macromolecular chains (PP), and macroradicals (PP macroradicals) existed in a big cube. The following reactions might occur besides β-scission as was discussed earlier (including but not limited to): first, the primary radicals could react with TMPTA monomers to form TMPTA homopolymers; second, the primary radicals could react with PP chains to form macroradicals; third, the TMPTA monomers could react with the PP macroradicals to form short branched chains (grafting reactions); and fourth, macroradicals grafted with TMPTA could react with each other or with those containing no TMPTA short chains via recombination to form long-chain branched PP (branching reactions). However, as the concentrations of BPO and TMPTA in this study were not high, the reactions above might be relatively slower. Therefore, the primary radicals, monomers, and all the macroradicals could be considered to be confined in a small space encased with a small dashed cube, known as the cage effect [28]. While when scCO2 was added, its strong plasticizing effect and diffusivity promoted the mobility of all these species [26, 34]. As a result, they could move more frequently in a larger spatial scale, which could be imagined to be in a larger solid cube. Consequently, the probability for macroradicals to react with monomers (grafting) and macroradicals (branching) was substantially increased. Actually, the presence of scCO2 not only promoted the grafting and branching reactions, but also restrained the competing reaction, i.e., β-scission of the macroradicals, through stabilizing the macroradicals. As reported in a previous study [28], the macroradicals either were stabilized via reaction with monomers (grafting), or eliminated via recombination (branching). As discussed about the results of FTIR and HT-GPC, both the grafting and branching reactions were promoted in the presence of scCO2. It could be said that as long as the grafting reactions was promoted, the branching reactions would also be promoted quantitatively and statistically, as long branched chains were probably produced from the grafting points.
Additionally, the branching reactions in the extruder involved two macroradicals, whereas the β-scission only involved one. Thereby, as the presence of scCO2 could improve the mobility of all the species, it could favor the branching reactions, while the β-scission might not be significantly affected. Moreover, as the viscosity of PP melt was decreased with scCO2, the reactive temperature was significantly reduced [29, 35]. Considering that the activation energy of β-scission was higher than that of other reactions, lower temperature could favor the branching reactions rather than the β-scission. Based on the above discussions, scCO2 played a positive role in enhancing the branching reaction and suppressing the degradation.
Rheological behavior
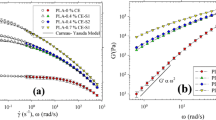
It is well known that the rheological properties are sensitive to changes in molecular chains. Shear and elongational rheological behaviors were studied with the pure PP and modified PP samples in the present work. The storage modulus (G’) of the pure PP and modified PP samples as a function of frequency is shown in Fig. 7a. It was found that pure PP and PP1 showed the typical terminal behavior, consistent with their linear chain structure. With the introduction of TMPTA, the modified PP samples were deviated from the typical terminal behavior. G’ increased at low frequencies with scCO2 for the modified PP samples, and the terminal region shifted to lower frequencies, indicating longer relaxation times due to formation of long chain branches during reactive extrusion. It was noted here that the modified PP samples (without purification) were cut into small pieces and then were Soxhlet extracted in boiling xylene for 24 h, and no visible gel was observed for all the samples. It could indicate that no significant cross-linked structure was formed.
Besides the storage modulus, the complex viscosity (η *) is also sensitive to the long-chain branched structure. Figure 7b shows the complex viscosity plots of the pure PP and modified PP samples. Compared to the pure PP, the complex viscosity of PP1 decreased severely and the Newtonian-zone became broader, indicating that only with the radical initiator, the molecular weight of PP was decreased due to chain scission. With the introduction of TMPTA, η * of PP2 was increased obviously at low frequencies, which demonstrated that long chain branches had been formed and the degradation was suppressed to some extent. Further, η * of the modified PP samples increased gradually with increased scCO2, and the transition from Newtonian-zone to Shear-thinning zone was shifted to lower frequencies, indicating that more long chain branches were formed with scCO2. The results were consistent with the previous literature [36], which reported that the presence of a very low amount of long chain branches can change the zero shear viscosity and the degree of shear thinning relative to linear polymers with similar molecular weight.
