Abstract
The development of a convenient method for oil-removal is of great significance for environmental protection. Here, we present a simple method for the removal of oils from water surface based on sponges that we fabricated by solution-immersion processes. The sponges exhibited high selectivity and absorption capacities for various kinds of oils when they were employed as absorptive materials. More importantly, the superhydrophobic sponge could be sustained 400 cycles of compressing test without losing their superhydrophobicity, exhibiting the high elasticity, robustness, and durability. To extend application field, superhydrophobic filter paper was used for oil–water separation. Interestingly, tunable wettability was received when oleophobic silica was employed instead of hydrophobic silica. We expected that this low-cost process can be used for oil-spill cleanup.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superhydrophobic surfaces with a contact angle (CA) greater than 150° attracted tremendous attention for researchers because of its greater importance in fundamental research [1, 2]. With increasing industrial development, frequent oil spills and the industrial discharge of organic solvents have resulted in serious environmental and ecological problems [3–7]. To address this challenge, a large number of absorbents including active carbon [8], zeolites [9], and natural fibers [10] have been used, but they suffer from poor selectivity and low absorption. Recently, surfaces with superhydrophobicity and superoleophilicity have attracted increasing interest in the field of oil–water separation because of their high oil–water separation efficiency and selectivity [11–13]. Lots of works were developed, such as active carbon [14], carbon composites [15–20], and graphene-based sponge [21, 22]. Nevertheless, the above-mentioned materials have limitations for their high-cost or time-consuming processes.
Polyurethane sponge is a kind of commercially available 3D porous material with high absorption ability and good flexibility. Polyurethane sponges have in this respect attracted much interest. However, polyurethane sponges are intrinsically superhydrophilic and superoleophilic, which make them impractical for selective removal of oils from water. Therefore, it is essential to design the wettability of polyurethane sponge. Jiang et al. developed a self-assembly technique to fabricate graphene-based polyurethane foam with a macroscopically ordered 3D structure [23]. Pan et al. reported the facile removal of oils from water surfaces via superhydrophobic polyurethane sponges [24]. Calcagnile et al. presented a novel composite based on commercially available polyurethane foams, which can separate oil from water [25]. However, the practical applications are limited by sophisticated procedures and expensive materials.
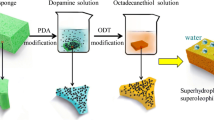
Here, we developed dip-coating method to fabricate superhydrophobic and superoleophilic monolithic material based on polyurethane sponge. Hydrophobic silica nanoparticles and polyfluorowax (PFW) were controllably anchored on the polyurethane sponge skeletons to regulate the characteristic from superhydrophilic to superhydrophobic. PFW was used to cure weak adhesion between superhydrophobic coatings and sponge. To extend application field, the coatings can also formed on the filter paper. Interestingly, oleophobic sponge was received when oleophobic silica was employed instead of hydrophobic silica.
Experimental section
Material
The PU sponge was purchased from a local store and used as received. PFW was provided by Micro Power Inc., USA. Silicon dioxide nanopowder (10–20 nm particle size), Trichloro(1H,1H,2H,2H-perfluorooctyl)silane were obtained from Aldrich and used as received. Chlorotrimethylsilane was provided by shanghai kefeng Co., Ltd.
Sample preparation
Preparation of hydrophobic and oleophobic silica nanoparticles
1.73 g Chlorotrimethylsilane added dropwise into a mixture of ethanol (65 ml) and silica (2 g) under magnetic stirring. Then reflux at 80 °C for 3 h. The product was collected by centrifugation, and dried at 60 °C. Oleophobic silica was synthesized by previous reports [26].
Preparation of monolithic material
In a typical synthesis of the monolithic material, 0.1 g PFW and 0.08 g hydrophobic/oleophobic silica were ultrasonically dispersed in 20 ml ethanol. Then pieces of commercially available polyurethane sponge were placed into the above solution immediately. After 5 min, the sponge was taken out and dried at 130 °C for 0.5 h. The superhydrophobic filter paper was fabricated through a simple process of filtration. The resulting hydrophobic silica (0.02 g) and PFW (0.02 g) were ultrasonically dispersed in 30 ml ethanol, and the suspension was poured onto the filter paper in the Büchner funnel. White layer was formed on the filter paper and dried at 130 °C for 0.5 h.
Characterization
CA was measured with 10 μl droplets of water using a Krüss DSA 100 (Krüss Company, Ltd., Germany) apparatus at ambient temperature. The morphology of the surface was observed by field-emission scanning electron microscope JEOL JSM-6701F FESEM. X-ray photoelectron spectra (XPS) were obtained on an ESCALAB250xi spectrometer equipped with a focused monochromatic Al X-ray source (1489.6 eV). The compressive tests of the superhydrophobic polyurethane sponges were performed using a XS (08) XD-3 testing machine, equipped with two flat-surface compression stages. The optical images were captured by a digital camera (Canon).
