Abstract
Controlling the physical properties of the functional materials is required for broadening their industrials and technological applications. High purity copper oxide (CuO) nanoparticles (NPs) of monoclinic phase and average crystallite size of 35 nm were prepared by a simple sol–gel technique. A solution casting method was applied to prepare carboxymethyl cellulose (CMC), polyvinyl alcohol (PVA)/CMC, and CuO/PVA/CMC nanocomposite films. X-ray diffraction (XRD) pattern of CMC shows a broad XRD peak around 2θ = 20.7° which is influenced by PVA and CuO NPs addition. The surface morphology and dispersion of CuO NPs in PVA/CMC films were investigated by scanning electron microscopy (SEM). CMC film shows transparency of 87 % increased after PVA addition, and then decreased with CuO NPs incorporation. The insulating properties, refractive index, and optical constants of CMC are controlled by mixing with PVA and doping with CuO NPs. In addition, the current–voltage (I–V) characteristics of the PVA/CMC blend and nanocomposite films show a non-ohmic behavior. The conduction mechanism in PVA/CMC blend is Schottky emission at low temperature, while the Poole–Frenkel effect plays an important role in CuO-doped films at high temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development and investigation of new nanocomposite films based on polymer blend attract considerable and increased attentions. Mixing of two or more polymers to develop a new blend with enhanced properties is usually less time-consuming than a realization of new polymer chemistry. The addition of small amounts from a nano-sized material could improve the overall performance of the polymeric materials. Hence, broadening the technological applications of these materials compared to their constituents’ individual polymers [1–5].
Cellulose is the most abundant biomass material in nature [6]. Carboxymethyl cellulose (Na-CMC) is the most popular and the cheapest ionic-type smart cellulose ether. It is produced by reacting alkali cellulose with sodium mono-chloroacetate under rigidly controlled conditions. It has a high transparency, good film-forming property, and high mechanical strength [7, 8]. Na-CMC is also a non-toxic and biodegradable material [9]. These properties and functions make it ideal for use in a broad range of applications like coating development [10], food packaging purposes [11], wound dressing [12], drug delivery systems [13], gas separation properties [14], liquid petroleum gas sensing [15], agriculture applications [16], and as a binder for Si-based Li-ion battery anodes [17]. On the other hand, polyvinyl alcohol (PVA) is a synthetic, colorless, water-soluble polymer. The non-toxicity, biocompatibility, and photo-stability upon UV–visible light irradiation, beside the thermal stability and semi-crystalline nature, make PVA as a strong candidate for many applications [18, 19]. Also, PVA/CMC is a flexible blend that could be involved in many applications [20, 21].
Study the effects of different dopants on the physical and chemical properties of CMC have attracted the interests of many researchers. Preparation of a solid biopolymer electrolytes (SBE) based on CMC doped with adipic acid was reported by Rozali et al. [22]. The influence of copper (Cu) [23], chitosan [24], carbon nanotube (CNTs) [25], NiCl [26], and TiO2 [27] on the properties of CMC was also reported. In addition, Yadava et al. [28] prepared graphene oxide (GO, at 1 wt%)/CMC/alginate composite with improved tensile strength and Young’s modulus by 40 and 1128 %, respectively. Zhan et al. [29] prepared GO (at 1.6 wt%)/polyacrylamide (PAM)/CMC nanocomposite with improved compressive strength. Another working group reported that doping of [PVA/chitosan (CN)/lithium perchlorate (LiClO4)] composite with TiO2 could be used in lithium ion batteries as it facilitates the migration of lithium ions in the polymer matrix [30].
Among the nano-sized materials, copper oxide (CuO) is a semiconductor with band gap of 1.2–2.1 eV and exhibits p-type conductivity due to the excess of oxygen or copper vacancies in its structure [31–33]. Besides its low production cost and non-toxicity, CuO shows good electronic and thermal properties, chemical stability, high dielectric constant of 18.1, and a refractive index of 1.4 [34]. Therefore, nanostructured CuO has been widely used for various applications such as supercapacitors [35], solar energy applications [36], spintronics and high T C superconductors [37], and nanofluids and heat transfer applications [38].
Based on the above literature, the present work is devoted to investigate the influence of PVA addition and CuO NPs on the physical properties of CMC polymer as an attempt to improve its optical and electrical properties. UV–Vis absorption spectroscopy is an elementary tool providing important information regarding the optical properties and the electronic band structure of the materials. The electrical conduction in polymers has been extensively studied in recent years to understand the nature of charge transport in these materials. In this branch, several mechanisms have been suggested for the charge transport in polymers [39–42].
