Abstract
Nano-sized precipitates in Al–Mg–Si alloys can effectively increase the mechanical property of these alloys. However, nanoscale dimensions and orientation variations greatly impede the understanding of crystal structures and phase relations of precipitates. In this paper, the structural stability, mechanical, and electronic properties of twelve Al–Mg–Si compounds in Al–Mg–Si alloys are examined systematically using first-principles calculations. The calculated results of Mg2Si and MgAlSi agree well with the previous experimental and theoretical results. The Mg4Si7 with \( P\bar{1} \) symmetry, MgAl2Si2 with C2/m symmetry, and Mg4AlSi3 with Pccn symmetry are identified as the more energetically and mechanically favorable phases suggested by our calculations. According to the predictions, MgSi2 and MgAlSi exhibit higher bulk moduli, 73.1 and 67.7 GPa, due to the tightly bounding Si–Si/Si–Al covalent networks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Al–Mg–Si alloys are extensively used in many industrial applications, such as lightweight construction, automotive, aircraft, and architecture, because of the excellent formability, mechanical strength, corrosion resistance, and weldability [1–3]. During a specific heating treatment process (known as aging hardening), the presence of diverse nano-sized needle- or plate-like metastable precipitates from the aluminum matrix greatly hinders the movement of dislocations and thus enhances the mechanical property of alloys [4]. Generally, the generic precipitation follows the sequence of SSSS → Mg/Si clusters → GP-zones → pre-β″ → β″ (Mg5Si6) → {U1, U2, B′, β′} → β (Mg2Si, stable), where SSSS refers to a supersaturated solid solution and GP-zones are aggregates of solute atoms in the aluminum matrix. Among the precipitations, β″ phase, generally present in alloys aged to peak hardness, has been determined to be a monoclinic structure with an ideal composition, Mg5Si6 [4]. However, in specific age-hardening process, low annealing temperatures and short annealing time limit the appearance of β″ phase, and thus the pre-β″ phase is formed with varied composition other than Mg5Si6, e.g., Mg4Si7, Mg2Al3Si6, and Mg4AlSi6 [5, 6]. The phases U1, U2, and B′ refer to “type A”, “type B”, and ‘‘type C’’ precipitates, respectively [7]. Furthermore, rod-like β′ is reported to appear in over-aged specimens and is determined to be hexagonal phase with a composition of Mg9Si5 [2]. The equilibrium phase in this system, the β phase, Mg2Si, has a cubic anti-fluorite (CaF2) structure (space group Fm3m). Very recently, two new compounds, (Mg9Si5)HP and (Mg4AlSi3)HP are reported by Ji et al. [8] under high pressure (5 GPa) and high temperature (900–1100 °C) conditions. In general, the microstructures of clusters and precipitates are often investigated by atom probe tomography [1, 9, 10] and high-resolution transmission electron microscopy [11, 12]. Nevertheless, small grain-size and many possible orientations of precipitates may limit the determinations of structure and physical properties.
First-principles calculation is a valuable prediction approach to understand precipitate energetics and studying their structural transformations [13, 14]. The bonding characteristics of the key precipitates in Al–Mg–Si alloys were investigated by Frøseth et al. [15] using augmented plane-wave under the framework of density functional theory calculations about one decade ago. The energetics of most of the precipitates in Al–Mg–Si system was studied by Ravi and Wolverton [6] using density functional-based calculations in both the local density approximations (LDA) and generalized gradient approximations (GGA). van Huis et al. [5, 16] systematically investigated stability and structural relations of the matrix-embedded precipitate phases in the Al–Mg–Si alloys during the evolution process by means of first-principles calculations. Zhao et al. [17] calculated the structure, stability, and finite-temperature thermodynamic properties of the key precipitates in the Al–Mg–Si alloy. Ehlers examined interface configuration stabilities and determined the interfacial energies over the full precipitate cross-section for the phase β″ in the Al–Mg–Si alloy system [14]. Most of the efforts were focused on the thermodynamic stability and structural relationship between the precipitates and the Al matrix. However, to our knowledge, there are no comparative investigations on the mechanical properties and underlying relations between these phases of Al–Mg–Si compounds. Therefore, by means of first-principles calculations based on density functional theory, in this paper, we report the enthalpies of formation, elastic constants, and electron structures of the whole precipitate phases, i.e., pre-β″/(Mg4AlSi6, Mg2Al3Si6, Mg4Si7), β″/Mg5Si6, β′/Mg9Si5, U1/MgAl2Si2, U2/MgAlSi, U3/MgSi2 [2], B′/Mg9Al3Si7, β/Mg2Si, and two high pressure phases—(Mg9Si5)HP and (Mg4AlSi3)HP. The systematic studies on the Al–Mg–Si compounds here would help the further understanding of the structural behaviors and mechanical properties of the metastable precipitate phases.
