Abstract
A novel palladium-based catalysts supported on Fe3O4/ZnO nanoparticles have been prepared by a simple method. The catalyst was characterized by transmission electron microscopy, X-ray powder diffraction, X-ray photoelectron spectroscopy, atomic absorption spectrophotometry, FT-IR, and BET analysis. The catalyst afforded efficient synthesis of 2-oxazolines and benzoxazoles from aromatic nitriles under solvent-free conditions. The significant features of this method are short reaction times, good to high yields of the products, simple operation, solvent-free condition, non-toxicity, reusability of the catalyst without significant loss of catalytic activity, and using ultra small amount of Pd (0.004 g of catalyst contains 9.16 × 10−3 mmol Pd which was determined by ICP).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benzoxazoles and 2-oxazolines are important classes of N-heterocycle compounds that are exist in variety of natural products, biologically active compounds, drug and agrochemicals, enzyme inhibitors as well as other applications [1, 2]. For example, anticancer agent NSC-693638 [3], HIV reverse transcriptase inhibitor L-697,661 [4], and antitumor agent BE-70016 [5, 6], include the benzoxazole and oxazoline core structure, respectively (Fig. 1). These compounds have received much attention due to their unique structures and biological activities.
A large number of methods for the synthesis of bezoxazoles and 2-oxazolines are known via the condensation of a 2-aminophenol or amino alcohol with nitriles (For synthesis of 2-oxazolines from nitriles see [7], for synthesis of benzoxazole from nitriles see [8–12]), carboxylic acids [13–15], and their derivatives [16–21]. Witte and Seeliger in 1974 [7] reported for the first time a simple one-pot reaction, where a nitrile is converted to the corresponding 2-oxazoline by reaction with an amino alcohol in the presence of a Lewis-acid catalyst. Using nitriles, which a wide range of them, are commercial available makes this reaction an ideal method to prepare benzoxazoles and 2-oxazolines. Although these methods have considerable progress, but there are still suffer from some disadvantages such as long reaction time, high temperature, corrosive reagents or catalysts, tedious work-up, and specially these methods cannot be utilized to synthesize both benzoxazoles and 2-oxazolines. Recently, Prasad and coworkers [22] reported the synthesis of aryl 2-oxazolines from aromatic nitriles and aminoalcohols using magnetically recoverable Pd/Fe3O4. In particular, magnetically Pd/Fe3O4 are attractive as the most catalyst for this reaction; however, it suffer from some drawbacks such as using toxic organic solvent (toluene), long reaction time (10 h), and large amount of Pd/Fe3O4 (3 mol%).
In general, heterogeneous catalysts are more favored over homogeneous catalysis because of their ease of handling and reusability. But heterogeneous catalysts suffer from a disadvantage in comparing to the homogeneous catalysts. The former have less active site than their homogeneous counterparts. To overcome this drawback, nanomaterials have emerged as sustainable alternatives to conventional heterogeneous catalysts and catalyst supports [23–26]. The nanomaterials present high specific surface area of the active component, thereby enhance the contact between reactants and catalyst support [27]. Although a higher surface area affords nanomaterials with more active sites, they are easy to agglomerate and hard to be separated. One strategy has focus on combining the advantages of different type of materials in order to prepare a new catalyst. In this regard, the use of magnetic nanoparticles which they can be readily separated at the end of reaction by an external magnet is very promising.
In recent years, our research group has been reported the catalytic activity of both bulky and nano sized zinc oxide. ZnO as a cheap and environmentally friendly metal oxide shows very active catalyst for many different organic transformations [28–30]. Encouraged by these results, herein we demonstrate the preparation and well characterization of Fe3O4/ZnO supported Pd(0) nanoparticles. To the best of our knowledge, this is first report preparing nano Pd/Fe3O4/ZnO catalyst. This new catalyst shows high catalytic activity as an effective heterogeneous catalyst for the synthesis of both 2-oxazoline and benzoxazole derivatives under solvent-free condition.
