Abstract
Utilizing a high-dilution condition method, a new aza-oxo macrocycle, referred to as L, was synthesized as a chemosensor. The chemosensing capabilities of L were thoroughly investigated using fluorescence studies. The obtained results demonstrate that L effectively responds to the presence of zinc ions, leading to a significant increase in fluorescence intensity. Comparative studies were conducted to investigate the impact of various metal cations, including Cr(III), Mn(II), Fe(II), Fe(III), Co(II), Ni(II), Cu(I), Cu(II), Zn(II), Cd(II), Gd(III), Na(I), K(I), Ba(II), Mg(II), Al(III), Pb(II), Sn(II), Hg(II), and Ag(I), on the fluorescence of L in an aqueous ethanol environment. During our investigations, a binding constant value of 7.81 × 105 M−1, with a 1:1 stoichiometry for Zn2+–L interactions, was established. Additionally, a low detection limit of 2.51 × 10−8 M and a rapid response time were observed. Furthermore, the chemical inputs of Zn2+ and Cu2+ ions meet the conditions of an INHIBIT molecular logic gate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive research has been conducted on the biological significance of the Zinc ion (Zn2+). Among the transition metal ions present in the human body, Zinc is the second most abundant after iron. Zn2+ plays a critical role as one of the primary cations in catalytic centers and structural cofactors of multiple Zn2+-containing enzymes, as well as DNA-binding proteins such as transcription factors. Zinc is an essential element in many biological processes, including gene transcription, immune function, and mammalian reproduction [1]. Additionally, it plays a critical role in brain function and pathology. Interestingly, zinc is also involved in certain pathological processes, such as Alzheimer’s disease, epilepsy, ischemic stroke, and infantile diarrhea [2]. Although most Zn2+ is bound tightly to enzymes and proteins, some tissues such as the brain, intestine, pancreas, and retina contain free zinc pools. The d10 electron configuration of Zn2+ makes it spectroscopically silent. In the recent decades, the design and development of fluorescent chemosensor for the imaging and detection of environmental and biological metal ions has emerged as an interesting field [3]. As a result, many fluorescent chemosensors have been extensively investigated for the detection of Zn2+. Fluorescence chemosensors can be classified as “turn-on” or “turn-off” depending on how the metal binding to the ligand influences the fluorescent intensity of the fluorophore [4, 5] There are various ways in which a molecule can exhibit fluorescence sensing property such as through Photoinduced electron transfer (PET), Excited state proton transfer effect (ESIPT), –CH = N isomerisation and more [6,7,8,9]. The receptor serves as the central processing unit (CPU) of a chemosensor. The cyclic framework of the receptor provides an intriguing platform for the complexation of metal ions. In particular, the aza-crown macrocyclic ligands have an interesting complexation character, which makes them potential ligands for the complexation with metal ions. Numerous chemosensor probes have been suggested that possess the capability to detect zinc ions in a wide range [9,10,11,12,13,14,15,16,17,18,19,20,21,22]. This communication presents the development of a new chemosensor that utilizes an aza-crown macrocycle receptor for fluorescence turn-on detection of Zn2+ ions. The detection limit is in the nanomolar range, even in the presence of several other metal ions (Scheme 1).
Experimental
Instruments and chemicals
Reagent grade solvents and reagents were purchased and used without any purification for the syntheses. Analytical grade solvents were used for fluorescence spectroscopic measurements. NMR spectra were obtained using a Bruker AV300 MHz spectrometer. Infrared spectra in KBr pellets (4000–400 cm−1) were recorded using a BIO-RAD FTS-40A spectrophotometer. Positive ion FAB mass spectra were recorded using a Kratos-MS-50 spectrometer. The fluorescence spectra were recorded on a Varian spectrofluorometer. Both emission and excitation bands were set at 5 nm.
Synthesis
2,2′-(Propane-1,3-diylbis(oxy))bis(1-naphthaldehyde) (D)
According to the documented process, dialdehyde D was synthesized [23].