It is well accepted that the rheological behavior of polymers significantly affects the foam structure. Specifically, elongational rheology is quite useful in determining whether or not the samples could be well foamed. Strain hardening has been shown to be a significant indicator of high melt strength [37, 38]. If strain hardening was observed for the modified PP samples, it meant that their melt strength was indeed increased, which could allow the polymer melt to sustain bubble growth and deformation, thus facilitating foaming to produce foams with uniform closed-cell structure. Figure 7c shows the elongational viscosities of the pure PP and modified PP samples at an elongational rate of 0.5 s−1 at 190 °C. It was found that no strain-hardening behavior was observed in the pure PP, due to its linear molecular structure. In comparison, the modified PP samples showed pronounced strain hardening, which became more obvious with increased scCO2 for the modified PP samples from PP2 to PP5, indicating that the presence of scCO2 favored the branching reactions. The enhancement in strain hardening was attributed to the existence of the long-chain branched structure. Under an external elongational force, the entanglement of the long branched chains resisted deformation, generating excess stress, and thus, strain hardening appeared [15]. Based on the discussions above, the strain hardening was observed for the modified PP samples from PP2 to PP5, it meant that the specimens were endorsed with high melt strength. Thereby, it is highly expected that the modified PP prepared in the reactive extrusion could be well foamed with uniform cell structure.
Foaming behavior
As is known to all, it is difficult to produce linear PP foams with a high expansion ratio due to weak melt strength. The cell walls of linear PP foams are not strong enough to endure any elongational force during bubble growth, and thus the bubbles tend to coalesce or collapse during foam processing. Consequently, linear PP foams usually have high open-cell contents and non-uniform cell distribution, and the processing window for linear PP foaming is quite narrow. In order to prepare PP foams with a fine cell structure and a high expansion ratio, strong melt strength is required to sustain elongational stress and deformation during foaming, and hence long-chain branched PP is supposed to be a good alternative. Based on the previous study, Park et al. [4] found that compared to linear PP, branched PP exhibited excellent foaming behavior, with high foam expansion ratios, broad foaming windows, and well-defined cell structure. In this study, as discussed in Fig. 7c, the modified PP samples showed significant strain hardening, which could prevent the melt from rupture and stabilize the bubbles during foaming. Therefore, it is highly expected that foams with fine cell structure could be obtained with the modified PP prepared via reactive extrusion.
The pure PP and modified PP foams were prepared by extrusion foaming at various scCO2 contents, and the SEM images of the cell structure are shown in Fig. 8. It displayed that the suitable foaming temperature for different PP foams was decreased with scCO2 contents, due to the enhanced plasticizing effect of scCO2 on the melt. It was observed that substantial improvements in cell structure were observed in modified PP foams compared to pure PP foams. With 3.0 wt% scCO2, the pure PP foam exhibited unsatisfactory cell morphology with extensive open cells and non-uniform cell distribution, resulted from its low melt strength. In comparison, the modified PP foam showed a better cell structure with closed cells in a majority. With 5.0 wt% scCO2, the collapse of cells in the pure PP foams was more obvious than that in the modified PP foams, even though their expansion ratios were similar. At higher scCO2 content of 7.0 wt%, the cell walls of the pure PP foams could not endure the strong extensional force caused by the release of large amounts of gas at the die temperature of 164 °C, resulting in severe coalescence of cells and a low expansion ratio. However, for the modified PP foam prepared with 7.0 wt% scCO2, its high melt strength prevented the cell walls from being broken and endowed the foams with a well-defined structure and large uniform cells in size. These results demonstrated that the modified PP did exhibit a better foaming behavior than the linear PP due to their high melt strength attributed to the long-chain branched structure.
