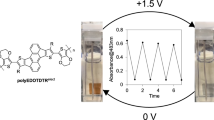
Abstract
A novel thiophene derivative (Fe–Th) with ferricyanide end group is successfully synthesized. The polythiophene derivative (Fe–PTh) is additionally obtained in aqueous solution by polymerization of Fe–Th, and shows a nanoparticle structure with a diameter from 10 to 100 nm. A simple solid-state electrochromic device is fabricated using the Fe–PTh as electrochromic material, and displays a novel three-color electrochromism from blue, green, and red with the increased potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polythiophene and its derivatives are an important class of organic conjugated polymers, which exhibit superior electronic and optical properties as well as good thermal and environmental stability. Among them, the polythiophene derivatives containing a functional group in position 3 of the ring are particularly attractive for special applications, for instance acting as electrochromic materials. In recent years, a large number of polythiophene derivatives have been designed and synthesized as electrochromic materials, but mostly present the monotonous color changes between blue and red [1, 2]. To satisfy the growing needs and enrich the increasing use for different applications in the high technology fields, the modified polythiophene structure with the updated characters is of great significance, and has always been one of the issues of concern.
On the other hand, as an inorganic electrochromic material, prussian blue (PB) with the special skeletal structure of Fe(III)–N–C–Fe(II)–C–N–Fe(III) possesses excellent electrocatalytic properties, good biocompatibility, high chemical stability and low cost of preparation, and thus is widely used in the fields of electrochromism [3], electrocatalysis [4], solid battery [5], biosensor [6], and electroanalysis [7, 8]. To enhance the applications of PB, many researches were focused on improving its response time and coloration efficiency [9]. In the case of people constantly in pursuit of improving and extending the property and application of electrochromic materials, development of new composite materials has become one of the most intensive approaches in order to create the new functions owing to the synergic effect originating from recombination. Recently, exploitation of organic/inorganic nanocomposite materials provides a new way to achieve the practical application of electrochromic device [10–14]. Some researches have especially been based on the composite of PB and conducting polymers instead of just improving each component, and present distinctive electrochromic capabilities [15–19]. For example, the electrochromic devices incorporating the electropolymerized poly(3,4-ethylenedioxythiophene) (PEDOT) film [20, 21] or poly(3,3-diethyl-3,4- dihydro-2H-thieno-[3,4-b] [1,4] -dioxepine) (PProDOT-Et2) film [22] with the ion storage PB layer has been fabricated, showing a color switch between light blue and deep blue under electric field. By depositing the PB films on polypyrrole (PPy) film [23] or polyaniline(PANI) film [24], the prepared electrochromic device could achieve the color changes from bluish green to pale brown, or from colorless to green and then to navy blue with the increased potential. However, in majority of the composites above, the conducting polymers and PB are in close contact through electrostatic interaction, while the composite connecting through direct chemical bonds is scarcely reported.
In this work, we design and synthesize a thiophene derivative with bromine group and a ferricyanide salt with dimethylamino pyridine as ligand, obtaining a new thiophene derivative with ferricyanide end group (Fe–Th, Scheme 1). This thiophene derivative combines the thiophene group and ferricyanide group through direct chemical bonding, and could form polythiophene and PB structure in the presence of FeCl3 (Fe–PTh). Using the Fe–PTh as an electrochromic material, a simple solid-state electrochromic device is fabricated and its electrochromic properties are discussed.
Materials and methods
Materials
4-(dimethylamino)-pyridine (DMAP), 3-thiophenemethanol, triethylamine, and poly(methyl methacrylate) (PMMA) were purchased from Sinopharm Chemical Reagent Co. Sodium pentacyano-ammineferroate (II) hydrate and bromoacetyl chloride were procured from Tokyo Chemical Industry Co. Methanol, dichloromethane (DCM), Iron(III) chloride (FeCl3), hydrochloric acid (HCl), and lithium perchlorate (LiClO4) were obtained from Nanjing Chemical Reagent Co. Propylene carbonate (PC) was purchased from Aladdin Chemical Co. All reagents were used as received and all aqueous solutions were prepared with deionized water.