Macrocycle of L
The aza-crown macrocyclic ligand (L) was synthesized by a modified literature method. Firstly, a solution of 2,6-diaminopyridine (0.109 g, 1 mmol) in 20 mL of methanol was added to a stirred solution of precursor dialdehyde D (0.398 g, 1 mmol) in 300 mL of MeOH/DMF (6:4 v/v). The mixture was gently refluxed for 48 h, after which it was cooled to room temperature.
Once the reaction mixture was cooled to room temperature, sodium borohydride (0.83 g, 0.02 mol) was added to the stirred solution over a period of 5 min, and stirred it for 20 min (scheme 1). After that, an excess of water (500 mL) was added to the solution and slowly raised the pH to around 11 by adding potassium hydroxide. The resulted mixture was extracted with chloroform (3 × 25 mL). The chloroform extracts were collected and dried over anhydrous sodium sulfate. During the process of reducing the volume of the extracts to 1/5 on a rotary evaporator, solid products were formed when the concentrate was allowed to stand overnight. Finally, yellow powders were formed as the end product (yield: 74%). Melting point = 164 °C. Anal. Calcd. For C30H34N2O2: C, 76.73; H, 7.51; N, 8.95%. Found: C, 76.31; H, 7.56; N, 8.72%; FT-IR (KBr), cm−1: 3397 (ν, stretching, NH), 1610(ν, bending, NH). 1H NMR (CDCl3, 300 MHz) δ (ppm): 2.40 (b, 2Ha), 4.41 (t, 4Hb), 5.14 (s, 4Hc), 7.25–8.10 (m, 15H, Ar). 13C NMR (CDCl3, 300 MHz) δ (ppm): 29.87, 55.56, 67.05, 114.47, 122.02, 123.20, 123.84, 127.00, 128.41, 129.42, 130.10, 132.90 and 154.27 (Figs. S1–S4).
DFT computation
The geometry optimization of L and [ZnL]2+ was performed using Gaussian 09 software [24,25,26] and DFT/B3LYP method. For C, H, N, O, a 6-311G basis set was employed, while for Zn, an effective potential (ECP) set known as Lanl2dz basis set [27,28,29,30,31] was used. Vibrational frequency calculations were conducted to confirm that the optimized geometries represented the local minima and only positive eigenvalues were obtained. Theoretical UV–Vis spectra were calculated using conductor-like polarizable continuum model (CPCM) by the time-dependent-DFT/B3LYP method [30,31,32], and the fractional contributions of various groups to each molecular orbital were calculated using GAUSSSUM [33, 34].
Results and discussion
Synthesis and characterization
High-dilution conditions or pseudo-high-dilution conditions have been frequently associated with successful macrocyclizations. The use of very diluted concentrations of the final open-chain precursor will always favor intramolecular processes leading to the macrocycle instead of intermolecular reactions producing oligomeric/polymeric species, some of which could eventually form higher order macrocycles. High dilution approaches usually involve slow addition of the components in a large volume of solvent. High-dilution conditions or pseudo-high-dilution conditions have often been linked to successful macrocyclizations. By employing highly diluted concentrations of the final open-chain precursor, intramolecular processes leading to the formation of the macrocycle are favored over intermolecular reactions that result in the production of oligomeric/polymeric species. These species could potentially form higher order macrocycles. High dilution approaches typically involve the gradual addition of components in a significant volume of solvent.