Experimental
Materials and instruments
NMR spectra were recorded on a Bruker Avance DPX-250 (1H NMR 250 MHz and 13C NMR 62.9 MHz) in pure deuterated solvents with tetramethylsilane (TMS) as internal standards. Scanning electron micrographs were obtained by SEM instrumentation (SEM, XL-30 FEG SEM, Philips, at 20 kV). A transmission electron microscopy TEM was also used for TEM (Philips CM10) image. Spectroscopic methods including X-ray diffraction (XRD, D8, Advance, Bruker, axs) and FT-IR spectroscopy (Shimadzu FT-IR 8300 spectrophotometer) were employed for characterization of the heterogeneous catalyst. Surface area measurements were conducted according to the Brunauer–Emmett–Teller (BET) gas (nitrogen) adsorption method. The porous structural parameter used in this paper was taken from Barret–Joyner–Halenda (BJH) data. Metal contents were obtained by an ICP analyzer (Varian, vista-pro). Melting points determined in open capillary tubes in a Büchi-535 circulating oil melting point apparatus. The purity determination of the substrates and reaction monitoring were accomplished by TLC on silica gel PolyGram SILG/UV254 plates or by a Shimadzu gas chromatograph (GC-10A) instrument with a flame ionization detector using a column of 15 % carbowax 20 M chromosorb-w acid washed 60–80 mesh. Column chromatography was carried out on short columns of silica gel 60 (70–230 mesh) in glass columns (2–3 cm diameter) using 15–30 g of silica gel per one gram of crude mixture. Chemical materials were purchased from Fluka, Aldrich, Alfa Aesar, and Merck Companies.
Preparation of Fe3O4/ZnO nanoparticle [31]
ZnO/Fe3O4 nanoparticle was synthesized by direct-precipitation method. 9.35 mL of ferric chloride aqueous solution (0.3 M) and 9.35 mL of anhydrous sodium sulfite aqueous solution (0.1 M) were mixed together and stirred till the color of aqueous solution changed from light yellow to crimson. Then commercially ZnO (Merck 108849, 0.031 mmol, 0.24 g) were immersed in the above solution at room temperature for 5 min concomitant stirring. Then 14.02 mL ammonia solution was added into the above mixture and kept at 90 °C for 2 h with stirring. The obtained black precipitates were collected after being washed with distilled water and absolute alcohol several times and dried at 80 °C for 3 h.
Preparation of Pd/Fe3O4 nanoparticle
The nitrate solution of palladium (0.0025 g/mL) and Fe3O4/ZnO (0.01 g/mL) were sonicated for 5 min. Then two solutions were mixed under vigorous stirring at room temperature. Subsequently, HCl solution (0.1 M) was added drop-wise to the mixed solution until the final pH of the solution was about 2.5. Then the products were washed with deionized water and ethanol, two times and dried at 80 °C for 3 h.
Preparation of Pd/Fe3O4/ZnO nanoparticle
The nitrate solution of palladium (0.0025 g/mL) and Fe3O4/ZnO (0.01 g/mL) were sonicated for 5 min. Then two solutions were mixed under vigorous stirring at room temperature. Subsequently, Na2CO3 solution (0.1 M) was added drop-wise to the mixed solution until the final pH of the solution was about 6.8. The products were washed with deionized water and ethanol two times and dried at 80 °C for 3 h.
General procedure for the synthesis of benzoxazoles and 2-oxazolines 3 and 5
To a round-bottom flask, a mixture of benzonitrile (1 mmol), 2-aminophenol or aminoethanol (1 mmol), and Pd/Fe3O4/ZnO nanoparticle (0.004 g) was stirred at 120 °C in an oil bath. After the reaction was completed, the reaction mixture was diluted with EtOH (10 mL) and then the catalyst removed by an external magnet. The solvent was evaporated and the crude product was purified by column chromatography. All products were characterized by 1H NMR, 13C NMR techniques.
All compounds are known and were characterized by comparison of their physical and spectroscopic data with the authentic described in the literature [32–36].