Synthesis of pentacyano(4-(dimethylamino)-pyridine)ferrate complex (Fe-DMAP)
The preparation of Fe-DMAP was adapted from Ref. [25]. Sodium pentacyano-ammineferroate(II) hydrate (1.0 g, 3.8 mmol) was added into a solution of DMAP (1.0 g, 8.2 mmol) in methanol (75 mL) at 40 °C. The suspension was stirred overnight yielding a dark purple solution. The solution was concentrated under reduced pressure to 10 mL, in which 40 mL chloroform then was added. The insoluble solid was filtered off through a plug of Celite, and the filtrate was concentrated to 10 mL and precipitated by dropping into 500 mL DCM. The purple solid was collected and dried under vacuum. Yield 0.79 g (56 %). 1H NMR (500 MHz, CD3OD, δ ppm): 8.08(2H, d), 6.65(2H, d), 3.01(6H, s).
Synthesis of thiophen-3-ylmethyl 2-bromoacetate (Br–Th)
3-thiophenemethanol (2 mmol) and triethylamine (2.4 mmol) were dissolved in DCM (8 mL) and cooled to 0 °C by an ice bath. Bromoacetyl chloride (2.4 mmol) containing DCM (2 mL) was added in a drop-wise fashion with a syringe. The reaction was allowed to proceed under nitrogen atmosphere at 40 °C for 24 h. The product in DCM was introduced into a separatory funnel and washed with saturated NaHCO3 (aq). The DCM layer was separated and dried over sodium sulfate, then concentrated under reduced pressure. The product, being called by Br–Th, was further purified by a silica column chromatography in a solvent mixture of petroleum ether/ethyl acetate (50/1, v/v) and dried finally under vacuum. Yield 0.18 g (37 %). 1H NMR (500 MHz, CDCl3, δ ppm): 7.37(2H, d), 7.14(1H, d), 5.24(2H, s), 3.89(2H, s).
Synthesis of thiophene derivative with ferricyanide end group (Fe–Th)
Br–Th (0.047 g, 0.2 mmol) and Fe-DMAP (0.037 g, 0.1 mmol) were dissolved in methanol (10 mL). The reaction was held for 24 h at 80 °C under magnetic stirring. After completion of reaction, the solvent was evaporated and the product was washed with ether. The resulting solid was dried under vacuum at 40 °C for 24 h, being called by Fe–Th. Yield 0.02 g (33 %). 1H NMR (500 MHz, CD3OD, δ ppm): 7.76(2H, d), 7.37(2H, d), 7.11(1H, d), 6.69(2H, d), 4.83(2H, s), 3.74(2H, s), 3.03(6H, m).
Polymerization of Fe–Th
0.1 g Fe–Th was dissolved in 5 mL water, in which 0.08 g FeCl3 was then added. The reaction was held for 24 h at 40 °C. After that, centrifugation was used for concentrating the products, which had been washed with water several times to remove excess FeCl3. The dark blue product was dried under vacuum, being called as Fe–PTh.
Preparation of gel polymer electrolyte
The PMMA-based gel polymer electrolyte was prepared according to the literatures [26]. 2.12 g LiClO4 salt was dissolved into 20 mL PC to form a solution which was then heated to 100 °C. In this hot solution, 2.9 g PMMA was added and well-stirred until a transparent gel was formed.
Characterizations
1H NMR spectroscopic analyses were performed at ambient temperature on 500 MHz spectrometer to confirm the monomer and polymer composition. Fourier transform infrared (FTIR) spectra and UV–vis absorption spectra were recorded on Bruker VECTOR22 spectrometer and UV3600 spectrometer (Shimadzu, Japan), respectively. A solid-state electrochromic device (Fig. 1) was fabricated by dropping 5 mg/mL Fe–PTh water dispersion and PMMA-based gel polymer electrolyte, respectively on two ITO glass plates to form their film and then piecing on the films facing each other, in which Fe–PTh and the PMMA-based gel polymer electrolyte act as electrochromic material and ion conductor layer, respectively.