Cell density is a value to show the number of survived cells (per unfoamed unit volume) that undergo cell nucleation, collapse, and coalescence. Figure 9a summarizes the cell density of the pure PP and modified PP foams as a function of the die temperature. For all the foams prepared at various contents of scCO2, the cell density increased with the decrease in die temperature, due to the increased melt strength of PP melts at low die temperature. Furthermore, it was found that the cell densities of the modified PP foams were higher than that of the pure PP foams. For example, with 5.0 wt% scCO2, the cell densities of the pure PP and modified PP foams prepared at the die temperature of 169 °C were 4.5 × 106 and 8.9 × 106 cells/cm3, respectively. In other words, the cell density of the pure PP foam was nearly half of that of the modified PP foam. The results indicated that the presence of long chain branches on modified PP increased the melt strength and suppressed cell coalescence effectively during the foaming process, resulting in the increased cell density of the modified PP foams.
Expansion ratio is a significant parameter to describe the amount of gas retained during foam expansion, which is usually contributed by cell nucleation, growth, and opening. Figure 9b summarizes the expansion ratio of the pure PP and modified PP foams as a function of die temperature. The classic mountain-shaped curves of expansion ratio as a function of the die temperature were obtained for the pure PP and modified PP foams. For foams prepared with 3.0 wt% scCO2, the modified PP foam was expanded at higher temperatures than the pure PP foam due to its high melt strength, even though their maximum foam expansion ratio was similar. With 5.0 wt% scCO2, the expansion ratio for pure PP foams obtained at various die temperatures ranged from 10 to 18 folds, whereas the modified PP foams ranged from 10 to 22 folds. When the scCO2 content was 7.0 wt%, the maximum expansion ratios for the pure PP and modified PP foams were 21 and 24, respectively. The marked increase in expansion ratio of modified PP foams relative to pure PP foams was mainly attributed to the long chain branches, which increased the strain hardening, reinforced the cell walls, suppressed cell opening, and reduced cell wall rupture and escape of the gas from the melt.
It was observed from Fig. 9 that compared to pure PP, the die temperature suitable for foaming for the modified PP expanded to higher temperature sections. For example, with 7.0 wt% scCO2, pure PP could not be well foamed at the die temperature of 164 °C, while the modified PP foam exhibited a satisfactory cell structure, indicating that the high melt strength of the modified PP contributed from long chain branches facilitated foam expansion even at high die temperatures. The foaming window, which can be seen as the range of the processing temperature for the samples to be well foamed with very thin walls and uniform cell distributions, was usually introduced to evaluate the foaming behaviors of pure PP and modified PP. In the case of pure PP foam produced with 3.0 wt% scCO2, as shown in Fig. 9a, good foamed samples could be obtained at a die temperature of 168–171 °C, indicating that the foaming window for pure PP was only 4 °C. In comparison, the temperature suitable for the modified PP foaming was increased to 6 °C (from 168 to 173 °C). The expansion of the foaming window was more obvious at a higher content of scCO2 (7.0 wt%), which was increased from 5 °C for the pure PP to 9 °C for the modified PP. These results verified that the presence of long chain branches broadened the foaming window of PP.
Conclusion
In this study, a novel strategy was designed for the preparation of long-chain branched PP via reactive extrusion with scCO2 to improve the foamability. The scCO2 works as both the foaming agent and the medium of the branching reaction in reactive extrusion. It was found that the presence of scCO2 dramatically decreased the processing temperature, efficiently hindered the degradation, and significantly promoted the grafting and long chain branching. Rheological studies showed that with increased content of scCO2, the modified PP showed increased melt viscosity and elongational melt strength. The study of foaming behavior of the pure PP and modified PP samples proved that the long chain branching favored the foamability of the modified PP samples, and the foaming window was expanded in the presence of scCO2. Therefore, it demonstrated potential for an improved foaming technology via preparation of long-chain branched PP samples through reactive extrusion with scCO2.