Results and discussion
Preparation and characterization of nano Pd/Fe3O4/ZnO catalyst
The magnetic Pd/Fe3O4/ZnO nanoparticle, used for the synthesis of benzoxazoles and oxazolines, was synthesized via two steps from cheap and commercial sources. In the first step, Fe3O4/ZnO was prepared according to the literature [31]. Then Pd were immobilized on Fe3O4/ZnO nanoparticle by controlling the pH of the solution about 6.8 in aqueous media. In order to evaluate the Pd contents of the catalyst, the catalyst was treated with concentrated HCl to digest the metals and the analyzed by induced coupled plasma (ICP) and atomic absorption spectroscopy (AAS). The result revealed that the loading amount of Pd on the catalyst was found to be ~2.29 mmol/g. This catalyst was also characterized by XRD, TEM, XPS, FT-IR, and BET analysis.
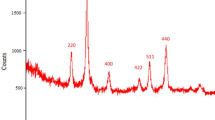
The XRD patterns of Fe3O4/ZnO, Pd/Fe3O4, and Pd/Fe3O4/ZnO nanoparticles are shown in Fig. 2. The peaks appeared at 29.9°, 35.3°, 42.8°, 53.0°, 58.3°, and 62.1° can be assigned to scattering from the (220), (311), (400), (422), (511), and (440) lattice planes of face-centered cubic (fcc) Fe3O4. Besides, the peaks at 31.8° (100), 34.3° (002), 36.0° (101), 56.7° (110), and 62.7° (103) corresponding to hexagonal wurtzite phase ZnO, respectively. As seen from Fig. 2b, the diffraction peaks at 2θ = 39.3°, and 45.9° are the characteristic signals of the face-centered cubic crystalline phase of Pd. The XRD peaks for Pd/Fe3O4/ZnO (Fig. 1) were similar to those of the pattern of Fe3O4 and ZnO. The diffraction intensity of Pd/Fe3O4/ZnO is not as strong as that of the pure samples. This can be attributed to the effect of doping. No characteristic peaks from other impurities are detected in the patterns. Due to the ultrafine grain size, the XRD diffraction peak of Pd could not be identified in the XRD pattern of Pd/Fe3O4/ZnO nanoparticle catalyst. Furthermore, using Debye–Scherrer formula: D = 0.9λ/βcosθ (where D is the average crystallite size, λ is the wavelength of Cu Kα, β is the full width at half maximum (FWHM) of the diffraction peaks, and θ is the Bragg’s angle), the average sizes of Fe3O4/ZnO and Pd/Fe3O4/ZnO are estimated to be about ~23.0 and ~25.9 nm, respectively. This is indicating that the Fe3O4 and ZnO crystalline do not change a little before and after palladium modification. Calculation the size particles of Pd by XRD was impossible because of very small amounts of Pd loaded on ZnO so the peak of Pd(0) is not strong.
The surface morphologies of Pd/Fe3O4/ZnO nanoparticle are shown in Fig. 3. It should be noted that, due to various reasons such as the same morphology and size distribution of each Fe3O4, ZnO and Pd nanoparticles, partially the same contrast of the electron beam through the Fe3O4, ZnO, and Pd nanoparticles during the TEM analysis and finally owing to the phenomenon such as relative coagulation of the synthesized nanoparticles, no significant difference was observed between the morphology and structure of Fe3O4/ZnO and Pd nanoparticles during characterization by TEM (Fig. 2b), even after enhancing the contrast by Au sputtering.