Results and discussion
DMAP molecule has a pyridine nitrogen and a tertiary nitrogen. The former can coordinate with iron ion, while the latter can act as a reaction site for further synthesis. We introduce the halogen group at position 3 of the thiophene ring, and then combine the ferricyanide salt and thiophene ring by forming a quaternary ammonium salt. In this way we obtain a new type of thiophene derivative which is water soluble due to the presence of ferricyanide end group.
Figure 2a shows the FTIR spectra of the as-synthesized products. For Fe-DMAP, the peaks at 2114 and 557 cm−1 due to C ≡ N stretching and Fe–CN stretching, respectively indicate the attendance of ferrocyanide salt. The peak at 1631 cm−1 corresponds to the pyridine ring vibrations. For Br–Th and Fe–Th, the peak at 1747 cm−1 is attributed to C = O stretching, demonstrating the presence of such function groups in these two products. The peak of pyridine ring is shifted from 1631 to 1647 cm−1, evidencing the change of the tertiary nitrogen on the pyridine ring to quaternary ammonium. The above characteristic bands demonstrate that the target molecules have been successfully synthesized. The peak at 806 cm−1 in Fe–Th is attributed to the out-of-plane vibrations of thiophene rings C–H at 2,5 position [27]. The absence of this peak in Fe–PTh indicates an occurrence of the chemical coupling of thiophene ring preferentially at 2,5 positions, verifying the polymerization of the derivative.
The morphology of Fe–PTh examined by TEM is presented in Fig. 2b, showing a nanoparticle structure with a diameter from 10 to 100 nm. The Fe–Th has a certain surfactant property due to the hydrophilic part of ferricyanide and the lipophilic part of thiophene ring, which makes the Fe–Th molecules into a micelle structure in aqueous. As a result of the interaction with Fe3+, the thiophene part is polymerized and the PB structure is formed at the same time. The final product presents a kind of nanoparticle structure (Scheme 2).
The UV–vis absorption spectra of the products are shown in Fig. 3. For Fe-DMAP, an absorption peak appears at 660 nm, which is due to an intervalence charge transfer mechanism between Fe(II) and Fe(III) [28]. This absorption peak is red shifted to around 720 nm for Fe–Th and Fe–PTh, since the generated quaternary ammonium group disperses the charges of the pyridine ring, thus reducing the transition energy of the complex and its polymer. Because of the easier electron transfer, the Fe–PTh possesses a good optical performance.
Most of the polythiophene derivatives generally present a red or blue color in the reduction or oxidation state. In the case of PB, its electrochromism only shows the color changes from dark blue to green up on changes from reduction to oxidation state (Fig. 4a). By contrast, the as-prepared Fe–PTh displays a novel electrochromic behavior due to its special molecular structure, that is, showing blue color at 0 V, passes through a green intermediate state at 1.8 V and finally exhibiting brown at 3.0 V, as shown in Fig. 4b. The device also shows a fast color switch time in about 1 s. Considering the PB end connecting with the thiophene ring through direct chemical bonds in the Fe–PTh, the coloration efficiency of our device could be regarded as the sum of the coloring efficiencies of the individual layers.
The UV spectra of the Fe–PTh at different voltages are presented in Fig. 5. In the spectra, the absorption at 450 nm expresses the π–π* transition of the polythiophene backbone and the broad absorption at 750 nm indicates the charge transfer between Fe(II) and Fe(III). It can be seen that, these two absorptions display different relative absorption intensity with the increased potential, and their combinations could result in the different color of the material [24]. The PB enhances the red absorbance, resulting in a darker and truer blue color. As the potential grows up, the intensity of the absorption of polythiophene backbone around 450 nm increases, while the absorption of PB around 750 nm changes little, so the color of the material turns to red. With the change of the relative intensity of these two peaks, the color of Fe–PTh changes to blue at 1 V, green at 2 V, and brown at 3 V. Therefore, Fe–PTh has multi-color electrochromic properties by covering an even wider portion of the color space, which is potential for applications in the intelligent window, color electronic paper, and other fields.