Computational details
First-principles calculations were performed using CASTEP code based on density functional theory (DFT) [18]. The exchange and correlation functions were treated by generalized gradient approximation with the parameterization by Perdew–Burke–Ernzerhof (GGA-PBE) [19, 20]. The electronic configurations of each element were 3s23p1 for Al, 2p63s2 for Mg, and 3s23p2 for Si, respectively. The convergence of calculations is initially checked by a 10 × 10 × 10 k-point and cutoff energy of 350 eV. The ground state and elastic constants (C ij ) of all considered Al–Mg–Si compounds are then calculated for each fully relaxed structure. Bulk and shear moduli were estimated based on Voight–Reuss–Hill (VRH) approximation [21]. Elastic moduli and Poisson’s ratio were obtained based on the relationship: E = 9BG/(3B + G), v = (3B − 2G)/(6B + 2G). The universal elastic anisotropy index (A U) [22] is obtained from the B and G of Voigt and Reuss with the equation: A U = 5G V/G R + B V/B R − 6.
Structural stability
Mechanical stability, a necessary condition for a stable crystal to exist in experiments, was firstly checked for all of the precipitates according to the Born–Huang criterion [23, 24]. The mechanical stability criteria are given as follows:
For cubic phase,
For hexagonal phase,
For tetragonal phase,
For trigonal phase,
For orthorhombic phase,
For monoclinic phase,
If all the individual elastic moduli of a specific phase are satisfied, the above criteria indicate its stability. The calculated elastic constants of the Al–Mg–Si compounds are given in Table 1. It can be found that pre-β″/Mg2Al3Si6, pre-β″/Mg4AlSi6, β″/Mg5Si6, U2/MgAlSi, U3/MgSi2, B′/Mg9Al3Si7, β′/Mg9Si5, β/Mg2Si, and (Mg9Si5)HP satisfy the Born–Huang criterion, indicating their mechanical stability. However, pre-β″/Mg4Si7 (C 44 = −127.7 GPa), U1/MgAl2Si2 (C 55 = −26.5 GPa), and (Mg4AlSi3)HP (C 66 = −8.4 GPa) are mechanically unstable, which are surprising to appear in the experimental observations [5–8]. To get a further understanding of the structural configurations of pre-β″/Mg4Si7, U1/MgAl2Si2, and (Mg4AlSi3)HP, 2 × 2 × 2 supercells were conducted to produce mechanically stable phases: \( P\bar{1} \)-type (Space group number: 2) Mg4Si7, C2/m-type (Space group number: 12) MgAl2Si2 and Pccn-type (Space group number: 56) Mg4AlSi3, which are found to be energetically more favorable than the previously identified structures with about 29, 32, and 10 meV/atom, respectively (as shown in Fig. 1a). Further dynamical stability for these structures was also validated, because no soft mode was observed in their phonon curves (as shown in Fig. 1b–d). Considering the small size of precipitates and possible orientations in experiment, \( P\bar{1} \)-Mg4Si7, C2/m-MgAl2Si2, and Pccn-Mg4AlSi3 should be more likely to precipitate and will be discussed in the following sections.