The process of synthesizing various macrocycles involves the use of dialdehydes as a key component. Dialdehydes are commonly used in organic synthesis to create complex structures, such as macrocycles, which are large cyclic molecules containing several atoms. These macrocycles are important in many fields, including materials science and drug discovery, as they exhibit unique properties that make them useful in a wide range of applications. Overall, synthesizing various macrocycles using dialdehydes is a complex and fascinating process that requires careful planning and execution. For the synthesis of the aza-crown macrocycle, 2,2′-(propane-1,3-diylbis(oxy))bis(1-naphthaldehyde) (D) was utilized. The aza-crown macrocyclic ligand of L was obtained by employing pyridine-2,6-diamine as the closing agent in reaction with the dialdehydes of D. In Fig. S1, the IR spectrum of the macrocycle is displayed. The synthesis of the macrocycle is evidenced by the appearance of the peak related to secondary amine groups, as well as the absence of the peak corresponding to aldehyde groups. The starching and bending vibrations of the amine, at 3396 and 1610 cm−1 respectively, are indicative of this synthesis. The structure of the compound is in complete agreement with the NMR data. Three signals in the aliphatic region of L’s 1H NMR spectral data were exactly matched with the structure of L. The region of 7.29–8.07 ppm observed the signals of aromatic protons. Three distinct signals at defined chemical shifts (δ 29.87, 55.66 and 67.05,), which were assignable to different C-atoms present in the compound, were shown in the 13C NMR spectrum. Ten signals were observed in the region of 114.47–154.27 ppm for the signals of aromatic carbons.
The complex of [ZnL]2+ was prepared by reacting ZnCl2.6H2O in ethanol solvent with L. The IR spectrum of [ZnL]2+ complex was prepared and compared with the IR spectrum of L. Two bands at 3396 and 1610 cm−1 related to stretching and bending vibrations of secondary amine groups were shown in the IR spectrum of L, which have been shifted to new frequencies of 3381 and 1624 cm−1 in the zinc complex. The shifts can be related to the formation of the coordination bond between the zinc atom and amine nitrogen atoms (Fig. S1).
Theoretical studies
In Fig. 1, the optimized structures of L and [ZnL]2+ obtained via DFT are presented, and Table S1 shows a selection of calculated bond distances and angles. The electronic property of the compounds has been analyzed based on the composition and energy of the MOs. In Fig. 1, the spatial distributions and orbital energies of the HOMO−1, HOMO, LUMO, and LUMO+1 of L and [ZnL]2+ were determined and displayed. The distribution of the HOMOs and LUMOs of L and [ZnL]2+ was clearly demonstrated to be mainly located over the naphthalene and pyridine moieties. The energy gaps between the HOMOs and LUMOs of L and [ZnL]2+ (Table 1) were used to calculate the values of the UV absorption spectrum peaks. The observed absorption bands in the UV–Vis spectra were compared with the related absorption bands. The UV absorption spectra of the ligand and the complex, which were calculated by the TD-DFT method, have been shown to be in good agreement with the experimental data and accurately explain the experimental results, as indicated by the calculation outcomes. An average discrepancy of 5 nm between the theoretical and experimental λ max is caused by several influencing factors, such as solvent effects, intermolecular interactions, etc., as a result of the strategy employed.
Spectral properties of fluorescent L
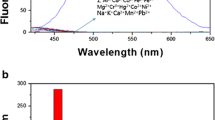
Significant roles have been played by macrocycles containing donor atoms in the development of the field of chemosensing of cations. A weak fluorescence band at λmax = 360 nm was observed in the fluorescence emission spectrum of L when excited at 280 nm at a concentration of 10 μM at room temperature. The fluorescence emission spectra of L were carried out in different solvents, including EtOH, MeOH, H2O, CH3CN, CHCl3, and DMF.
The solvent-dependent fluorescence emission was exhibited by the chemosensor, as shown in Fig. 2. To adjust the pH of the solution, an EtOH-H2O (9:1, v/v) mixture solution was selected.
pH changes can affect the host–guest interaction, making it an important factor that can influence fluorescent chemosensors in general. Therefore, it is necessary to investigate the pH effect to identify the optimal conditions when fluorescence measurements are employed. The changes in fluorescence intensity of probe L towards pH were investigated in an EtOH-H2O (9:1, v/v) solution and the optimum condition was selected at neutral pH, as shown in Fig. 3.