Experimental procedures
Materials preparation
A simple sol–gel process was applied to get CuO nanoparticles (NPs). In brief: 29.1 g Cu(CH3COO)2·H2O [M W = 199.65, Barcelona, PRS Panreac] and 18.91 g of high purity oxalic acid [C2H2O4·2H2O, M W = 126.07, LOBA Chemie, India] were dissolved in 100 mL double distilled (DD) water under a magnetic stirring at 60 °C for 1.0 h. The obtained solution was aged for 12 h then stirred at 70 °C for 0.5 h. The obtained gel was calcined at 500 °C for 2 h in an air furnace and left to cool to room temperature (RT). The synthesized CuO NPs were kept in a zipper bag to avoid the moisture effect.
Four polymeric films with the composition; CMC, PVA/CMC, 0.25 wt% CuO, and 0.50 wt% CuO-doped PVA/CMC were synthesized by solution casting method. The first film was prepared by dissolving 3 g CMC [Na-carboxymethyl cellulose of commercial grade, supplied by El Nasr Pharmaceutical Chemicals Co., Egypt] in 100 mL DD water at 80 °C and a magnetic stirring for 1.0 h till the complete dissolution of CMC. To form the blend films; 0.6 g PVA [Avondale Laboratories, England, average M W(average) = 17000] and 2.4 g CMC were dissolved in 100 mL DD water at 80 °C and a magnetic stirring for 1.0 h till the complete dissolution of the mixed polymers. The desired CuO NPs content M (0.25 and 0.50 wt%) was calculated using the following equation:
where m d and m b are the weight of the dopant material (CuO NPs) and the blend (20 % PVA + 80 % CMC), respectively. Finally, the solutions cast into glass petri dishes and placed in an oven to dry at 60 °C for 24 h in air. The films were peeled off from the petri dishes, and care was taken to obtain polymer films of uniform thickness. It was not possible to prepare composites with higher (>0.5 wt%) CuO NPs loadings because of the high viscosity of the blend solution and to avoid the nanoparticles agglomeration.
Measurements
The X-ray diffraction (XRD) patterns of CuO NPs and the prepared films were obtained using a PANalytical’s X’Pert PRO system. High-resolution transmission electron microscopy (HRTEM; JEM 2100, Jeol, Japan) was used to test the grains size and morphology of the as-synthesized CuO NPs. Scanning electron microscopy (SEM; Inspect S, FEI, Holland) and fracture cross-section images were taken for all prepared films. The thickness of the films was evaluated using a digital micrometer with accuracy ±0.001 mm.
The absorbance (Abs.) and reflectance (R) spectra of the samples were recorded at RT using a Shimadzu UV-3600 UV–VIS–NIR spectrophotometer in the wavelength range 200–800 nm with an accuracy of ±0.2 nm. Barium sulfonate was used as a reference to provide a nominal 100 % reflectance measurement. Optical properties of CuO NPs were studied by collecting Abs. spectra of a solution of 3.5 mg CuO NPs dispersed in 50 mL de-ionized water. The absorption coefficient (α) was determined using the equation [43];
where ρ is the density of CuO (6.31 g/cm3) [34], l is the optical path length, and c is the solution concentration.
A computerized Keithley 2635A system source meter is used to measure the current–voltage (I–V) characteristics of PVA/CMC blend and CuO-doped PVA/CMC nanocomposite films. It was connected to a host computer through GPIB/USB cable. The I–V characteristics were recorded in the temperature range 303–373 K and the voltage range 0–20 V. The samples were coated with silver paste to achieve ohmic contact. The samples with the holder were put inside an electrical furnace where the temperature measured by a digital thermometer.
Results and discussions
Structural properties
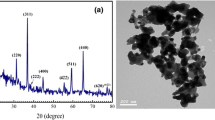
Figure 1a shows the XRD pattern of the sol–gel prepared CuO NPs. The observed sharp peaks indicate the good crystallinity of the synthesized powder. The 2θ values, d-spacing, and the relative intensity of the peaks are listed in Table 1. Clearly, the diffraction peaks can be identified for monoclinic (tenorite) phase of CuO (JCPDS file no. 45-0937) with lattice parameters a = 4.685 Å, b = 3.426 Å, c = 5.13 Å, and β = 99.55°. These lattice parameters are obtained using the following relation [44]:
where d is the d-spacing obtained from XRD data and h, k, and l are the miller indices of the crystallographic planes shown in Fig. 1a. Diffractions corresponding to Cu, Cu2O, Cu(OH)2, or Cu(CH3COO) were not observed, suggesting the formation of high purity CuO.
