Abstract
A series of Sr3Gd(1−x)Sm x Na(PO4)3F (x = 0.01, 0.03, 0.05, 0.07, 0.09) phosphors were synthesized through a conventional high-temperature solid-state reaction. The excitation and emission spectra indicate that these phosphors can be efficiently excited in the range from 350 to 430 nm which matches perfectly with the near-UV light, and emit orange–red light with CIE coordinates (0.5811, 0.4181). The concentration quenching mechanism of Sm3+ ions was ascribed to the dipole–dipole interaction. The activation energy E for thermal quenching is calculated to be 0.242 eV. And the lifetime of the Sr3Gd(1−x)Sm x Na(PO4)3F (x = 0.03, 0.05, 0.07, 0.09) phosphor was determined to be 0.8669, 0.8643, 0.8525, 0.8232 ms, respectively. These orange–red-emitting phosphors might be promising for use in n-UV white LEDs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The past decade has seen a rapid evolution of solid-state lighting sources. White light-emitting diodes (w-LEDs) have many merits such as high efficiency, long lifetime, significant power-saving capability, and environmental friendliness compared with both traditional incandescent and currently implemented fluorescent lamps [1]. Therefore, White light-emitting diodes (w-LEDs) have become another trend for general illumination and have attracted much attention [2, 3]. The most common fabrication technique is by combing an InGaN-based blue diode and yellow phosphor materials [4]. At present, the commercial yellow phosphor can be generated using a blue InGaN LED chip in combination with a yellow phosphor of cerium (III)-doped yttrium aluminum garnet (YAG:Ce3+) [5]. Unfortunately, it exhibits some deficiencies such as high correlated color temperature (CCT > 4500 K) and poor color rendering index (CRI < 80) due to the absence of the red emission [6, 7]. Therefore, to explore high-quality red phosphors with high efficiency, high rendering index, and low CCT is one of the main direction of research and development of practical white LED. An approach to work out this problem is combing a near-ultraviolet (n-UV) emitting LED with a tricolor (blue, green, and red) emitting phosphors when making LEDs, which exhibits excellent color rendering index and an adjustable correlated color temperature [8]. In recent years, the researches of blue and green phosphors which apply to w-LED have been relatively mature [9]. Accordingly, it is an urgent task to explore a high-efficient red-emitting phosphor which can be efficiently excited by the n-UV LED (350–420 nm) chips.
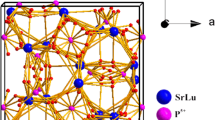
It is well known that Sm3+ ion is an important emitting activator because of the stronger absorption of n-UV region [10, 11]. It exhibits high quantum efficiency and produces reddish-orange light emitted due to its 4f-f transitions which has attracted extensive research [12, 13]. Phosphors based on fluorophosphates have many excellent properties, for instance strong visible luminescence, low sintering temperature synthesis, and high chemical stability which have attracted great attention in recent years [14, 15]. And it has a general formula, that is R10[PO4]6Z2 and the structure, isotypic with Ca10(PO4)6F2 which has been determined by Naray-Szabo [16]. Recently, You et al. reported the luminescence properties of Sr3GdNa(PO4)3F: Eu2+, Tb3+ phosphors and in this paper, the structure of Sr3GdNa(PO4)3F was determined [17]. According to this, we synthesized a series of Sr3GdNa(PO4)3F: Sm3+ phosphors to act as a novel red-emitting phosphor and the luminescence properties were investigated in detail.
Experimental
A series of samples of Sr3Gd(1−x)Sm x Na(PO4)3F (SGNP: xSm3+, x = 0.01, 0.03, 0.05, 0.07, 0.09) were synthesized through a conventional high-temperature solid-state reaction. The raw materials, SrCO3 (AR), NaF (AR), Gd2O3 (AR), Sm2O (99.9 %) (NH4)2HPO4 (AR) were weighed stoichiometrically with 15 % excess of NaF and ground evenly in agate mortar. Then the ground powder was firstly sintered at 600 °C for 3 h in crucibles, and then at 1000 °C for 3 h under a reductive atmosphere. After calcining, finally the sample was ground thoroughly into powder for subsequent measurement after being cooled down to room temperature in the furnace.