The surface structure of the Pd/Fe3O4/ZnO nanoparticles was also investigated using XPS analysis and the results are shown in Fig. 4. All of the peaks on the curve can be ascribed to Pd, Fe, Zn, O, and C elements, while C 1 s at 285.0 eV is due to hydrocarbon from the XPS instrument itself. It is clear that all the peaks are ascribed on Zn, O, Fe, Pd, and no peaks characteristic of impurities are observed (Fig. 4a). Figure 3b shows peaks at 335.4 and 340.7 eV for Pd 3d5/2 and Pd 3d3/2, respectively. In general, the binding energy (BE) of Pd 3d 2+5/2 appeared at 337.0 eV according to the BE handbook of the XPS instrument. Thus, the BE of Pd 3d5/2 appeared at 335.4 eV clearly indicates that the palladium is in the zero oxidation state. BE of Fe 2p3/2 and Fe 2p1/2 is 711.1 and 724.6 eV, respectively, which corresponds to the XPS spectrum of Fe3O4 that is shown in Fig. 3c. In Fig. 4d, the peak position of Zn 2p is shown. Because of strong spin–orbit coupling, the Zn 2p peak split into Zn 2p3/2 and Zn 2p1/2 with a doublet peak energy separation of ~23 eV. The peak positions at 1021.1 and 1043.4 eV corresponds to the Zn 2p3/2 and Zn 2p1/2, respectively, which confirm that the Zn in the catalyst mainly exists in the form of Zn2+. Finally, the peak centered at 530.5 eV is closely associated with the lattice oxygen of ZnO and Fe3O4. The peak centered at about 532.7 eV is attributed to the oxygen of surface hydroxyl group (Fig. 4c).
The FT-IR spectrum provides direct proof for the existence of related substances (Fig. 5). The strong band appearing at 587 cm−1 is characteristics of Fe–O vibrations, revealing the existence of Fe3O4. The peak at ~3351 and 1128 cm−1 is attributed to the stretching vibrations of OH, which is assigned to OH absorbed by Fe3O4 and ZnO nanoparticles. The absorption band at 452 cm−1 was assigned to Zn–O stretching vibration. The weak band near 1598 cm−1 is assigned to H–O–H bending vibration mode which was presented due to the adsorption of moisture, when FT-IR sample disks were prepared in an open air atmosphere. These observations provided the evidence for the presence of hydration in the structure.
From further analysis of the synthesized catalyst, BET surface area, pore volume, and pore size of the Pd/Fe3O4/ZnO nanoparticle sample were measured and the results are given in Table 1. The BET surface areas of the Pd/Fe3O4/ZnO nanoparticle catalyst was measured about 62.79 m2/g.
In order to evaluate the Pd contents of the catalyst, the catalyst was treated with concentrated HCl to digest the metals and the analyzed by ICP. The result revealed that the loading amount of Pd on the catalyst was found to be about 2.29 mmol/g.
The magnetic properties were also measured using vibrating sample magnetometer (VSM). Figure 6 shows the hysteresis loops of the Pd/Fe3O4/ZnO nanoparticles measured at room temperature. These magnetic properties allow a fast separation of the catalyst from the reaction media and the re-dispersion of the catalysts in solution without severe assembly and/or aggregation in successive reactions (Fig. 6b, c).
Catalytic activity of Pd/Fe3O4/ZnO nanoparticle
After successful synthesis and well characterization of Pd/Fe3O4/ZnO nanoparticle, its catalytic activity for the synthesis of oxazoline and benzoxazoles as important classes of heterocycles was examined. At first, the reaction between benzonitrlie (1a) and 2-aminophenol (2) was chosen as model compounds and parameters such as solvents and temperature were examined. Furthermore, different nano materials were tested as well (Table 2). The results demonstrated that the Pd/Fe3O4/ZnO nanoparticle (0.004 g, which contains 9.16 × 10−3 mmol Pd) at 120 °C under solvent-free conditions was optimal to convert benzonitrile and 2-amino phenol to the corresponding benzoxazole product.