The crystallite size (L) of CuO NPs is calculated using Scherrer formula; L = 0.9λ/(FWHM × cos θ), where FWHM is the full width at half maximum intensity. The calculated L values are listed in Table 1, and the average crystallite size (L av) is found to be ~35 nm. To investigate the shape and morphology of CuO powder, HR-TEM image was taken. Figure 1b demonstrates that the CuO NPs have nearly the same shape and their sizes are consistent with the XRD results given in Table 1. Similar results were reported for flower-like CuO nanostructure prepared by annealing of Cu(OH)2 at 500 °C [36] and for the micro-nanostructured CuO/C spheres prepared by an aerosol spray pyrolysis [45]. In addition, Chand et al. [46] synthesized CuO nanostructures of different morphologies by a hydrothermal method via controlling pH values of the solution.
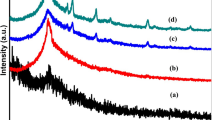
The influence of PVA and CuO NPs on the structural properties of CMC was studied by XRD and SEM spectroscopies. XRD patterns of pure CMC, PVA/CMC, and CuO-doped PVA/CMC nanocomposite films are depicted in Fig. 2a–d. For CMC film, there is a broad peak exists around 2θ = 20.7°, Fig. 2a. Another weak intense peak at 2θ ≈ 36° is existing indicating the semi-crystalline structure of CMC. Similar results were reported for CMC films [47]. Also, these two peaks were observed in the XRD pattern of CMC prepared by Grumezescua et al. [48]. Addition of 20 % PVA shifts the broad peak in XRD pattern of CMC to a lower value of 2θ (~20°) and the weak intense peak to higher 2θ value (40.5°), Fig. 2b. This confirms the presence of a typical semi-crystalline structure of the blend and suggests that PVA has strong interaction with CMC [49]. This is consistent with the pervious published data [50]. Also, doping of PVA/CMC blend with CuO NPs re-shifted the maximum intensity peak to higher 2θ values, i.e., 21.5° and 20.8° after doping with 0.25 and 0.50 wt% CuO, respectively (Fig. 2c, d). Moreover, it is seen that the most intense CuO XRD peaks, appeared in Fig. 1a, are also appearing in the XRD pattern of 0.5 wt% CuO-doped film, Fig. 2d. The average inter-crystallite separation (S) of the films was calculated from the position of the maximum of the halo [50]:
where λ = 1.54 Å is the wavelength of the used X-ray radiation. The values of S are found to be 5.36, 5.54, 5.16, and 5.33 Å for CMC, PVA/CMC, 0.25 and 0.50 wt% CuO-doped PVA/CMC films, respectively. These values confirm the effect of PVA and CuO NPs addition on the crystallinity of CMC film.
SEM was performed to examine the distributions and dispersions of CuO NPs on the surfaces and inside PVA/CMC blend. Figure 3a–d shows the SEM images and the fracture cross sections (the insets) of CMC, PVA/CMC, and the nanocomposite films. It is observed from Fig. 4c that the 0.25 wt% CuO NPs content is good dispersed on the surface of the PVA/CMC blend. However, small number of agglomerations begin to appear with increasing CuO NPs content to 0.5 wt%. These results indicate that the addition of PVA and CuO NPs with small amount could be used to control the structural properties of CMC polymer.
Optical properties
From the experimental values of reflectance (R) and absorbance (Abs.), the transmittance (T) and the extinction coefficient (k) of the films were calculated using the following relations [51]:
where the absorption coefficient α = Abs./t and t is the measured values of the film thicknesses, listed in Table 2. The dependence of the transmittance percentage (T%) on the wavelength, λ, for the CMC pure, the blend (PVA/CMC) and the nanocomposite films are depicted in Fig. 4a. In the wavelength region 520–700 nm, CMC film shows T ≈ 87 %, which increased to about 89 % after the addition of 20 % PVA. This indicates the good miscibility between PVA and CMC chains and supports the results of XRD analysis [52]. This result is in a good agreement with that reported earlier. The pure PVA showed T = 90 % in the wavelength region 400–800 nm [53] and also, it is reported that a blend of (50 % CMC + 50 % PVA) showed a transmittance of ~85 % [54]. On the other hand, doping the (PVA/CMC) blend with CuO NPs leads to the decrease of T to about 77 %. The incorporation of CuO NPs inside blend matrix may act as scattering centers and leads to the observed decrease in T% [55]. Figure 4b shows the dependence of k and absorption spectra (the inset) of the films on λ. The absorption of pure CMC is higher than that of the pure blend and the absorption increases with increasing CuO NPs content in the PVA/CMC blend. Also, it is observed from Fig. 4b that a clear absorption band is existed for all films at ~275 nm and assigned to π–π* electronic transitions. This band decreases after adding PVA to CMC and increases with increasing CuO NPs content. Similar results were reported for Sb2O3/CMC [56] and ZnS/CMC [47] nanocomposite films. In addition, these results indicate that the optical properties of CMC could be effectively controlled by the addition of PVA and CuO NPs.