References
Colton JS (1989) The nucleation of microcellular foams in semi crystalline thermoplastics. Mater Manuf Process 4:253–262
Song N, Zhu L, Yan XL et al (2008) Effect of blend composition on the rheology property of polypropylene/poly (ethylene-1-octene) blends. J Mater Sci 43:3218–3222. doi:10.1007/s10853-008-2554-9
Ding J, Ma WH, Song FJ, Zhong Q (2013) Effect of nano-calcium carbonate on microcellular foaming of polypropylene. J Mater Sci 48:2504–2511. doi:10.1007/s10853-012-7039-1
Park CB, Cheung LK (1998) A study of cell nucleation in the extrusion of polypropylene foams. Polym Eng Sci 37:1–10
Arroyo CS, Saja JAD, Velasco JI et al (2012) Moulded polypropylene foams produced using chemical or physical blowing agents: structure–properties relationship. J Mater Sci 47:5680–5692. doi:10.1007/s10853-012-6357-7
Lu B, Chung TC (1999) Synthesis of long chain branched polypropylene with relatively well-defined molecular structure. Macromolecules 32:8678–8680
Langston JA, Colby RH, Chung TCM et al (2007) Synthesis and characterization of long chain branched isotactic polypropylene via metallocene catalyst and T-reagent. Macromolecules 40:2712–2720
Langston JA, Colby RH, Shimizu F et al (2007) One-pot synthesis of long chain branch PP (LCBPP) using Ziegler-Natta catalyst and branching reagents. Macromol Symp 260:34–41
Weng WQ, Hu WG, Dekmezian AH, Ruff CJ (2002) Long chain branched isotactic polypropylene. Macromolecules 35:3838–3843
Auhl D, Stange J, Münstedt H et al (2004) Long-chain branched polypropylenes by electron beam irradiation and their rheological properties. Macromolecules 37:9465–9472
Yoshii F, Makuuchi K, Kikukawa S et al (1996) High-melt-strength polypropylene with electron beam irradiation in the presence of polyfunctional monomers. J Appl Polym Sci 60:617–623
Wong B, Baker WE (1997) Melt rheology of graft modified polypropylene. Polymer 38:2781–2789
Krause B, Stephan M, Volkland S et al (2006) Long-chain branching of polypropylene by electron-beam irradiation in the molten state. J Appl Polym Sci 99:260–265
Graebling D (2002) Synthesis of branched polypropylene by a reactive extrusion process. Macromolecules 35:4602–4610
Lagendijk RP, Hogt AH, Buijtenhuijs A, Gotsis AD (2001) Peroxydicarbonate modification of polypropylene and extensional flow properties. Polymer 42:10035–10043
Su FH, Huang HX (2011) Supercritical carbon dioxide-assisted reactive extrusion for preparation long-chain branching polypropylene and its rheology. J Supercrit Fluid 56:114–120
Wang D, Xie XM, Jow JD et al (2008) Styrene-assisted melt free-radical grafting of pentaerythritol triacrylate onto polypropylene and its crystallization behavior. J Appl Polym Sci 108:1737–1743
Santos ASF, Agnelli JAM, Trevisan DW, Manrich S (2002) Degradation and stabilization of polyolefins from municipal plastic waste during multiple extrusions under different reprocessing conditions. Polym Degrad Stab 77:441–447
Mantia FPL, Gardette JL (2002) Improvement of the mechanical properties of photo-oxidized films after recycling. Polym Degrad Stab 75:1–7
Shi D, Hu GH, Li RKY (2006) Concept of nano-reactor for the control of the selectivity of the free radical grafting of maleic anhydride onto polypropylene in the melt. Chem Eng Sci 61:3780–3784
Shi D, Li RKY (2006) Nano-reactors for controlling the selectivity of the free radical grafting of maleic anhydride onto polypropylene in the melt. Polym Eng Sci 46:1443–1454