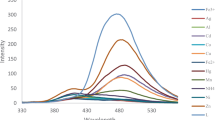
Various metal nitrates (Na+, K+, Cs+, Mg2+, Ba2+, Al3+, Sn2+, Hg2+, Cr3+, Fe2+, Fe3+, Mn2+, Co2+, Ni2+, Cu+, Cu2+, Zn2+, Cd2+, Pb2+, Hg2+ and Gd3+) were used to evaluate the chemosensing ability of L using fluorescence spectroscopies. Figure 4 displays the photoactivated fluorescence emission intensity of L in the presence of the various metals. The majority of the tested ions did not result in notable changes to the fluorescence emission of the chemosensor. However, metal ions of Fe2+, Fe3+, Ni2+, and Cu2+ were found to quench the emission intensity of the chemosensor. In contrast, the addition of Zn2+ resulted in a noteworthy increase in emission intensity, setting it apart from the other metal ions.
To determine the fluorescence enhancement factors (FEF) for L in the presence of different metal ions, the formula ((IX − IL)/IL) was used, where IL represents the fluorescence intensity of L prior to the addition of metal ions at 360 nm, and Ix represents the intensity of L after the addition of metal ions. In the presence of Zn2+ ions, the FEF value for L reached its highest point. To gain a better understanding of L interaction with other metal ions, Fig. 5 showcases its FEF values in relation to these other metals.
Interference effect of other metal ions
The main practical limitation for the use of chemosensors in realistic applications is often related to both their affinity and selectivity. To determine the possibility of using compound L as a practical ion-selective fluorescent chemosensor for Zn2+, competition experiments of L were conducted in the presence of different metal ions. Sample solutions of various metal ions (Na+, K+, Cs+, Mg2+, Ba2+, Al3+, Sn2+, Hg2+, Cr3+, Fe2+, Fe3+, Mn2+, Co2+, Ni2+, Cu+, Cu2+, Zn2+, Cd2+, Pb2+, Hg2+ and Gd3+) were treated with the compound L and Zn2+. The corresponding fluorescence emission was measured (Fig. 6). Only Fe3+, Fe2+, Ni2+ and Cu2+ caused a decrease in the fluorescence emission, while no considerable change was observed in the fluorescence emission of (L + Zn2+) in the presence of other used metals.
The fluorescence titration experiments of L with Zn2+ were carried out by adding aliquots of micromolar concentration of Zn2+ ions. Changes in the fluorescence emission intensity of L with incremental additions of Zn2+ ions were shown in Fig. 7. As Zn2+ concentration increased from 0 to 10 μM, the fluorescence emission was gradually enhanced. The fluorescence emission reached its maximum value around 1 equivalent of Zn2+ and then remained at a plateau at increasing zinc levels, indicating that a 1:1 complex is formed between L and Zn2+ (Fig. 7).
The continuous variation method, also known as the job’s plot, has been the most commonly applied method for the determination of stoichiometry of complex chemical entities for over decades. It has been proven to be successful in the analysis of stable metal–ligand complexes. The molar ratio was selected by [Zn2+]/([Zn2+] + [L]) and measured from 0 to 0.9. A 1:1 complex formation is indicated by the Job’s plot of L with Zn2+ (Fig. S5). The fluorescence quantum yields for L and L– Zn2+ complex in EtOH–H2O solution (9:1, v/v) at 23° were determined by the ratio method. Fluorescence quantum yields values of 0.03004 and 0.12742 were obtained for L and Zn2+–L complex, respectively.
Limit of detection, detection range and response time
The limit of detection (LOD) and detection range (DR) of the Zn2+ ions were estimated from the linear part of the calibration plot (Fig. S6) using the chemosensor. The equation used for this estimation was LOD = kS/m, where k = 3, S is the standard deviation of the blank sample and m is the slope of the calibration graph in the linear range. At low concentration ranges, it is indicated that Zn2+ ions can be detected with high sensitivity over a wide range of concentrations (2.51 × 10−8 M) by the linear correlation.