The phase structure of the samples were performed by X-ray powder diffraction (XRD) in a Bruker-D2 Phase (Cu Kα radiation, 30 kV, 20 mA), and the recorded range of 2θ is from 10° to 80° with a scan rate of 0.08°/s. The photoluminescence (PL) spectra and emission (PL) were measured using a Hitachi F-7000 fluorescence spectrophotometer with a 150 W xenon lamp used as the excitation source. The temperature-dependent photoluminescence properties spectra and luminescent decay curves were recorded on FluoroLog-3 spectrofluorometer (HORIBA, USA), which were combined with the heating apparatus (TAP-02) and a R928P photomultiplier for signal detection, and the Nano LED (N-370) acts as the excitation source when luminescent decay curves were measured.
Results and discussion
Figure 1 shows the X-ray diffraction (XRD) patterns of the prepared phosphors together with the JCPDS card no. 50-1595. According to the recent report by You et al. [17], the standard data of Sr3(La,Ce)Na(PO4)3(F,OH) (JCPDS 50-1595) can be indexed to the X-ray diffraction (XRD) patterns of the prepared phosphors because of all the diffraction peaks of the powder agreeing well with the standard JCPDS card no. 50-1595 and no impurity phase is presented. Therefore, the pure samples were carried out and a few of Sm3+ ions were doped into matrix, which do not cause any significant change on the phase formation.
Figure 2 depicts the photoluminescence performances of Sr3Gd(1−x)Sm x Na(PO4)3F (x = 0.05) phosphor at room temperature. Several excitation peaks can be observed when monitoring at 598 nm which are located at 345 nm (6H5/2 → 4H9/2), 362 nm (6H5/2 → 4D3/2), 374 nm (6H5/2 → 6P7/2), 403 nm (6H5/2 → 4F7/2), 417 nm (6H5/2 → 6P5/2), 442 nm (6H5/2 → 4G5/2) from the perspective and they are attributed to the f → f forbidden transitions of Sm3+ [12, 18]. Among them, the highest intensity of transition was centered at 403 nm evidently. And as shown in Fig. 3, we can see the distinct absorption band being centered around 403 nm in the region from 350 to 410 nm of the diffuse reflectance spectrum of Sr3GdNa(PO4)3F and Sr3Gd(1−x)Sm x Na(PO4)3F (x = 0.01, 0.03, 0.05, 0.07, 0.09). These findings indicate that the prepared phosphor can be effectively excited by near-UV LED chips (350–420 nm), which is significant for the fabrication of near-UV chips in w-LEDs. There are three emission peaks in the emission spectrum located around 563, 598, and 646 nm, which correspond with 4G5/2 → 6H J (J = 5/2, 7/2, and 9/2) transitions when excited at 403 nm. And to the best of our knowledge, the 4G5/2 → 6H5/2 (563 nm) mainly obeys the magnetic-dipole transition (MD), the 4G5/2 → 6H7/2 (598 nm) is a partly magnetic dipole and partly electric-dipole transition (ED), the 4G5/2 → 6H9/2 (646 nm) originates from purely ED transition [12, 19]. It is believed that the intensity of 4G5/2 → 6H7/2 (598 nm) is the highest of three transitions. Furthermore, the CIE 1931 chromaticity of Sr3Gd0.95Sm0.05Na(PO4)3F phosphor under 403 nm excitation is calculated to be (0.5811, 0.4181), as shown in Fig. 4. All of these results indicate that the prepared phosphor can be applied to orange–red-emitting phosphor.