To verify the thermodynamic stability of the considered precipitated phases in the alloys, the formation enthalpies (ΔH f) are calculated as listed in Table 2 and compared with available experimental and literature first-principles calculation results [5, 6, 17, 25–33]. The formation enthalpy of Mg x Al y Si z phase is defined as
where E total(Mg x Al y Si z ) is the total energy of Mg x Al y Si z at equilibrium lattice constants; E total(Mg), E total(Al), and E total(Si) are the calculated total energies of hcp-Mg (S.G. P63/mmc), fcc-Al (S.G. Fm-3m), and diamond-Si (S.G. Fd-3m); and x, y, and z (x + y + z = 1) are the atomic fractions of Mg, Si, Al, respectively. Generally, our obtained results agree well with previous ones. Figure 2 shows the formation enthalpies of the precipitated phases in comparison with GGA (VASP) results by van Huis et al. [5]. It can be seen that all three pre-β″ phases (Mg4Si7, Mg4AlSi6, and Mg2Al3Si6) show positive formation enthalpy, which were identified in the early stage during age- hardening process based on both experimental and theoretical results [3, 16, 34, 35]. Following the precipitation sequence, except for MgSi2 (an only theoretically predictive phase and has not been observed experimentally), the formation enthalpy of the metastable precipitates becomes negative, suggesting the stable behaviors against the decomposition into their elemental compositions under ambient conditions. The formation enthalpy of the precipitates follows the sequence of pre-β″ (Mg2Al3Si6 > Mg4AlSi6 > Mg4Si7) > β″/Mg5Si6 > {U1/MgAl2Si2 > B′/Mg9Al3Si7 > U2/MgAlSi > β′/Mg9Si5} > β/Mg2Si. Our calculated results also confirmed that Mg2Si phase has the most negative ΔH (−0.163 eV/atom; −15.73 kJ/mol-atom), verified by experiment as the equilibrium phase. The sequence of the calculated formation enthalpy is quite consistent with the experimental observations [1, 15, 36] and results by van Huis et al. [5]. It is also interesting to know that the structural symmetry of the key precipitates in Al–Mg–Si alloys changes following the precipitation sequence, i.e., from triclinic (\( P\bar{1} \)-Mg4Si7) and monoclinic (C2/m-Mg2Al3Si6; C2/m-Mg4AlSi6, C2/m-Mg5Si6 and C2/m-MgAl2Si2) to orthorhombic (Pnma-MgAlSi and Imma-MgSi2), then hexagonal (\( P\bar{6} \)-Mg9Al3Si7 and P63/m-Mg9Si5), and finally cubic (\( Fm\bar{3} \) m-Mg2Si), which may result from the structural stabilization of the temperature-dependent entropic contributions to the free energy.
(color online) Calculated formation enthalpies of the precipitates in Al–Mg–Si alloys comparing with theoretical results in Ref. [5] by GGA
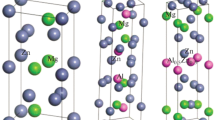
The calculated structural parameters and Wyckoff positions of different precipitates, after full stress and position relaxation, are shown in Table 3 and Supporting Information. In general, our results are in good accordance with the previous theoretical results [2, 4–6, 8, 14, 37–40]. The lattice constant of a of monoclinic Mg4AlSi6 deviates by ~12 % comparing with the experimental values, as suggested by Ref. [4], which may result from the interfacial and strain energies generated during precipitate process, influencing the crystal structure of the precipitate phases, the matrix phase, and the interface between them. As shown in Fig. 3, the crystal structures of the pre-β″ phases (\( P\bar{1} \)-type Mg4Si7 in Fig. 3a, Mg4AlSi6 in Fig. 3b, and Mg2Al3Si6 in Fig. 3c) are composed of corrugated Si layers, and the Si layers are alternately stacked with Mg atoms paralleling to c axis. The Si layers can