Cartier H, Hu GH (1998) Styrene-assisted melt free radical grafting of glycidyl methacrylate onto polypropylene. J Polym Sci Part A 36:1053–1063
Li D, Han BX, Liu ZM (2001) Grafting of 2-hydroxyethyl methacrylate onto isotactic poly(propylene) using supercritical CO2 as a solvent and swelling agent. Macromol Chem Phys 202:2187–2194
Liu T, Hu GH, Tong GS et al (2005) Supercritical carbon dioxide assisted solid-state grafting process of maleic anhydride onto polypropylene. Ind Eng Chem Res 44:4292–4299
Spadaro G, Gregorio RD, Galia A et al (2000) Gamma radiation induced maleation of polypropylene using supercritical CO2: preliminary results. Polymer 41:3491–3494
Tong GS, Liu T, Hu GH et al (2007) Supercritical carbon dioxide-assisted solid-state free radical grafting of methyl methacrylate onto polypropylene. J Supercrit Fluid 43:64–73
Dorscht BM, Tzoganakis C (2003) Reactive extrusion of polypropylene with supercritical carbon dioxide: free radical grafting of maleic anhydride. J Appl Polym Sci 87:1116–1122
Cao K, Shen ZC, Yao Z et al (2010) New insight into the action of supercritical carbon dioxide for grafting of maleic anhydride onto isotactic polypropylene by reactive extrusion. Chem Eng Sci 65:1621–1626
Nalawade SP, Picchioni F, Janssen LPBM (2006) Supercritical carbon dioxide as a green solvent for processing polymer melts: processing aspects and applications. Prog Polym Sci 31:19–43
Wang K, Wu F, Zhai WT, Zheng WG (2013) Effect of polytetrafluoroethylene on the foaming behaviors of linear polypropylene in continuous extrusion. J Appl Polym Sci 129:2253–2260
Liu JY, Lou LJ, Yu W et al (2010) Long chain branching polylactide: structures and properties. Polymer 51:5186–5197
Wang XC, Tzoganakis C, Rempel GL (1996) Chemical modification of polypropylene with peroxide/pentaerythritol triacrylate by reactive extrusion. J Appl Polym Sci 61:1395–1404
Tian JH, Yu W, Zhou CX (2006) The preparation and rheology characterization of long chain branching polypropylene. Polymer 47:7962–7969
Dong ZX, Liu ZM, Han BX et al (2004) Modification of isotactic polypropylene films by grafting methyl acrylate using supercritical CO2 as a swelling agent. J Supercrit Fluid 31:67–74
Adams PMW, Dealy JM, Groot AW, Redwine OD (2000) Effect of molecular structure on the linear viscoelastic behavior of polyethylene. Macromolecules 33:7489–7499
Malmberg A, Gabriel C, Steffl T et al (2002) Long-chain branching in metallocene-catalyzed polyethylenes investigated by low oscillatory shear and uniaxial extensional rheometry. Macromolecules 35:1038–1048
Gotsis AD, Zeevenhoven BLF, Hogt AH (2004) The effect of long chain branching on the processability of polypropylene in thermoforming. Polym Eng Sci 44:973–982
Li SZ, Xiao MM, Wei DF et al (2009) The melt grafting preparation and rheological characterization of long chain branching polypropylene. Polymer 50:6121–6128
Acknowledgements
Financial supports from the National Natural Science Foundation of China (51473181), the Natural Science Foundation of Zhejiang Province (LQ14E030006), and the Natural Science Foundation of Ningbo (2014A610131) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, K., Wang, S., Wu, F. et al. A new strategy for preparation of long-chain branched polypropylene via reactive extrusion with supercritical CO2 designed for an improved foaming approach. J Mater Sci 51, 2705–2715 (2016). https://doi.org/10.1007/s10853-015-9584-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9584-x