Figure 5 shows the emission intensities of Sr3Gd(1−x)Sm x Na(PO4)3F at different doping concentrations of Sm3+ ions (x = 0.01, 0.03, 0.05, 0.07, 0.09) at room temperature (λ ex = 403 nm). It can be seen that the emission intensity of Sr3Gd(1−x)Sm x Na(PO4)3F increases initially with the rising concentration of Sm3+ ions then decreases and achieves the maximum when x = 0.05 due to the occurrence of the concentration quenching, as shown in the inset.. Consequently, the optimum concentration of Sm3+ is determined to be about 0.05. The behavior of concentration quenching is mainly generated by the process of nonradiative energy migration among the Sm3+ ions at the high concentration. And the concentration quenching mechanism is ascribed to two main aspects: one is multipolar interaction and the other is exchange interaction [20]. The critical transfer distance between donor and acceptor should be shorter than 0.5 nm if the energy transfer due to the exchange interaction. Accordingly, in order to confirm the concentration quenching mechanism of phosphor, the calculation of the critical distance (R c) is essential. According to Blasse’s theory, the critical distance (R c) between the Sm3+ ions can be calculated using the following equation [21]
where V is the volume of the unit cell, N represents the number of Sm3+ ions in the Sr3GdNa(PO4)3F unit cell, x c is the critical concentration. For Sr3GdNa(PO4)3F host, N = 2, V = 56.4795 nm3, x c = 0.05, therefore, the value of the critical transfer distance R c for Sm3+ is calculated to be 5.1287 nm. Obviously, the value calculated is much larger than 0.5 nm. Therefore, the concentration quenching among the Sm3+ ions in the phosphor is ascribed to the multipolar interaction. On the basis of Dexter’s theory, the energy transfer expressions of multipolar interaction can be divided into dipole–dipole, dipole–quadrupole, and quadrupole–quadrupole interactions. Furthermore, according to Van Uiter’s report, the emission intensity (I) per activator ion follows the equation [22]:
where x is the activator concentration, I/x is the emission intensity per activator concentration, k and β are constants for a given host crystal in the same excitation condition, θ = 6, 8, and 10 represent dipole–dipole, dipole–quadrupole, and quadrupole–quadrupole interactions, respectively. The Eq. (3) can be obtained by means of taking the Eq. (2) to the form of logarithm.
In order to acquire the value of θ, choosing x = 0.05, 0.07, 0.09 and its corresponding emission intensity, respectively. The dependence of lg (I/x) on lg x plotted is shown in Fig. 6. Therefore, the value of θ was calculated to be 4.08, which is approximate to 6, namely, the pattern of energy transfer is dipole–dipole. For more clarity, the concentration quenching of Sm3+ions was ascribed to the dipole–dipole interaction.
Figure 7 shows the emission spectra of Sr3Gd0.95Sm0.05Na(PO4)3F phosphor for various temperatures excited at 403 nm; the inset shows temperature-dependent relative emission intensity of Sr3Gd0.95Sm0.05Na(PO4)3F phosphor. The integrated emission intensity of Sr3Gd0.95Sm0.05Na(PO4)3F phosphor sustainably decreases with increasing temperature between from 25 to 250 °C with excitation at 403 nm, which is partly due to the thermal quenching in the configurational coordinate diagram [5]. Firstly, the luminescence center of excited state is thermally activated by the means of phonon interaction and then releases through the crossing point between the excited state and the ground state in the configurational coordinate diagram, finally bringing about the luminescence quenching [7]. The clarification of thermal quenching behavior and the value of activation energy can be achieved through the Arrhenius equation [23] :
where I 0 is the first luminescence intensity and I(T) is the luminescence intensity of Sm3+ in the presence of temperature, k is Boltzmann,s constant (8.617 × 10−5 eV/K), and c is a constant, E is the activation energy for thermal quenching. The value of E can be estimated through the Eq. (5) which is rearranged by the Eq. (4) as follows:
Figure 8 shows the plots of ln [(I 0 /I)−1] versus 1/kT for Sr3Gd0.95Sm0.05Na(PO4)3F phosphor. Accordingly, the activation energy E for thermal quenching is calculated to be 0.242 eV. Thus the thermal quenching of prepared phosphors was small, which was required when used in w-LEDs. Additionally, the CIE chromaticity coordinate (x, y) of Sr3Gd0.95Sm0.05Na(PO4)3F phosphor at different temperatures respectively are given in Table 1. It is clear that the CIE chromaticity coordinate has a slighter motion with the increscent of temperature so as to keep good color quality. Therefore, the prepared phosphor has a good thermal stability and chemical stability which is significant when it is applied to in w-LEDs.