The kinetics of fluorescence enhancement at 360 nm upon analyzing different concentrations of Zn2+ was studied to evaluate the response time of the chemosensor towards Zn2+ (Fig. S7). It was observed that the time required to reach equilibrium increases with the increase of Zn2+ concentrations, making the response time of L concentration-dependent towards Zn2+. In less than 15 s, a stable reading could be obtained in all cases. Therefore, real-time tracking of Zn2+ could be done using this chemosensor. It was observed from Fig. S7, that once a plateau is reached, the fluorescence intensity at 360 nm remains almost unchanged which indicates that the chemosensor is photostable under irradiation with visible light.
Binding constants measurements
The association constant (Ka) for L–Zn2+ was calculated using the Benesi-Hildebrand plot at room temperature. The fluorescence titration curves of L (10 µM) were obtained by adding various concentrations of Zn2+ (0–1.2 µM). Gradual increase in the emission of L was observed when various concentrations of Zn2+ ions were injected. It can be discovered from Fig. S8 that the constant value of emission intensity was reached at concentrations of more than 10 µM of Zn2+.
The fluorescence emission of L in the presence of the excess amount of Zn2+ is denoted by Imax. The fluorescence emission intensity of L in the absence and presence of Zn2+ ions are denoted by I and I0, respectively. A linear relationship was observed (R2 = 0.99) when 1/[Zn2+] was plotted versus fluorescence emission of [1/(I − I0)]. The binding constant (Ka) was shown as 7.81 × 105 M−1 in the Benesi–Hildebrand plot. A strong binding ability of L with Zn2+ was shown by the obtained Ka.
The chemical reversibility behavior
The chemical reversibility behavior of the binding of L and Zn2+ in the EtOH-H2O solution was studied. It was anticipated that the addition of EDTA will sequester Zn2+ of the metal complex, liberating the free L, due to the high stability constant of the EDTA-Zn2+ complex. With this intention, excess of EDTA was added to the [ZnL]2+. Instant decrease in the fluorescence emission of the solution was observed upon the addition of EDTA, whereas the fluorescence signal could be recovered by readdition of excess Zn2+ (Fig. 8), demonstrating the chemical reversibility of the binding of L with Zn2+.
The LOD values reported in the present work for Zn2+ were compared and listed in Table 2 with the previously reported values. Table 1 indicates a suitable situation of L among the other chemosensors in terms of low LOD value [9,10,11,12,13,14,15,16,17,18,19,20,21,22].
NMR studies
The 1H NMR spectra of L were recorded in the absence and presence of Zn2+ ions. As shown in Fig. 9, upfield shift was observed in the resonance signals corresponding to aliphatic protons upon the addition of Zn2+ (Fig. 9). This result indicates the coordination of Zn2+ to nitrogen atoms and oxygen atoms from the macrocyclic ligand. The weak fluorescence exhibited by chemosensor L was due to fluorescence quenching caused by photoinduced electron transfer from the lone pair of electrons on the nitrogen and oxygen atoms to naphthalene. The PET mechanism is blocked by binding zinc ions to the chemosensor, resulting in great fluorescence enhancement of naphthalene.
Molar conductivity
The molar conductivity (Ʌm) of the 10−3 M solution of the zinc complex in DMF was measured at room temperature. The zinc complex was found to have a molar conductivity of 235–280 Ω−1 cm2 mol−1, indicating the 1:2 ionic natures in the complex. These values, indicative of the presence of two outer sphere chloride anions, are in agreement with the results obtained.
Application
The concentration of Zn2+ in drinking water should be within safe limits to ensure it does not pose a health risk. The World Health Organization (WHO) has established a guideline value of 3 mg L−1 for zinc in drinking water. This guideline takes into consideration both the potential health effects and the practicality of achieving this level. In order to assess the suitability of L as a chemosensor, we employed it as a fluorescence probe for the detection of Zn2+ ions in drinking water. The obtained results were compared with those obtained using the atomic absorption spectroscopic method (as shown in Table 3). We are pleased to report that the results obtained with L were highly satisfactory, with relative errors of less than 5.4%.