The E g values of the CuO NPs and the CuO/PVA/CMC nanocomposite films are calculated using Tuac’ relation:
where hν is the energy of incident photons and m = 1/2 for indirect and 2 for direct allowed transitions. Assuming direct transitions between the valence band and the conduction band in CuO, E g is determined by extrapolating the straight portion of (αhν)2 verses (hν) plot to the energy axis at α = 0 (Fig. 5). The estimated E g value for CuO NPs is ~2.0 eV which is consistent with the reported values for CuO in the literature [31, 32]. Also, Chand et al. [46] reported E g values for CuO NPs between 2.90 and 3.60 eV depending on the pH value of the solution. The dependence of the absorption spectra of CuO NPs on λ is shown in the inset of Fig. 5. There is an absorption band around 340 nm. Similar values were reported for the cocoon-shaped CuO hollow architectures and CuO NPs. In these reports, the position of the absorption band depends on the particles size of the prepared CuO [32, 57].
The indirect and direct optical band gaps of the prepared films were investigated using Eq. (6). The linear dependence of both (αhν)2 and (αhν)0.5 on hν at higher photon energies, Fig. 6a, b indicates that, both direct and indirect optical transitions are possible for these films. The straight-line portions of the curves are extrapolated to zero to give the optical band gaps, E g (direct) and E g (indirect), and the obtained values are listed in Table 2. As seen from Fig. 6 and Table 2, E g (direct) of CMC film is 5.36 eV which increases to 5.40 eV after adding 20 % PVA, and then decreases to 5.10 eV after doping with CuO NPs. Incorporation of CuO NPs may introduce energy levels inside the band gap of PVA/CMC matrix, leading to the narrowing of its E g . The same behavior is also observed for E g (indirect). Luna-Martínez et al. [47] reported the decrease of E g (direct) with increasing ZnS NPs content in CMC film. In addition, these results are consistent with our previous works, where the reported E g value for PVA is 5.1 eV [50] and that of the (50 % CMC + 50 % PVA) blend is 4.84 eV [54].
Refractive index and the optical constants of the films
The absorbance (Abs.) and the reflectance (R) data were analyzed to determine the refractive index (n) and some dispersion parameters. The reflectance relates to n by the following equation [58]:
Solving the above equation gives:
Figure 7a shows the refractive index distributions of the films. Also, the n values at λ = 500 nm are listed in Table 2. It is noted that n decreases from 1.645 for CMC to 1.576 for PVA/CMC blend, and then it increases with increasing CuO NPs content inside the blend till reach 1.852. Also, n of the films decrease with increasing λ in the most of visible range of the spectra. It has been concluded that when the incident light interacts with a material that has a large amount of particles, the refraction will be higher and hence the refractivity of the films will increase [59]. Similar to that were reported for CdO/PVC and α-Fe2O3/PEG/PVA nanocomposite films [55, 60].
The variation of n with λ can be expressed by the following dispersion relation [60]:
where n ∞ is the long wavelength refractive index and λ o is the average inter-band oscillator wavelength. The parameters n ∞ , λ o, as well as S o [the average oscillator strength; S o = (n 2∞ − 1)/λ 2o )] were obtained from the slope and the intercepts of (n 2 − 1)−1 versus λ −2 curves shown in Fig. 7b. The values of these parameters are also given in Table 2. The frequency dependence of the optical dielectric constant is a parameter that gives some information about the electronic excitations inside the materials. The real (ε re) and imaginary (ε im) parts were calculated using the formulae [61].
It is seen, from Fig. 8a and b, that with increasing hν, ε im values increase and then decrease at the higher values of hν. The behavior of ε re with hν is somewhat different. An increase in ε re is followed by a decrease till reaching a minimum value at hν = 4.2 eV for PVA/CMC film and at hν = 4.5 eV for the other films. After that, ε re values increase again then slightly decrease at the end for 0.5 % CuO-doped film. Both ε re and ε im of CMC decrease with PVA addition but increase with increasing CuO NPs content which may be attributed to the high dielectric constant of CuO [34]. The ε im values are smaller by a factor of ~2 × 10−4 than ε re values. The 0.50 wt% CuO-doped film has the largest ε re and ε im values over a wide energy range.
I–V characteristics and evaluation of the conduction mechanism
The current–voltage (I–V) characteristics of PVA/CMC blend and CuO-doped films at some selected temperatures are shown in Fig. 9a–c. By increasing the applied potential difference (V), the current (I) increases slowly at first then increases with higher rates where the non-ohmic behavior is clear. As the temperature increases, the current (I) increases till reaching a certain temperature depending on the CuO NPs content then it decreases again. Finally (I) increases as the dopant concentration increases.