The PL decay curves of Sr3Gd(1−x)Sm x Na(PO4)3F at different doping concentrations of Sm3+ ions (x = 0.03, 0.05, 0.07, 0.09) were measured so as to investigate the luminescence dynamics. Figure 8 shows the decay curve of Sr3Gd(1−x)Sm x Na(PO4)3F (x = 0.03, 0.05, 0.07, 0.09) phosphor. And the lifetime can be defined as follows:
where I is the luminescence intensity at a time t, τ 1 and τ 2 are exponential components of the decay time, A 1 and A 2 are constants. On the basis of Eq. (7) which is rearranged by the Eq. (6), the lifetime of the Sr3Gd(1−x)Sm x Na(PO4)3F (x = 0.03, 0.05, 0.07, 0.09) phosphor were determined to be 0.8669, 0.8643, 0.8525, 0.8232 ms, respectively.
It is clear that the lifetime decreases with increased concentration. According to a spin selection rule, the activator Sm3+ ions have a long decay time induced by a forbidden transition of Sm3+ which has a very low probability and decay time in the level of ms [6]. Obviously, the lifetime of prepared phosphors are short enough for potential applications in w-LED Fig. 9.
Conclusions
In summary, samples of Sr3Gd(1−x)Sm x Na(PO4)3F (SGNP: xSm3+, x = 0.01, 0.03, 0.05, 0.07, 0.09) were synthesized through a conventional high-temperature solid-state reaction. The photoluminescence properties, temperature-dependent luminescence, and decay times were investigated in detail. The obtained phosphors have a distinct excitation band centered at 403 nm ranging from 350 to 430 nm which can match perfectly with the n-UV LED chips. The CIE 1931 chromaticity of the prepared phosphor under 403 nm excitation was calculated to be (0.5811, 0.4181). And phosphors can emit intense orange–red light with an optimal concentration of 0.05 under the excitation of 403 nm. The concentration quenching is ascribed to dipole–dipole interaction. The prepared phosphor has a good thermal stability and chemical stability. All of these results indicate that the prepared phosphor can be applied to orange–red-emitting n-UV white LEDs.
References
Schubert EF, Kim JK (2005) Solid-state light sources getting smart. Science 308:1274–1278
Nakamura SJ, Senoh M, Mukai T (1993) High-power InGaN/GaN double-hetero strructure violet light-emitting-diodes. Appl Phys Lett 62:2390–2392
Zhang ZY, Wang JG, Yin YL, Zhou JJ, Zhou SP, Pan YX (2013) A novel green-to-yellow emitting phosphor: BaSi2SN2.67:Eu2+ for potential application in UV-LEDs. Opt Mater 35:1273–1275
Xia ZG, Ji JH, Huang ZhH, Atuchin VV (2014) New yellow-emitting whitlockite-type structure Sr1.75Ca1.25(PO4)2: Eu2+ phosphor for near-UV pumped white light-emitting devices. Inorg Chem 53:5129–5135
Sun JY, Cui DP (2014) Synthesis, structure, and thermally stable luminescence of Dy3+-doped Na3YSi2O7 host compound. J Am Ceram Soc 97:843–847
Sun JY, Zhang XY, Xia ZG, Du HY (2012) Luminescent properties of LiBaPO4: RE (RE = Eu2+, Tb3+, Sm3+) phosphors for white light-emitting diodes. J Appl Phys 111:013101–013103
Sun JY, Han L, Xu QG, Di QM (2014) Synthesis and luminescence properties of Na3YSi2O7: Sm3+ phosphor. Ceram Int 40:14261–14265
Shang MM, Li GG, Geng DL, Yang DM, Kang XJ, Zhang Y, Lian HZ, Lin J (2012) Blue emitting Ca8La2(PO4)6O2: Ce3+/Eu2+ phosphors with high color purity and brightness for white LED: soft-chemical synthesis, luminescence, and energy transfer properties. J Phys Chem C 116:10222–10231