Results and discussion
Figure 1a and b shows the images of superhydrophobic polyurethane sponge (HPS), oleophobic polyurethane sponge (OPS), and boat-like superhydrophobic filter paper before and after put into the mixture of water and hexadecane (colored by oil red O). As shown in Fig. 1b, the HPS and boat-like filter paper sank below the oil–water interface, but the OPS floated on hexadecane surface. Liquids with different surface tension on OPS surface were shown in Figure S1a, and CA was shown on Figure S1b. All droplets attained in spherical when placed on the surface of OPS. The bright, reflective surface visible underneath the droplets is a signature of trapped air and the establishment of a composite solid–liquid–air interface [27].
In order to understand the wetting behavior of sponges, the surface morphology and chemical composition were considered, which are two main factors governing the surface wettability. Surface morphology of HPS is not flat and large numbers of particles were inserted on it, which can trap a large fraction of air and constitute the hierarchical roughness required for a superhydrophobic surface (Fig. 2a and b). XPS analysis demonstrated that the elements of surface are carbon, oxygen, fluorine, and silicon, and the content of fluorine element is up to 34.68 % (Fig. 2c). This low surface energy, when combined with the rough textures, results in the sponge with superhydrophobicity. As for OPS, similar surface morphology was obtained (Fig. 2d and e). However, the content of fluorine element is 75.03 % (Fig. 2f). Thus, the obtained sponge displayed oleophobicity.
As expected, the HPS separated oils from the water surface easily. By dipping the sponge into a mixture of water and oil (xylene dyed with oil red O), the oil was quickly absorbed by the sponge in a few seconds. More interestingly, the absorbed oil in the sponge was readily collected by a simple squeezing process (Fig. 3). Similar to HPS, the oil–water separation process was achieved using superhydrophobic filter paper (Figure S2).
To further demonstrate the oil-absorption ability of HPS, we investigated its sorption capacities for different oils and organic solvents. The sorption efficiency was determined by weighting the sample before and after oil absorption. The sorption capacities range from 22 to 75 times the weight of the HPS for a variety of oils and organic solvents (Fig. 4a). Liquids being test were mainly stored in the macrospores of the HPS, so the differences of sorption capacities were related to the densities of liquids. The sorption capacities were superior to traditional products. The sorption capacities are superior to those of activated carbon (<1 times), marshmallow-like macro porous gels (6–14 times), and graphene/α-FeOOH aerogel (13–27 times). Recyclability of HPS of organic solvents is key requirements in practical oil cleanup applications. We evaluated the absorption capacity of HPS for the three kinds of organics and two oils by a simple squeezing process. As shown in Fig. 4b, no obvious change in saturated absorption capacity was found when the sponges absorbed and desorbed organic solvents for cycles. For pump oil and rap oil, the saturated absorption capacity decrease to 7.25 and 13.44, respectively. Such a decrease is due to the residual oil inside the sponge, which cannot be removed by mechanical squeezing process.
The mechanical property of HPS was evaluated by stress–strain measurement (Fig. 5). The stress–strain curve exhibits a plateau followed by a gradually increasing slope until a strain of about 75 %. The sponge can be compressed to more than 75 % volume reduction at low stress values (4–24 kPa), due to their high porosity and structural flexibility. The curves demonstrate good recovery of elasticity in the process of repeated compression. Inset of Fig. 5 shows the CA and photographs of the HPS before and after mechanical compression. A slight decrease in the water CA is found after 400 cycles of compressing test, indicating excellent durability of the sponge. We believe that this kind of sponge is a promising candidate for large-scale removal of organic contaminants from water.
Conclusion
We demonstrated a facile approach to fabricate robust superhydrophobic polyurethane sponges through solution-immersion process. PFW here was used to increase adhesion between superhydrophobic coatings and sponges. It was found that the superhydrophobicity and elasticity of the resulting sponge are retained after 400 cycles of compression test. The as-prepared sponge could be applied for the removal of oil spills from water surface with high oil-absorption capacity. Interestingly, superhydrophobic filter paper and oleophobic sponge were also fabricated using the same procedure. We expect this method can be adopted for the fabrication of monolithic materials with multifunction integration.