be also viewed as parallelogram-Si4 (denoted as P-Si4 later) zigzag chains connected by rhombus-Si4 (denoted as R-Si4 later) planar chains. The structures of Mg4AlSi6 and Mg2Al3Si6 are reasonably built from \( P\bar{1} \)-type Mg4Si7 with Si1, Mg2, and Mg3 atom substituted by Al atoms, respectively. Experimentally, in the precipitation process, Si and Mg tend to form clusters at the beginning. Initially, the clusters will be Si-rich due to its poorer solubility in Al and have higher diffusion speed. Subsequently, Mg will diffuse into the Si-rich clusters to form Mg/Si clusters [41]. Then Al atoms diffusing from the matrix replaced Mg or Si atoms in Mg/Si clusters. The bond lengths of Si–Si in P-Si4 unit are 2.37 and 2.68 Å for \( P\bar{1} \)-type Mg4Si7, 2.42 and 2.55 Å for Mg4AlSi6, and 2.47 and 2.72 Å for Mg2Al3Si6, correspondingly. The bond lengths of Si–Si (or Si–Al) in R-Si4 unit (or R-Si2Al2 unit) in \( P\bar{1} \)-type Mg4Si7, Mg4AlSi6, and Mg2Al3Si6 are 2.53, 2.70, and 2.87 Å, respectively. The close structural features imply the small barrier of the phase transformation among Mg4Si7, Mg4AlSi6, and Mg2Al3Si6 in experiment. Additionally, the structure of Mg5Si6 (Fig. 3d) is also constituted with alternated layers of Si and Mg atoms, which can be derived from \( P\bar{1} \)-type Mg4Si7 with Si1 atoms substituted by Mg1 atoms.
The C2/m-type MgAl2Si2 (Fig. 3e) consists of bilayers of puckered graphene-like sheets of AlSi, with Mg atoms capping the puckered hexagons. The bond lengths of Si–Al within [AlSi]3 six-rings are 2.49 (×2) and 2.50 (×4) Å, respectively, while the Si–Al bonds length connecting two [AlSi] layers is 2.62 Å.
In the MgSi2 phase (Fig. 3f), rectangle-Si4 units build a tightly bound bonding network, and zigzag chains of Mg atoms run through the Si channels. MgAlSi phase (Fig. 3g), close to the MgSi2 structure, can be described as half of the diagonal Si atoms in the rectangle-Si4 units substituted by Al atoms. The bond lengths of Si–Si (Si–Al) in MgSi2 (in MgAlSi) are 2.45, 2.47, and 2.56 Å (2.55, 2.63 and 2.71 Å), respectively.
The crystal structure of Mg9Al3Si7 (Fig. 3h) can be described as the linear [Al3Si7] chains separated by Mg atoms along c direction. The Si–Al bond lengths are 2.53, 2.55, and 2.59 Å. The Si atoms in Mg9Si5, (Mg9Si5)HP, and Mg2Si (Fig. 3i, l, j) can be classified to three different types: isolated Si atoms, Si2 dimers, and not fully occupied linear chains. The Si–Si bond lengths in Si2 dimers are 2.46 Å for Mg9Si5, 2.46 and 2.47 Å for (Mg9Si5)HP. The Si–Si bond length in linear chains in (Mg9Si5)HP is about 2.20 Å. Finally, the crystal structure of (Mg4AlSi3)HP (Fig. 3k) is composed of alternating [AlSi2] and [Mg4Si] layers, with each Al atom having five nearest Si atoms with Si–Al bond length 2.50 (×2) and 2.75 (×2) Å (within [AlSi2] layer) and 2.83 Å (between [AlSi2] and [Mg4Si] layer), and each Si atom two nearest Al atoms and one Si atom (Si–Si bond length is 2.44 Å).
Elastic properties
The calculated individual elastic constants within the strain–stress method are listed in Table 1 for different Al–Mg–Si compounds. In general, all of the mechanically stable phases show the relatively higher values of C11, C22, and C33 than that of C44, indicating relatively higher incompressibility along a-, b-, and c-axis. Moreover, C22 (163.6 GPa) of Mg2Al3Si6 is the largest value among the individual elastic constants, suggesting its low compressibility along b-axis. Additionally, the largest C44 value, 50.3 GPa, is observed in MgAlSi, indicating its relatively strong shear strength.