The increase in I as temperature increases means that these films are thermally activated, i.e., they have semiconducting properties. By raising the temperature, an increase in the segmental motion of the polymer chains occurred [24, 62]. The chains now have energy to push against hydrostatic pressure exerted by its neighboring molecules and created a small amount of space for segmental motion to occur. Also the mobility of CuO NPs may be enhanced [63]. These two factors result in the formation of more connected networks. Another important factor in increasing I is the increase in the concentration of charge carriers [64] due to the heating process. The decrease in the current after certain temperature may be due to some structural change in the crystalline phase inside PVA/CMC matrix [65].
As the content of CuO NPs in the films increases, more connected networks will form. This is due to the higher probability of physical contacts of these NPs in the polymer matrix. Such contacts could form paths that facilitate the motion of charge carriers and result in the current increase. Another important factor is that the addition of CuO NPs may introduce energy levels inside the band gap of PVA/CMC matrix, as discussed in the previous sections, leading to the narrowing of its E g and the increase of the current.
The log (V) − log (I) plots for PVA/CMC blend and CuO-doped nanocomposite films are shown in Fig. 10. Such plots help us to find the nonlinear coefficient parameter (r) which is given using the relation;
where C is a constant. It is clear that, there are two regions in each graph so we have two values r 1 and r 2 . The values of nonlinear coefficient parameters are listed in Table 3 and indicate the non-ohmic behavior of the I–V characteristics of the films. It was noticed also that r 2 is larger than r 1 . The values of r increase as temperature and the content of CuO NPs increase.
It should be mentioned that, the I–V characteristics obey the relation [66];
where e is the charge of electron, E is the applied electric field and γ is a constant characteristic of the conduction mechanism. Plotting V 0.5 − log (I) will help us to know the concerned conduction mechanism. Figure 11 shows V 0.5 − log (I) plots of PVA/CMC blend and CuO-doped nanocomposite films. Linear trends are obvious, especially at higher potentials. It was reported in the literature that if the experimental value of γ is about 2.09 × 10−5 eV, the charge transport mechanism operating in the polymer is Schottky emission [67–69]. The charges are injected from the electrodes into the polymer over a potential barrier, the magnitude of which is field-dependent. But, if the value of γ is about 4.09 × 10−5 eV, the conduction mechanism is Poole–Frenkel effect [39], the charge carriers can be released from traps into the dielectric material. The experimental values of γ are found from the slope of the linear trends in Fig. 11 and are listed in Table 4. It is suggested that, the conduction mechanism is Schottky emission in PVA/CMC blend especially at low T. But after the doping with CuO NPs and raising the temperature, Poole–Frenkel effect plays an important role in explaining the conduction mechanism in our films.
Conclusions
CuO NPs of average crystallite size 35 nm and optical band gap (E g) of 2.0 eV were prepared by a sol–gel method, and then mixed with PVA/CMC films. The addition of 20 % PVA has shifted the main XRD peak of CMC from 2θ = 20.7° to 20°. The optical transmittance (T %) of CMC increased from 87 to 89 % after mixing with PVA and decreased to 77 % after doping with 0.5 wt% CuO NPs. The E g values were of similar trend as T%. The refractive index of CMC decreased from 1.645 to 1.576 after PVA addition, and then increased to 1.852 after doping the PVA/CMC with CuO NPs. The optical constants of CMC film were also influenced by PVA and CuO NPs. The current–voltage characteristics of the films were of non-ohmic behavior. The conduction mechanism is Schottky emission in PVA/CMC film at low temperature and Poole–Frenkel effect plays a role in CuO-doped films at high temperatures. Based on these results, there is a possibility for controlling the structural, optical, and electrical properties of CMC polymer by adjusting the PVA content and CuO NPs loading. Hence, the industrial and environmental applications of CuO-doped PVA/CMC could be broadened.