Conclusions
A novel thiophene derivative with ferricyanide end group is designed and synthesized. The afforded water-soluble thiophene derivative with ferricyanide end can be polymerized in aqueous phase in the form of a nanoparticle structure. The as-prepared polythiophene derivative possesses a good optical property with the interaction between thiophene and PB. The construction and characterization of the electrochromic device is also conducted on the basis of the polymer Fe–PTh. This material exhibits a three-color electrochromic property, suggesting its potentiality for application as new electrochromic materials.
References
Ohmori Y, Uchida M, Muro K, Yoshino K (1991) Effects of alkyl chain-length and carrier confinement layer on characteristics of poly(3-alkylthiophene) electroluminescent diodes. Solid State Commun 80(8):605–608
Garnier F, Tourillon G, Gazard M, Dubois JC (1983) Organic conducting polymers derived from substituted thiophenes as electrochromic material. J Electroanal Chem 148(2):299–303. doi:10.1016/s0022-0728(83)80406-9
Ellis D, Eckhoff M, Neff VD (1981) Electrochromism in the mixed-valence hexacyanides.1. voltammetric and spectral studies of the oxidation and reduction of thin-films of prussian blue. J Phys Chem 85(9):1225–1231. doi:10.1021/j150609a026
Shankaran DR, Narayanan SS (2002) Amperometric sensor for thiosulphate based on cobalt hexacyanoferrate modified electrode. Sens Actuators B-Chem 86(2–3):180–184
Neff VD (1985) Some performance-characteristics of a Prussian blue battery. J Electrochem Soc 132(6):1382–1384. doi:10.1149/1.2114121
Lin MS, Shih WC (1999) Chromium hexacyanoferrate based glucose biosensor. Anal Chim Acta 381(2–3):183–189. doi:10.1016/s0003-2670(98)00745-4
Radoi A, Compagnone D, Devic E, Palleschi G (2007) Low potential detection of NADH with Prussian blue bulk modified screen-printed electrodes and recombinant NADH oxidase from Thermus thermophilus. Sens Actuators B 121(2):501–506. doi:10.1016/j.snb.2006.04.075
Castro SSL, Balbo VR, Barbeira PJS, Stradiotto NR (2001) Flow injection amperometric detection of ascorbic acid using a Prussian blue film-modified electrode. Talanta 55(2):249–254. doi:10.1016/s0039-9140(01)00407-6
Lin C-L, Liao L-C (2014) Preparation and characterization of micropatterned prussian blue thin films with enhanced electrochromic properties. Surf Coat Technol 259:330–334. doi:10.1016/j.surfcoat.2014.02.058
Chen W-K, Hu C-W, Hsu C-Y, Ho K-C (2009) A study on the electrochromic properties of polyaniline/silica composite films with an enhanced optical contrast. Electrochim Acta 54(18):4408–4415. doi:10.1016/j.electacta.2009.03.017
Zhu YB, Wolf MO (2000) Charge transfer and delocalization in conjugated (ferrocenylethynyl)oligothiophene complexes. J Am Chem Soc 122(41):10121–10125. doi:10.1021/ja0008564
Xia XH, Tu JP, Zhang J, Huang XH, Wang XL, Zhang WK, Huang H (2009) Multicolor and fast electrochromism of nanoporous NiO/poly(3,4-ethylenedioxythiophene) composite thin film. Electrochem Commun 11(3):702–705. doi:10.1016/j.elecom.2009.01.017
Gadgil B, Damlin P, Heinonen M, Kvarnström C (2015) A facile one step electrostatically driven electrocodeposition of polyviologen–reduced graphene oxide nanocomposite films for enhanced electrochromic performance. Carbon 89:53–62. doi:10.1016/j.carbon.2015.03.020
Lee JS, Choi Y-J, Park H-H, Chul Pyun J (2011) Electrochromic properties of poly(3,4-ethylenedioxythiophene) nanocomposite film containing SiO2 nanoparticles. J Appl Polym Sci 122(5):3080–3085. doi:10.1002/app.34130