Utilizing this convenient protocol, diverse benzoxazole derivatives were synthesized (Table 3). According to this Table, benzonitriles with both electron donating and withdrawing group converted to the corresponding benzoxazole in good to excellent yields. Also heterocyclic nitriles gave good results. Having established the efficacy of the Pd/Fe3O4/ZnO nanoparticle in the synthesis of benzoxazole derivatives, we next turned our attention to synthesis of another important heterocyclic compounds named 2-oxazoline. Under the same conditions, 2-oxazoline (5a) was produced in 90 % isolated yield after 4 h when the benzonitrile and 2-aminoalcohol were used as starting materials. So, we next set out to determine the scope and practicality of the method through the synthesis of small molecule of 2-oxazolines with diverse substituents. In all cases, the reaction gives the products in good to high yields and limited problems which may associate with using of solvents such as handling, cost, safety, and pollution. In all cases, electron withdrawing benzonitriles yielded better results than electron donating. Also the ortho substituted benzonitrils which have some steric effect produced the desired products in good yields. The reaction of hetero-aromatic nitriles such as 3-cyanopyrimidine and 4-cyanopyrimidine with 2-aminoethanol underwent in excellent yields. The synthesis of 2-oxazoline 3a was scaled to 15 mmol. The desired product was isolated in 87 % yield (entry 1).

To check the recyclability of the catalyst, as can be seen from Fig. 7, the reaction was performed with benzonitrile and 2-aminophenol at 120 °C for 3 h under solvent-free condition. After completion, the reaction mixture was diluted with EtOH (10 mL) and then the catalyst removed by an external magnet, washed with EtOAc and water, and finally dried at 80 °C. A new reaction was then performed with fresh reactants under the same conditions. As can be seen in Fig. 7a, Pd/Fe3O4/ZnO nanoparticle catalyst could be used more than 4 times without significant changes in activity (85–90 % yields). Also, the XRD pattern of the catalyst after fourth recovery is shown in Fig. 7b, and no changes or impurities were observed.
For practical applications of heterogeneous catalysts especially in industry, the heterogeneity is very important. To clarify this, two separate experiments were conducted with benzonitrile and 2-aminoalcohol. In the first experiment, the reaction was terminated after 2 h, the catalyst was separated from the reaction mixture by an external magnet and the reaction was continued with the filtrate for an additional 2 h. In the second experiment, the reaction was terminated after 2 h. In both cases, the desired product was obtained in the same yield (43 %). Pd was not detected in the filtrate in either experiment by ICP analyzer. These studies demonstrate that the reaction proceeds on the heterogeneous surface. In addition, no Pd metal was detected in the final products.
A proposed mechanism is shown in Scheme 1. The reaction presumably proceeds via activation of nitrile by Pd/Fe3O4/ZnO nanoparticle followed by imine formation, and the resulting imine further reacts with –OH group of aminophenol resulting in the formation of hydrobenzoxazole. Subsequently hydrobenzoxazole undergoes aromatization under aerial oxidation to give benzoxazole.
Conclusions
In this study, a novel magnetite Pd/Fe3O4/ZnO nanoparticle catalyst was synthesized by a simple and low cost process. As an example of its catalytic potential, Pd/Fe3O4/ZnO nanoparticle was evaluated and found to exhibit high catalytic activity toward the synthesis of two important classes of heterocyclic compounds (2-oxazoline and benzoxazole derivatives) in solvent-free condition in air. The present catalytic system effectively couples the advantages of heterogeneous (e.g., low cost, air-stability, easy separation by an external magnet, and good reusability) and supported a ultra small amount of Pd on a mixed metal oxides surface, making it a promising material for practical application. We are currently investigating other catalytic metals and metal combinations in an effort to demonstrate the versatility of the Fe3O4/ZnO support.