To further demonstrate the mechanical properties of Mg–Al–Si alloys, bulk modulus, shear modulus, Young’s modulus, Poisson’s ratio, and universal elastic anisotropy index (A U) were estimated from the calculated individual elastic constants (Table 4); the previous available results are also listed for comparison. It can be seen that our calculated results are in good agreement with previously theoretical and experimental results. For example, the calculated bulk moduli of Mg2Si and MgAlSi were 53.0 and 67.7 GPa, respectively, consistent with theoretical values reported by Anders et al., 54.3 and 69.1 GPa, respectively [15]. Also, bulk moduli of all precipitates are higher than that of pure Mg (45 GPa), but lower than that of pure Al (76 GPa) and diamond-Si (97.6 GPa). Furthermore, MgSi2 exhibits highest bulk modulus, 73.1 GPa, followed by MgAlSi, 67.7 GPa, due to the strong covalent Si–Si (Si–Al) bond in three-dimensional Si-framework (Si–Al-framework). It is interesting that the bulk modulus decreased gradually with the increasing Mg concentration by the fitted function B = 69.2 − 20.7x Mg (as shown in Fig. 4a), possibly induced by the decreasing of the Si–Si or Si–Al covalent bonding and the increasing of the Si–Mg ionic bonding. However, there is no apparent interaction between the shear modulus and the concentration of Mg (as shown in Fig. 4b). MgAlSi possesses a maximum value, 48.8 GPa, while Mg4AlSi6 exhibits a minimum value, 22.0 GPa.
The ratio between the shear and bulk modulus (B/G) has been proposed by Pugh [42] to predict brittle or ductile behavior of materials. According to the Pugh criterion, a high B/G value indicates a tendency for ductility, while a low B/G value is associated with brittleness. From Table 4, it can be seen that G/B value of Mg2Al3Si6, MgAlSi, Mg9Si5, and Mg2Si are under the critical value, 1.75, separating ductility from brittleness, indicating the brittle behaviors; while the other phases are ductile, with a higher value than the critical value. The elastic anisotropy index (A U) represents a universal measure to quantify the single-crystal elastic anisotropy. From Table 4, Mg2Si is an isotropic phase with the lowest A U value closed to zero (0.006), while Mg4AlSi6 has the largest A U absolute value (5.09) displaying the most anisotropic.
Electronic properties
Figure 5 displays the total and partial density of states (DOS) for all the precipitates and high pressure phases. We found that Mg2Si phase is the only phase showing the semiconducting feature. The calculated band structure of Mg2Si presents an indirect gap with an energy value of 0.22 eV, in agreement with the previous theoretical results (0.23 eV) [43], but lower than experimental value, 0.74 eV [44], because of the underestimation of band gap by GGA. \( P\bar{1} \)-Mg4Si7, Mg4AlSi6, Mg2Al3Si6, and Mg5Si6 phases exhibit metallic features, as evidenced by finite DOS values at Fermi level (E F), which originate mostly from 2p electrons of Si. The Si–Mg (or Si–Al) covenant interactions is relatively weak because of the less overlapping between the partial DOS of Si and Mg atoms (or Si and Al atoms). For MgSi2, it can be found that there is a wide overlap between Si-s and Si-p from −12 to −7 eV, showing strong sp3 hybridization, which mainly accounts for the largest bulk modulus among the considered Mg–Al–Si phases. Furthermore, there is a general feature for stable C2/m-MgAl2Si2, MgAlSi, Mg9Al3Si7, Mg9Si5, Mg2Si, Pccn-(Mg4AlSi3)HP, and (Mg9Si5)HP. Below the Fermi level, the valence bands were dominated by Si states with Si-s states at lower band and Si-p states at the higher band. Furthermore, for Mg9Si5 and (Mg9Si5)HP, the Fermi level nearly falls in the pseudogap valley, implying more stability of these two phases than others, quite consistent with the experimental findings [8]. Nevertheless, from Fig. 5, it can be observed that, for all the Al–Mg–Si precipitated phases, the contributions to the total DOS from the Mg states are not significant because Mg atoms donate electrons to Al–Si (or Si–Si) network to stabilize the structure, for example, about 1 and 0.67–0.79 electron transformed from one Mg atom to Al–Si (or Si–Si) network for MgAl2Si2 and Mg4Si7, respectively, by analyzing the atomic Milliken overlap population.