Sun JY, Zeng JH, Sun YN, Du HY (2013) Tunable luminescence of Ce3+/Mn2+-coactivated Sr3Gd(PO4)3 with efficient energy transfer for white-light-emitting diode. J Lumin 138:72–76
Sun JY, Zeng JH, Sun YN, Du HY (2013) Synthesis and luminescence properties of novel Y2Si4N6C: Sm3+ carbonitride phosphor. Ceram Int 39:1097–1102
Lei BF, Li B, Zhang HR, Li WL (2007) Preparation and luminescence properties of CaSnO3: Sm3+ phosphor emitting in the reddish orange region. Opt Mater 29:1491–1494
Yu RJ, Jeong JH, Jang K (2014) Photoluminescence characteristics of Sm3+ doped Ba3La(PO4)3 as new orange-red emitting phosphors. J Lumin 145:717–722
Dabre KV, Park K, Dhoble SJ (2014) Synthesis and photoluminescence properties of microcrystalline Sr2ZnWO6: RE3+ (RE = Eu, Dy, Sm and Pr) phosphors. J Alloy Compd 617:129–134
Jiao MM, Guo N, Lü W, Jia YC, Lv WZ, Zhao Q, Shao BQ, You HP (2013) Tunable blue-green-emitting Ba3LaNa(PO4)3F: Eu2+, Tb3+ phosphor with energy transfer for near-UV white LEDs. Inorg Chem 52:10340–10346
Mi RY, Zhao CL, Xia ZG (2014) Synthesis, structure, and tunable luminescence properties of novel Ba3NaLa(PO4)3F: Eu2+, Mn2+ phosphors. J Am Ceram Soc 6:1802–1808
Mayer I, Roth R, Brown W (1974) Rare earth substituted fluoride-phosphate apatites. J Solid State Chem 11:33–37
Jiao MM, Jia YC, Lu W, Lv W, Zhao Q, Shao BQ, You HP (2014) Sr3GdNa(PO4)3F: Eu2+, Mn2+: a potential color tunable phosphor for white LEDs. J Mater Chem C 2:90–97
Wu MQ, Liu XL, Gu M, Ni C, Liu B, Huang SM (2014) Characterization and luminescence properties of sol-gel derived M’-type LuTaO4: Ln3+ (Ln = Pr, Sm, Dy) phosphors. Mater Res Bull 60:652–658
Raut SK, Dhoble NS, Park K, Dhoble SJ (2014) Precipitation based synthesis and luminescence of Ln3+ (Eu, Ce, Dy, Sm, Tb) activated BaCa2Si3O9-Walstromite cyclosilicate phosphors. Mater Chem Phys 147:594–603
Xia ZG, Liu RS (2012) Tunable blue-green color emission and energy transfer of Ca2Al3O6F: Ce3+, Tb3+ phosphors for near-UV white LEDs. J Phys Chem C 116:15604–15609
Xia ZG, Zhuang JQ, Liao LB, Liu HK, Luo Y, Du P (2011) Synthesis and luminescence properties of Ba2Gd(BO3)2Cl: Eu2+ phosphor. J Electrochem Soc 158:J359–J362
Zhou J, Xia ZG, Yang MX, Shen K (2012) High efficiency blue emitting phosphor: Ce3+-doped Ca5.45Li3.55(SiO4)3O0.45F1.55 for near UV pumped light-emitting diodes. J Mater Chem 22:21935–21941
Li GG, Geng DL, Shang MM, Zhang Y, Peng C, Cheng ZY, Lin J (2011) Color tuning luminescence of Ce3+/Mn2+/Tb3+-triactivated Mg2Y8(SiO4)6O2 via energy transfer: potential single-phase white-light-emitting phosphors. J Phys Chem C 115:21882–21892
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 20976002), the Beijing Natural Science Foundation (No. 2122012), Key Projects for Science and Technology of Beijing Education Commission (KZ201310011013), Projects of Transformation and Industrialization of College Scientific & Technological Achievements, and Projects of the combination of Manufacture. Graduate Student Scientific Research and Academic Innovation Project Funding of Beijing Technology and Business University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, L., Xu, D., Xu, Q. et al. Synthesis and luminescence properties of Sr3GdNa(PO4)3F: Sm3+ phosphor. J Mater Sci 50, 2257–2262 (2015). https://doi.org/10.1007/s10853-014-8788-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8788-9