References
Evindar G, Batey RA (2006) Parallel synthesis of a library of benzoxazoles and benzothiazoles using ligand-accelerated copper-catalyzed cyclizations of ortho-halobenzanilides. J Org Chem 71:1802–1808
Wipf P (1998) In: Pelletier SW (ed) Alkaloids: chemical and biological perspectives, Pergamon: New York, p 187–228
Easmon J, Pürstinger G, Thies K-S, Heinisch G, Hofmann J (2006) Synthesis, structure–activity relationships, and antitumor studies of 2-benzoxazolyl hydrazones derived from alpha-(N)-acyl heteroaromatics. J Med Chem 49:6343–6350
Grobler JA, Dornadula G, Rice MR, Simcoe AL, Hazuda DJ, Miller MD (2007) Eco-friendly synthesis of 2-substituted benzothiazoles catalyzed by silica sulfuric acid. J Biol Chem 282:8005–8010
Leventhal L, Brandt MR, Cummons TA, Piesla MJ, Rogers KE, Harris HA (2006) An estrogen receptor-beta agonist is active in models of inflammatory and chemical-induced pain. Eur J Pharmacol 553:146–148
Sha C-K, Huang S-J, Zhan Z-P (2002) Anionic cyclization approach toward perhydrobenzofuranone: stereocontrolled synthesis of the hexahydrobenzofuran subunit of avermectin. J Org Chem 67:831–834
Witte H, Seeliger W (1974) Cyclische imidsäureester aus nitrilen und aminoalkoholen. Liebigs Ann Chem 1974:996–1009
Zhang D-X, Xiang S–K, Hu H, Tan W, Feng C, Wang B-Q, Zhao K-Q, Zhao K-Q, Hu P, Yang H (2013) An improved protocol for synthesis of N-arylamides and benzoxazoles by the copper-catalyzed reaction of aryl halides with nitriles. Tetrahedron 69:10022–10029
Lester RP, Camp JE (2013) Methods for the direct synthesis of benzoxazoles from halogenated nitriles in alcoholic solvents, ACS Sustainable. Chem Eng 1:545–548
Heng T, Xu Y-L, Pan C-x, Wang H-s, Pan Y-m (2012) Synthesis of benzoxazoles by the copper triflate catalysed reaction of nitriles and o-aminophenols. J Chem Res 3:370–373
Holljes EL, Wagner EC (1944) Some reactions of nitriles as acid anammonides. J Org Chem 9:31–49
Xiang S-K, Zhang D-X, Hu H, Shi J-L, Liao L-G, Feng C, Wang B-Q, Zhao K-Q, Hu P, Yang H, Yu W-H (2013) Synthesis of n-arylamides by copper-catalyzed amination of aryl halides with nitriles. Adv Synth Catal 355:1495–1499
Whelligan DK, Bolm C (2006) The synthesis of pseudo-geminal, pseudo-ortho and ortho phosphinyl-oxazolinyl-[2.2]paracyclophanes for use as ligands in asymmetric catalysis. J Org Chem 71:4609–4618
Kangani CO, Kelley DE, Day BW (2006) One pot direct synthesis of oxazolines, benzoxazoles, and oxadiazoles from carboxylic acids using the deoxo-fluor reagent. Tetrahedron Lett 47:6497–6499
Wen X, Bakali J, Deprez-Poulain R, Deprez B (2012) Efficient propylphosphonic anhydride (®T3P) mediated synthesis of benzothiazoles, benzoxazoles and benzimidazoles. Tetrahedron Lett 53:2440–2443
Hughey JL, Knapp S, Schugar H (1980) Dehydrogenation of 2-imidazolines to imidazoles with barium manganate. Synthesis 1980:489–490
Aidouni A, Demonceau A, Delaude L (2006) Microwave-assisted synthesis of n-heterocyclic carbene precursors. Synlett 3:493–495
Katritzky AR, Cai CM, Suzuki K, Singh SK (2004) Facile syntheses of oxazolines and thiazolines with N-acylbenzotriazoles under microwave irradiation. J Org Chem 69:811–814
Bastug G, Eviolitte C, Markó IE (2012) Functionalized orthoesters as powerful building blocks for the efficient preparation of heteroaromatic bicycles. Org Lett 14:3502–3505
Suresh D, Dhakshinamoorthy A, Pitchumani K (2013) A green route for the synthesis of 2-substituted benzoxazole derivatives catalyzed by Al3+-exchanged K10 clay. Tetrahedron Lett 54:415–6419