The calculated DOS of lower energy structures, \( P\bar{1} \)-Mg4Si7, C2/m-MgAl2Si2, and Pccn-(Mg4AlSi3)HP (Fig. 6b, d, f) was compared with that of the previously proposed phases (Fig. 6a, c, e). The DOS around Fermi level (E F) at right side is lower than that at left side in Fig. 4, and it shows a “splitting” into a pseudogap, thus underlying their stability. In (Mg4AlSi3)HP and C2/m-Mg4Si7, the pseudogap appears far below E F (about 1 eV), pointing to the electronic origin of their instability. Furthermore, for (Mg4AlSi3)HP, DOS at E F of Pccn-type structure Mg4AlSi3 was contributed more from Mg atoms than that of P4/ncc-type structure, and a similar profile between PDOS-Si and PDOS-Mg is observed, suggesting relatively stronger Si–Mg bond in Pccn-(Mg4AlSi3)HP, thereby more favorable mechanical stability. Similarly, relatively stronger Si–Al (Si–Mg) bond for MgAl2Si2 (Mg4Si7) with C2/m (\( P\bar{1} \)) symmetry can be induced from their electronic DOS. Therefore, one can understand that the enhancement of Si–Mg (or Si–Al) bond interactions in \( P\bar{1} \)-Mg4Si7, C2/m-MgAl2Si2, and Pccn-(Mg4AlSi3)HP contributes to their energetic and mechanical stabilities.
Conclusions
In summary, we systematically investigated the crystal structure, phase stability, mechanical properties, and electronic structure of the precipitates and high pressure phases of in Al–Mg–Si alloys by first-principles calculations. The sequence of our calculated formation enthalpy of the precipitates is coincident with the experiment trend. Previously suggested phases C2/m-Mg4Si7, \( P\bar{3} \) m-MgAl2Si2, and P4/ncc-(Mg4AlSi3)HP, are found to be mechanically unstable. The new structures of \( P\bar{1} \)-Mg4Si7, C2/m- MgAl2Si2, and Pccn- (Mg4AlSi3)HP are proposed in this paper. Among the precipitates, MgSi2 and MgAlSi exhibit higher bulk modulus, 73.1 and 67.7GPa due to the tightly bound Si–Si/Si–Al covalent networks. MgAlSi possesses the maximum shear modulus of 48.8 GPa, and Mg4AlSi6 exhibits minimum shear modulus 22.0 GPa. Furthermore, the bulk modulus decreased gradually with the increase in Mg concentration by the fitted function B = 69.2 − 20.7 x Mg (x, Mg atomic fraction), because of the interaction of Si–Si or Si–Al covalent bonding and ionic Si–Mg bonding.