References
Su FH, Yao K (2014) Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl Mater Interfaces 6:8762–8770
Liu CS, Su FH, Liang JZ (2014) Facile fabrication of a robust and corrosion resistant superhydrophobic aluminum alloy surface by a novel method. RSC Adv 4:55556–55564
Kwon G, Kota AK, Li YX, Sohani A, Mabry JM, Tuteja A (2012) On demand separation of oil-water mixtures. Adv Mater 24:3666–3671
Feng L, Zhang ZY, Mai ZH, Ma YM, Liu BQ, Jiang L, Zhu DB (2004) A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew Chem 116:2046–2048
Nguyen DD, Tai NH, Lee SB, Kuo WS (2012) Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ Sci 5:7908–7912
Cong HP, Ren XC, Wang P, Yu SH (2012) Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 6:2693–2703
Dong XC, Chen J, Ma YW, Wang J, Chan-Park MB, Liu XM, Wang LH, Huang W, Chen P (2012) Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water. Chem Commun 48:10660–10662
Fuertes AB, Marban G, Nevskaia DM (2003) Adsorption of volatile organic compounds by means of activated carbon fibre-based monoliths. Carbon 41:87–96
Adebajo MO, Frost RL, Kloprogge JT, Carmody O, Kokot S (2003) Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J Porous Mater 10:159–170
Deschamps G, Caruel H, Borredon ME, Bonnin C, Vignoles C (2003) Oil removal from water by selective sorption on hydrophobic cotton fibers. 1. Study of sorption properties and comparison with other cotton fiber-based sorbents. Environ Sci Technol 37:1013–1015
Yuan JK, Liu XG, Akbulut O, Hu JQ, Suib SL, Kong J, Stellacci F (2008) Superwetting nanowire membranes for selective absorption. Nat Nanotechnol 3:332–336
Chu Y, Pan QM (2012) Three-dimensionally macroporous Fe/C nanocomposites as highly selective oil-absorption materials. Acs Appl Mater Interfaces 4:2420–2425
Jiang GH, Hu RB, Xi XG, Wang XH, Wang RJ (2013) Facile preparation of superhydrophobic and superoleophilic sponge for fast removal of oils from water surface. J Mater Res 28:651–656
Sun H, Li A, Zhu Z, Liang W, Zhao X, La P, Deng W (2013) Superhydrophobic activated carbon-coated sponges for separation and absorption. ChemSusChem 6:1057–1062
Gui XC, Wei JQ, Wang KL, Cao AY, Zhu HW, Jia Y, Shu QK, Wu DH (2010) Carbon nanotube sponges. Adv Mater 22:617–621
Hu H, Zhao ZB, Wan WB, Gogotsi Y, Qiu JS (2013) Ultralight and highly compressible graphene aerogels. Adv Mater 25:2219–2223
Xiao N, Ling Z, Zhou Y, Qiu JS (2013) Synthesis and structure of carbon belts made of carbon nanofibers supported on carbon foams. Carbon 61:386–394
Xiao N, Zhou Y, Ling Z, Qiu JS (2013) Synthesis of a carbon nanofiber/carbon foam composite from coal liquefaction residue for the separation of oil and water. Carbon 59:530–536
Hu H, Zhao ZB, Wan WB, Gogotsi Y, Qiu JS (2014) Polymer/graphene hybrid aerogel with high compressibility, conductivity, and “sticky” superhydrophobicity. ACS Appl Mater Interfaces 6:3242–3249
Tao SY, Wang YC, Shi D, An YL, Qiu JS, Zhao YS, Cao Y, Zhang XF (2014) Facile synthesis of highly graphitized porous carbon monoliths with a balance on crystallization and pore-structure. J Mater Chem A 2:12785–12791
Hu H, Zhao ZB, Gogotsi Y, Qiu JS (2014) Compressible carbon nanotube-graphene hybrid aerogels with superhydrophobicity and superoleophilicity for oil sorption. Environ Sci Technol Lett 1:214–220
Li R, Chen CB, Li J, Xu LM, Xiao GY, Yan DY (2014) A facile approach to superhydrophobic and superoleophilic graphene/polymer aerogels. J Mater Chem A 2:3057–3064
Wu C, Huang XY, Wu XF, Qian R, Jiang PK (2013) Mechanically flexible and multifunctional polymer-based graphene foams for elastic conductors and oil-water separators. Adv Mater 25:5658–5662
Zhu Q, Pan QM, Liu FT (2011) Facile removal and collection of oils from water surfaces through superhydrophobic and superoleophilic sponges. J Phys Chem C 115:17464–17470
Calcagnile P, Fragouli D, Bayer IS, Anyfantis GC, Martiradonna L, Cozzoli PD, Cingolani R, Athanassiou A (2012) Magnetically driven floating foams for the removal of oil contaminants from water. ACS Nano 6:5413–5419
Ge B, Zhang ZZ, Men XH, Zhu XT, Zhou XY (2014) Sprayed superamphiphobic coatings on copper substrate with enhanced corrosive resistance. Appl surface sci 293:271–274
Cassie ABD, Baxter S (1944) Wettability of porous surfaces. Trans Faraday Soc 40:546–551
Acknowledgements
The authors acknowledge the financial support of the National Nature Science Foundation of China (Grant Nos. 51335010).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ge, B., Men, X., Zhu, X. et al. A superhydrophobic monolithic material with tunable wettability for oil and water separation. J Mater Sci 50, 2365–2369 (2015). https://doi.org/10.1007/s10853-014-8756-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8756-4