References
Hsieh TH, Huang YS, Shen MY (2015) Mechanical properties and toughness of carbon aerogel/epoxy polymer composites. J Mater Sci 50:3258–3266. doi:10.1007/s10853-015-8897-0
Bidsorkhi HC, Adelnia H, Pour RH, Soheilmoghaddam M (2015) Preparation and characterization of ethylene-vinyl acetate/halloysite nanotube nanocomposites. J Mater Sci 50:3237–3245. doi:10.1007/s10853-015-8891-6
Arjmandi R, Hassan A, Eichhorn SJ, Haafiz MKM, Zakaria Z, Tanjung FA (2015) Enhanced ductility and tensile properties of hybrid montmorillonite/cellulose nanowhiskers reinforced polylactic acid nanocomposites. J Mater Sci 50:3118–3130. doi:10.1007/s10853-015-8873-8
Abreu AS, Oliveira M, Rodrigues PV, Moura I, Botelho G, Machado AV (2015) Synthesis and characterization of polystyrene-block-poly(vinylbenzoic acid): a promising compound for manipulating photoresponsive properties at the nanoscale. J Mater Sci 50:2788–2796. doi:10.1007/s10853-015-8835-1
Zhou L, Liu H, Zhang X (2015) Graphene and carbon nanotubes for the synergistic reinforcement of polyamide 6 fibers. J Mater Sci 50:2797–2805. doi:10.1007/s10853-015-8837-z
Thomas S, Maria HJ, Joy J, Chan CH, Pothen LA (2013) Natural rubber materials: composites and nanocomposites, vol 2. Royal Society of Chemistry, London, UK
Aziz NAN, Idris NK, Isa MIN (2009) Ionic conductivity studies of chitosan-based polymer electrolytes doped with adipic acid. Ionics 15:643–646
Samsudin AS, Isa MIN (2012) Structural and electrical properties of carboxymethyl cellulose-dedecyltrimethyl ammonium bromide-based biopolymer electrolytes system. Int J Polym Mater 61(1):30–40
Mohammed FA (2001) Topical permeation characteristics of diclofenac sodium from Na-CMC gels in comparison with conventional gel formulations. Drug Dev Ind Pharm 27(10):1083–1097
Tiitu M, Laine J, Serimaa R, Ikkala O (2006) Ionically self-assembled carboxymethyl cellulose/surfactant complexes for antistatic paper coatings. J Colloid Interface Sci 301:92–97
Roy N, Saha N, Kitano T, Saha P (2012) Biodegradation of PVP-CMC hydrogel film: a useful food packaging material. Carbohydr Polym 89:346–353
Wang M, Xu L, Hu H, Zhai M, Peng J, Nho Y, Li J, Wei G (2007) Radiation synthesis of PVP/CMC hydrogels as wound dressing. Nucl Instrum Meth B 265:385–389
Sandeep C, Harikumar SL, Kanupriya (2012) Hydrogel: a smart drug delivery system. Int J Res Pharm Chem 2:603–614
Miremadi SI, Shafiabadi N, Amini-Fazl MS (2013) Gas separation properties of cross-linked and non-cross-linked carboxymethyl cellulose (CMC) membranes. Sci Iran C 20(6):1921–1928
Ravikiran YT, Kotresh S, Vijayakumari SC, Thomas S (2014) Liquid petroleum gas sensing performance of polyaniline carboxymethyl cellulose composite at room temperature. Curr Appl Phys 14:960–964
Raafat AI, Eid M, El-Arnaouty MB (2012) Radiation synthesis of superabsorbent CMC based hydrogels for agriculture applications. Nucl Instrum Meth B 283:71–76
Magasinski A, Zdyrko B, Kovalenko I, Hertzberg B, Burtovyy R, Huebner CF, Fuller TF, Luzinov I, Yushin G (2010) Toward efficient binders for Li-Ion battery Si-based anodes: polyacrylic acid. ACS Appl Mater Interfaces 2:3004–3010
Lee J, Bhttacharryya D, Easteal AJ, Metson JB (2008) Properties of nano-ZnO/poly(vinyl alcohol)/poly(ethylene oxide) composite thin films. Curr Appl Phys 8:42–47
Abdel-Kader FH, Shehap AM, Abellil MS, Mahmoud KH (2005) Relaxation phenomenon of poly(vinyl alcohol)/sodium carboxymethyl cellulose blend by thermally stimulated depolarization currents and thermal sample technique. J Appl Polym Sci 95:1342–1352
Abou Taleb MF, Abd El-Mohdy HL, Abd El-Rehim HA (2009) Radiation preparation of PVA/CMC copolymers and their application in removal of dyes. J Hazard Mater 168:68–75
El Salmawi KM (2007) Application of polyvinyl alcohol (PVA)/carboxymethyl cellulose (CMC) hydrogel produced by conventional crosslinking or by freezing and thawing. J Macromol Sci, Pure Appl Chem 44:619–624
Rozali MLH, Isa MIN (2014) Electrical behaviour of carboxymethyl cellulose doped adipic acid solid biopolymer electrolyte. Int J Mater Sci 4:59–64
El-Saied H, Basta AH, Hanna AA, El-Sayed AM (1999) Some semiconductor properties of carboxymethyl cellulose-copper complexes. Polym-Plast Technol 38:1095–1105