Guadagnini L, Salatelli E, Kharina A, Tonelli D (2014) Electrochemically deposited thiophene-based polymers as protective agents for Prussian Blue thin films. J Solid State Electrochem 18(10):2731–2742. doi:10.1007/s10008-014-2530-z
Thakur B, Sawant SN (2013) Polyaniline/Prussian-blue-based amperometric biosensor for detection of uric acid. ChemPlusChem 78(2):166–174. doi:10.1002/cplu.201200222
Lupu S, Mihailciuc C, Pigani L, Seeber R, Totir N, Zanardi C (2002) Electrochemical preparation and characterisation of bilayer films composed by Prussian Blue and conducting polymer. Electrochem Commun 4(10):753–758. doi:10.1016/s1388-2481(02)00440-x
Noel V, Randriamahazaka H, Chevrot C (2000) Composite films of iron(III) hexacyanoferrate and poly(3,4-ethylenedioxythiophene): electrosynthesis and properties. J Electroanal Chem 489(1–2):46–54. doi:10.1016/s0022-0728(00)00195-9
Lisowskaoleksiak A (2008) Impedance spectroscopy studies on hybrid materials consisting of poly(3,4-ethylenedioxythiophene) and iron, cobalt and nickel hexacyanoferrate. Solid State Ionics 179(1–6):72–78. doi:10.1016/j.ssi.2007.12.032
Deepa M, Awadhia A, Bhandari S, Agrawal SL (2008) Electrochromic performance of a poly(3,4-ethylenedioxythiophene)-Prussian blue device encompassing a free standing proton electrolyte film. Electrochim Acta 53(24):7266–7275. doi:10.1016/j.electacta.2008.04.020
Deepa M, Awadhia A, Bhandari S (2009) Electrochemistry of poly(3,4-ethylenedioxythiophene)-polyaniline/Prussian blue electrochromic devices containing an ionic liquid based gel electrolyte film. Phys Chem Chem Phys 11(27):5674–5685. doi:10.1039/b900091g
Chen K-C, Hsu C-Y, Hu C-W, Ho K-C (2011) A complementary electrochromic device based on Prussian blue and poly(ProDOT-Et2) with high contrast and high coloration efficiency. Sol Energy Mater Sol Cells 95(8):2238–2245. doi:10.1016/j.solmat.2011.03.029
Somani P, Mandale AB, Radhakrishnan S (2000) Study and development of conducting polymer-based electrochromic display devices. Acta Mater 48(11):2859–2871. doi:10.1016/s1359-6454(00)00098-7
DeLongchamp DM, Hammond PT (2004) Multiple-color electrochromism from layer-by-layer-assembled polyaniline/Prussian Blue nanocomposite thin films. Chem Mater 16(23):4799–4805. doi:10.1021/cm0496624
Liang G, Xu J, Wang X (2009) Synthesis and characterization of organometallic coordination polymer nanoshells of Prussian Blue using miniemulsion periphery polymerization (MEPP). J Am Chem Soc 131(15):5378–5379. doi:10.1021/ja900516a
Tung TS, Ho KC (2006) Cycling and at-rest stabilities of a complementary electrochromic device containing poly(3,4ethylenedioxythiophene) and Prussian blue. Sol Energy Mater Sol Cells 90(4):521–537. doi:10.1016/j.solmat.2005.02.018
Geetha S, Trivedi DC (2005) A new route to synthesize high degree polythiophene in a room temperature melt medium. Synth Metals 155(1):232–239. doi:10.1016/j.synthmet.2005.08.003
Robin MB (1962) Color and electronic configurations of prussian blue. Inorg Chem 1(2):337–342. doi:10.1021/ic50002a028
Acknowledgements
This project was supported by the National Natural Science Foundation of China (Nos. 21174059, 21374046), Program for Changjiang Scholars and Innovative Research Team in University, Open Project of State Key Laboratory of Supramolecular Structure and Materials (SKLSSM2015015), and the Testing Foundation of Nanjing University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Yan, H. & Lu, Y. The thiophene derivative with ferricyanide end group and its polymers: synthesis and electrochromic performance. J Mater Sci 50, 6920–6925 (2015). https://doi.org/10.1007/s10853-015-9242-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9242-3