Shelkar R, Sarode S, Nagarkar J (2013) Nano ceria catalyzed synthesis of substituted benzimidazole, benzothiazole, and benzoxazole in aqueous media. Tetrahedron Lett 54:6986–6990
Prasad AS, Bollikonda S (2012) Synthesis of aryl 2-oxazolines from aromatic nitriles and aminoalcohols using magnetically recoverable Pd/Fe3O4. Der Pharm Chem 4:93–99
Polshettiwar V, Varma RS (2009) Nanoparticle-supported and magnetically recoverable ruthenium hydroxide catalyst: efficient hydration of nitriles to amides in aqueous medium. Chem Eur J 15:1582–1586
Le Goff A, Artero V, Jousselme B, Tran PD, Guillet N, Métayé R, Fihri A, Palacin S, Fontecave M (2009) From hydrogenases to noble metal–free catalytic nanomaterials for h2 production and uptake. Science 326:1384–1387
Berseth PA, Harter AG, Zidan R, Blomqvist A, Araujo CM, Scheicher RH, Ahuja R, Jena P (2009) Carbon nanomaterials as catalysts for hydrogen uptake and release in NaAlH4. Nano Lett 9:1501–1505
Nadagouda MN, Polshettiwar V, Varma RS (2010) Self-assembly of palladium nanoparticles: synthesis of nanobelts, nanoplates and nanotrees using vitamin B1, and their application in carbon–carbon coupling reactions. J Mater Chem 19:2026–2031
Polshettiwar V, Varma RS (2010) Green chemistry by nano-catalysis. Green Chem 12:743–754
Hosseini-Sarvari M (2013) Catalytic organic reactions on ZnO. Curr Org Synth 10(5):697–723
Hosseini-Sarvari M, Roosta A (2014) Synthesis of 2-amino-4H-chromen-4-yl phosphonats via C–P bond formation catalyzed by nano-rods ZnO under solvent-free condition. Comb Chem High T Screen 1:47–52
Hosseini-Sarvari M, Moeini F (2014) Nano copper(I) oxide/zinc oxide catalyzed N-arylation of nitrogen-containing heterocycles with aryl halides and arylboronic acids in air. RSC Adv 4:7321–7329
Cao J, Fu W, Yang H, Yu Q, Zhang Y, Wang S, Zhao H, Sui Y, Zhou X, Zhao W, Leng Y, Zhao H, Chen H, Qi X (2010) Fabrication, characterization and application in electromagnetic wave absorption of flower-like ZnO/Fe3O4 nanocomposites. Mat Sci Eng B 175:56–59
Ge H, Liu P, Li X, Sun W, Li J, Yang B, Shi Z (2013) S–Co(II) cascade catalysis: cyclocondensation of aromatic nitriles with alkamine. Tetrahedron 69:6591–6597
Teimouria A, Najafi Chermahinib A, Salavatia H, Ghorbanianc L (2013) An efficient and one-pot synthesis of benzimidazoles, benzoxazoles, benzothiazoles and quinoxalines catalyzed via nano-solid acid catalysts. J Mol Catal A Chem 373:38–45
Moghadam M, Mirkhani V, Tangestaninejad S, Mohammadpoor-Baltork I, Kargar H (2009) InCl3 as an efficient catalyst for synthesis of oxazolines under thermal, ultrasonic and microwave irradiations. J Iran Chem Soc 2:251–258
Xu Q, Li Z (2009) A facile synthesis of 2-oxazolines using a PPh3-DDQ system. Tetrahedron Lett 50:6838–6840
Boissarie PJ, Hamilton ZE, Lang S, Murphy JA, Suckling CJ (2011) A powerful palladium-catalyzed multicomponent process for the preparation of oxazolines and benzoxazoles. Org Lett 13:6256–6259
Acknowledgements
We gratefully acknowledge the support of this work by the Shiraz University. We are also grateful to Mr. H. Sajedian Fard for helpful cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini-Sarvari, M., Khanivar, A. & Moeini, F. Magnetically recoverable nano Pd/Fe3O4/ZnO catalyst: preparation, characterization, and application for the synthesis of 2-oxazolines and benzoxazoles. J Mater Sci 50, 3065–3074 (2015). https://doi.org/10.1007/s10853-015-8866-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8866-7