Hafiza MN, Isa MIN (2014) Ionic conductivity and conduction mechanism studies of CMC/chitosan biopolymer blend electrolytes. Res J Recent Sci 3:50–56
Zhu C, Chen J, Koziol K, Gilman JW, Trulove PC, Rahatekar SS (2014) Effect of fibre spinning conditions on the electrical properties of cellulose and carbon nanotube composite fibres spun using ionic liquid as a benign solvent. Express Polym Lett 8:154–163
El-Bahy ZM, Mahmoud KH (2012) Dielectric characterization and catalytic activity studies of nickel chloride doped carboxymethyl cellulose films. Spectrochim Acta A 92:105–112
Abdel-Galil HE, Atta AA, Balboul MR (2014) Influence of nanostructured TiO2 additives on some physical characteristics of carboxymethyl cellulose (CMC). J Radiat Res Appl Sci 7:36–43
Yadava M, Rhee KY, Park SJ (2014) Synthesis and characterization of graphene oxide/carboxymethyl cellulose/alginate composite blend films. Carbohydr Polym 110:18–25
Zhang H, Zhai D, He Y (2014) Graphene oxide/polyacrylamide/carboxymethyl cellulose sodium nanocomposite hydrogel with enhanced mechanical strength: preparation, characterization and the swelling behavior. RSC Adv 4:44600–44609
Rathod SG, Bhajantri RF, Ravindrachary V, Sheela T, Pujari PK, Naik J, Poojary B (2015) Pressure sensitive dielectric properties of TiO2 doped PVA/CN-Li nanocomposite. J Polym Res 22:6
Mugwang’a FK, Karimi PK, Njoroge WK, Omayio O, Waita SM (2013) Optical characterization of copper oxide thin films prepared by reactive dc magnetron sputtering for solar cell applications. Int J Thin Film Sci Technol 2:15–24
Ganga BG, Santhosh PN (2014) Manipulating aggregation of CuO nanoparticles: correlation between morphology and optical properties. J Alloys Compd 612:456–464
Chaudhary YS, Agrawal A, Shrivastav R, Satsangi VR, Dass S (2004) A study on the photoelectrochemical properties of copper oxide thin films. Int J Hydrogen Energ 29:131–134
Zhang Q, Zhang K, Xu D, Yang G, Huang H, Nie F, Liu C, Yang S (2014) CuO nanostructures: synthesis, characterization, growth mechanisms. Fundamental properties and applications. Prog Mater Sci 60:208–337
Zhang YX, Huang M, Li F, Wen ZQ (2013) Controlled synthesis of hierarchical CuO nanostructures for electrochemical capacitor electrodes. Int J Electrochem Sci 8:8645–8661
Liu Y, Liao L, Li J, Pan C (2007) From copper nanocrystalline to CuO nanoneedle array: synthesis, growth mechanism, and properties. J Phys Chem C 111:5050–5056
Zhu H, Zhao F, Pan L, Zhang Y, Fan C, Zhang Y, Xiao JQ (2007) Structural and magnetic properties of Mn-doped CuO thin films. J Appl Phys 101:09H111
Vidyasagar CC, Naik YA, Venkatesh TG, Viswanatha R (2011) Solid-state synthesis and effect of temperature on optical properties of Cu–ZnO, Cu–CdO and CuO nanoparticles. Powder Technol 214:337–343
Hill KM (1971) Poole-Frenkel conduction in amorphous solids. Philos Mag 23:59–86
Caserta G, Rispoli B, Serra A (1969) Space-charge-limited current and band structure in amorphous organic films. Phys Stat Sol A 35:237–248
Perets YS, Matzui LY, Vovchenko LL, Prylutskyy YI, Scharff P, Ritter U (2015) The effect of boron nitride on electrical conductivity of nanocarbon-polymer composites. J Mater Sci 49:2098–2105. doi:10.1007/s10853-013-7901-9
Sinha S, Chatterjee SK, Ghosh J, Meikap AK (2015) Electrical transport properties of polyvinyl alcohol–selenium nanocomposite films at and above room temperature. J Mater Sci 50:1632–1645. doi:10.1007/s10853-014-8724-z
Makhlouf SA, Bakr ZH, Aly KI, Moustafa MS (2013) Structural, electrical and optical properties of Co3O4 nanoparticles. Superlattices Microst 64:107–117
Singh I, Bedi RK (2011) Studies and correlation among the structural, electrical and gas response properties of aerosol spray deposited self assembled nanocrystalline CuO. Appl Surf Sci 257:7592–7599
Lu Y, Zhang N, Zhao Q, Liang J, Chen J (2015) Micro-nanostructured CuO/C spheres as high-performance anode materials for Na-ion batteries. Nanoscale 7:2770–2776
Chand P, Gaur A, Kumar A (2013) Structural, optical and ferroelectric behavior of CuO nanostructures synthesized at different pH values. Superlatt Microst 60:129–138