References
Edwards GA, Stiller K, Dunlop GL, Couper MJ (1998) The precipitation sequence in Al-Mg-Si alloys. Acta Mater 46:3893
Vissers R, van Huis MA, Jansen J, Zandbergen HW, Marioara CD, Andersen SJ (2007) The crystal structure of the β′ phase in Al–Mg–Si alloys. Acta Mater 55:3815
Marioara CD, Andersen SJ, Jansen J, Zandbergen HW (2003) The influence of temperature and storage time at RT on nucleation of the β″ phase in a 6082 Al-Mg-Si alloy. Acta Mater 51:789
Zandbergen HW, Andersen SJ, Jansen J (1997) Structure determination of Mg5Si6 particles in Al by dynamic electron diffraction studies. Science 277:1221
van Huis MA, Chen JH, Zandbergen HW, Sluiter MHF (2006) Phase stability and structural relations of nanometer-sized, matrix-embedded precipitate phases in Al–Mg–Si alloys in the late stages of evolution. Acta Mater 54:2945
Ravi C, Wolverton C (2004) First-principles study of crystal structure and stability of Al-Mg-Si-(Cu) precipitates. Acta Mater 52:4213
Matsuda K, Sakaguchi Y, Miyata Y, Uetani Y, Sato T, Kamio A, Ikeno S (2000) Precipitation sequence of various kinds of metastable phases in Al-1.0mass% Mg2Si-0.4mass% Si alloy. J Mater Sci 35:179
Ji SD, Imai M, Zhu HK, Yamanaka SJ (2013) Structural characterization of magnesium-based compounds Mg9Si5 and Mg4Si3Al (superconductor) synthesized under high pressure and high temperature conditions. Inorg Chem 52:3953
Ringer SP, Swenser SP, Muddle BC, Polmear IJ, Sakurai T (1996) APFIM/TEM observations of a high strength-creep resistant Al-Cu-Mg-Si-Ge alloy. Mater Sci Forum 217–222:689
Hasting HS, Frøseth AG, Andersen SJ, Vissers R, Walmsley JC, Marioara CD, Danoix F, Lefebvre W, Holmestad R (2009) Composition of β″ precipitates in Al-Mg-Si alloys by atom probe tomography and first principles calculations. J Appl Phys 106:123527
Matsuda K, Gamada H, Fujii K, Uetani Y, Sato T, Kamio A, Ikeno S (1998) High-resolution electron microscopy on the Structure of Guinier-Preston Zones in an Al-1.6 Mass Pct Mg2Si alloy. Metall Mater Trans A 29A:1161
Coene W, Janssen G, Beeck MOD, van Dyck D (1992) Phase retrieval through focus variation for ultra-resolution in field-emission transmission electron microscopy. Phys Rev Lett 69:3743
Pan RK, Ma L, Bian N, Wang MH, Li PB, Tang BY, Peng LM, Ding WJ (2013) First-principles study on the elastic properties of B′ and Q phase in Al-Mg-Si (-Cu) alloys. Phys Scr 87:015601
Ehlers FJH, Dumoulin S (2014) Interface configuration stability and interfacial energy for the β″ phase in Al-Mg-Si as examined with a first principles based hierarchical multi-scale scheme. J Alloys Comp 591:329
Frøseth AG, Høier R (2003) Bonding in MgSi and Al-Mg-Si compounds relevant to Al-Mg-Si alloys. Phys Rev B 67:224106
van Huis MA, Chen JH, Sluiter MHF, Zandbergen HW (2007) Phase stability and structural features of matrix-embedded hardening precipitates in Al-Mg-Si alloys in the early stages of evolution. Acta Mater 55:2183
Zhao D, Zhou LC, Kong Y, Wang AJ, Wang J, Peng YB, Du Y, Ouyang YF, Zhang WQ (2011) Structure and thermodynamics of the key precipitated phases in the Al-Mg-Si alloys from first-principles calculations. J Mater Sci 46:7839. doi:10.1007/s10853-011-5765-4
Segall MD, Lindan PJD, Probert MJ, Pickard CJ, Hasnip PJ, Clark SJ, Payne MC (2002) First-principles simulation: ideas, illustrations and the CASTEP code. J Phys Condens Matter 14:2717
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865
Hill R (1952) The elastic behaviour of a crystalline aggregate. Proc Phys Soc A 65:349
Ranganathan SI, Ostoja-Starzewski M (2008) Universal elastic anisotropy index. Phys Rev Lett 101:055504
Born M, Huang K (1954) Dynamical theory of crystal lattices. Oxford University Press, London
Wu ZJ, Zhao EJ, Xiang HP, Hao XF, Liu XJ, Meng J (2007) Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys Rev B 76:054115