Luna-Martinez JF, Hernandez-Uresti DB, Reyes-Melo ME, Guerrero-Salazar CA, Gonzalez-Gonzalez VA, Sepulveda-Guzman S (2011) Synthesis and optical characterization of ZnS–sodium carboxymethyl cellulose nanocomposite films. Carbohydr Polym 84:566–570
Grumezescu AM, Andronescu E, Ficai A, Bleotuc C, Mihaiescu DE, Chifiriuc MC (2012) Synthesis, characterization and in vitro assessment of the magnetic chitosan–carboxymethyl cellulose biocomposite interactions with the prokaryotic and eukaryotic cells. Int J Pharm 436:771–777
Agarwal R, Alam MS, Gupta B (2013) Polyvinyl alcohol-polyethylene oxide-carboxymethyl cellulose membranes for drug delivery. Appl Polym Sci 129:3728–3736
Hassen A, El Sayed AM, Morsi WM, El-Sayed S (2012) Influence of Cr2O3 nanoparticles on the physical properties of polyvinyl alcohol. J Appl Phys 112:093525
Ali AI, Ammar AH, Abdel Moez A (2014) Influence of substrate temperature on structural, optical properties and dielectric results of nano-ZnO thin films prepared by Radio Frequency technique. Superlattices Microst 65:285–298
Abd Alla SG, Said HM, El-Naggar AM (2004) Structural properties of γ-irradiated poly (vinyl alcohol)/poly (ethylene glycol) polymer blends. J Appl Polym Sci 94:167–176
Mallakpour S, Madani M (2011) Use of l-tyrosine amino acid as biomodifier of Cloisite Na+ for preparation of novel poly(vinyl alcohol)/organoclay bionanocomposites film. J Mater Sci 46:4071–4078. doi:10.1007/s10853-011-5336-8
El Sayed AM (2014) Synthesis and controlling the optical and dielectric properties of CMC/PVA blend via γ-rays irradiation. Nucl Instrum Meth B 321:41–48
El Sayed AM, Morsi WM (2014) α-Fe2O3/(PVA + PEG) Nanocomposite films; synthesis, optical, and dielectric characterizations. J Mater Sci 49:5378–5387. doi:10.1007/s10853-014-8245-9
Chang PR, Yu J, Ma X (2009) Fabrication and characterization of Sb2O3/carboxymethyl cellulose sodium and the properties of plasticized starch composite films. Macromol Mater Eng 294:762–767
Deng C, Hu H, Zhu W, Han C, Shao G (2011) Green and facile synthesis of hierarchical cocoon shaped CuO hollow architectures. Mater Lett 65:575–578
Abdelaziz M (2011) Cerium (III) doping effects on optical and thermal properties of PVA films. Phys B 406:1300–1307
El Sayed AM, Morsi WM (2013) Dielectric relaxation and optical properties of polyvinyl chloride/lead monoxide nanocomposites. Polym Compos 34:2031–2039
El Sayed AM, El-Sayed S, Morsi WM, Mahrous S, Hassen A (2014) Synthesis, characterization, optical, and dielectric properties of polyvinyl chloride/cadmium oxide nanocomposite films. Polym Compos 35:1842–1851
Al-Ghamdi AA (2006) Optical band gap and optical constants in amorphous Se96-xTe4Agx thin films. Vacuum 80:400–405
Lampman S (2003) Characterization and failure analysis of plastics. ASM International, Ohio
Suzuki K, Tanaka N, Ando A, Takagi H (2012) Size-selected copper oxide nanoparticles synthesized by laser ablation. J Nano Part Res 14:863–874
Diaham S, Locatelli ML (2012) Concentration and mobility of charge carriers in thin polymers at high temperature determined by electrode polarization modeling. J Appl Phys 112:013710
Li Q, Xue QZ, Gao XL, Zheng QB (2009) Temperature dependence of the electrical properties of the carbon nanotube/polymer composites. Express Polym Lett 3:769–777
Nagaraj N, Reddy ChVS, Sharma AK, Rao VVRN (2002) DC conduction mechanism in polyvinyl alcohol films doped with potassium thiocyanate. J Power Sour 112:326–330
Abdel-Malik TG, Abdel-Latif RM, Sawaby A, Ahmed SM (2008) Electrical properties of pure and doped polyvinyl alcohol (PVA) films using gold and aluminum electrodes. J Appl Sci Res 4:331–336
Bronsgeest M (2014) Physics of Schottky electron sources: theory and optimum operation. CRC Press, Boca Raton
Pillai PKC, Jain K, Jain VK (1973) Thermally stimulated discharge current studies in shellac wax electrets. Phys Stat Sol A17:221–227
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Sayed, A.M., El-Gamal, S., Morsi, W.M. et al. Effect of PVA and copper oxide nanoparticles on the structural, optical, and electrical properties of carboxymethyl cellulose films. J Mater Sci 50, 4717–4728 (2015). https://doi.org/10.1007/s10853-015-9023-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9023-z