Zhang H, Wang Y, Shang SL, Ravi C, Wolverton C, Chen LQ et al (2010) Solvus boundaries of (meta)stable phases in the Al-Mg-Si system: first-principles phonon calculations and thermodynamic modeling. CALPHAD 34:20
Kubaschewski O, Villa H (1949) Heat of formation of binary alkaline earth compounds. Z Electrochem 53:32
Gerstein BC, Jelinek FJ, Habenschuss M, Shickell WD, Mullaly JR, Chung PL (1967) Thermal study of groups II—IV semiconductors. Lattice heat capacities and free energies of formation. Heat capacity of Mg2Si from 15°-300° K. J Chem Phys 47:2109
Blachnik R, Kunze D, Schneider A (1971) On the experimental results of a direct calorimetry method. Metall (Isernhagen) 25:119
Caulfield HJ, Hudson DE (1966) Sublimation in the intermetallic series SiMg2, SnMg2, and PbMg2. Solid State Commun 4:299
Ryabchikov IV, Mikulinski AS (1963) Measurement of the vapor pressure of magnesium over solid magnesium and magnesium-silicon and magnesium-calcium-silicon alloys. Izv Vyssh Uchebn Zaved Tsvet Met 1:95
Lukashenko GM, Eremenko VN (1964) Thermodynamic properties of magnesium-silicides. Russ J Inorg Chem 9:1243
Rao YK, Belton GR, Gokcen NA (ed) (1981) Thermodynamic properties of Mg − Si system. Chemical metallurgy: a tribute to carl wagner. The Metallurgical Society of AIME, New York
Feufel H, Godecke T, Lukas HL, Sommer F (1997) Investigation of the Al-Mg-Si system by experiments and thermodynamic calculations. J Alloys Comp 247:31
Marioara CD, Andersen SJ, Jansen J, Zandbergen HW (2001) Atomic model for GP-zones in a 6082 Al-Mg-Si system. Acta Mater 49:321
Marioara CD, Nordmark H, Andersen SJ, Holmestad R (2006) Post-β″ phases and their influence on microstructure and hardness in 6xxx Al-Mg-Si alloys. J Mater Sci 41:471
Chen JH, Costan E, van Huis MA, Xu Q, Zandbergen HW (2006) Atomic pillar-based nanoprecipitates strengthen AlMgSi alloys. Science 312:416
Andersen SJ, Marioara CD, Vissers R, Frøseth A, Zandbergen HW (2007) The structural relation between precipitates in Al-Mg-Si alloys, the Al-matrix and diamond silicon, with emphasis on the trigonal phase U1-MgAl2Si2. Mater Sci Eng A 444:157
Andersen SJ, Marioara CD, Frøseth A, Vissers R, Zandbergen HW (2005) Crystal structure of the orthorhombic U2-Al4Mg4Si4 precipitate in the Al-Mg-Si alloy system and its relation to the β′ and β″ phases. Mater Sci Eng A 390:127
Wolverton C (2001) Crystal structure and stability of complex precipitate phases in Al-Cu-Mg-(Si) and Al-Zn-Mg alloys. Acta Mater 49:3129
Villars P, Calvert LD (1985) Pearson’s handbook of crystallographic data for intermetallic phases, vol 3. American Society for Metals, Metals Park, p 2701
Edwards GA, Stiller K, Dunlop GL (1994) APFIM investigation of fine-scale precipitation in aluminium alloy 6061. Appl Surf Sci 76(77):219
Pugh SF (1954) XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos Mag Ser 7 45:823
Viennois R, Jund P, Colinet C, Tédenac JC (2012) Defect and phase stability of solid solutions of Mg2X with an antifluorite structure: an ab initio study. J Solid State Chem 193:133
Mahan JE, Vantomme A, Langouche G, Becker JP (1996) Semiconducting Mg2Si thin films prepared by molecular-beam epitaxy. Phys Rev B 54:16965
Acknowledgements
The authors acknowledge the financial support by the National Natural Science Foundation of China (NSFC) under Grants No. 51071140. The authors also thank Dr. Qingyang Hu of Carnegie institution of Washington and Dr. Jihua Hao of John Hopkins University for the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Bo Zhang, and Lailei Wu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, B., Wu, L., Wan, B. et al. Structural evolution, mechanical properties, and electronic structure of Al–Mg–Si compounds from first principles. J Mater Sci 50, 6498–6509 (2015). https://doi.org/10.1007/s10853-015-9209-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9209-4