Logic gate operation
The INHIBIT gate, also known as the NOT gate with control input, is a digital logic gate that produces a logic 0 output when its control input is at logic 1, and it behaves like a buffer (or pass-through gate) when its control input is at logic 0. In other words, the output of an INHIBIT gate is the complement of its control input, but only when the control input is high. This gate is commonly used in digital circuits for control and data processing applications. The molecular logic gate based on fluorescence emission intensity can be created using L molecule. Zn2+ ions (input 1) and Cu2+ ions (input 2) were chosen as the inputs and fluorescence emission intensity at 360 nm was selected as the output. When L molecule coordinates with Zn2+ (input 1), the fluorescence emission intensity at 360 nm is increased. Upon addition of Cu2+ (input 2), the fluorescence emission intensity is completely quenched. When Cu2+ alone was used for titration, the emission of L was also quenched. The emission intensity is only high enough (output 1 = 1) when Zn2+ (input 1 = 1 and input 2 = 0) is added. As shown in Fig. 10 (truth table), the changes of fluorescence intensity of L under the two chemical inputs Zn2+ and Cu2+ satisfied the INHIBIT logic gate function at the molecular level (Fig. 10).
Conclusion
A new N3O2 aza-crown macrocyclic ligand was synthesized and characterized by various spectroscopy methods. The macrocycle can detect Zn2+ ions in the aqueous ethanolic mixture by enhancement fluorescence emission at 360 nm. The mechanism was found to be the photo-induced electron transfer (PET). The association constant for [L–Zn2+] complex was calculated to be 7.81 × 105 M−1. The detection of limit for Zn2+ is 2.51 × 10−8 M and shows a suitable situation of L among the other chemosensors in terms of low LOD value. Molecular logic gates INHIBIT is proposed using Zn2+ and Cu2+ as inputs.
Data availability
All the data generated or analyzed during this study are included in the published article.
References
Frederickson, C.J., Koh, J.-Y., Bush, A.I.: Nat. Rev. Neurosci. 6, 449 (2005)
Bush, A.I., Pettingell, W.H., Multhaup, G., Paradis, M., Vonsattel, J.-P., Gusella, J.F., Beyreuther, K., Masters, C.L., Tanzi, R.E.: Science 265, 1464 (1994)
Piskacek, M., Zotova, L., Gábor, Z.: J. Cell. Mol. Med. 13, 693–697 (2009)
Kaur, K., Bhardwaj, V.K., Kaur, N., Singh, N.: Inorg. Chem. Commun. 26, 31–36 (2012)
Gupta, V.K., Sethi, B., Sharma, R.A., Agarwal, S., Bharti, A.: J. Mol. Liq. 177, 114–118 (2013)
Gupta, V.K., Karimi-Maleh, H., Sadegh, R.: Int. J. Electrochem. Sci. 10, 303–316 (2015)
Rout, K., Manna, A.K., Sahu, M., Patra, G.K.: Inorg. Chim. Acta 486, 733–741 (2019)
Kim, M.S., GeunJo, T., Yang, M., Han, J., Lim, M.H., Kim, C.: Spectrochim. Acta A Mol. Biomol Spectrosc. 211, 34–43 (2019)
Kim, A., Lee, H., Yun, D., Jung, U., Kim, K.-T., Kim, C.: Spectrochim. Acta. A Mol. Biomol. Spectrosc. 241, 118652 (2020)
So, H., Choa, H., Lee, H., Tran, M.C., Kim, K.-T., Kim, C.: Microchem. J. 155, 104788 (2020)
Maity, D., Mandal, S.K., Guha, B., Roy, P.: Inorg. Chim. Acta 519, 120258 (2021)
Fu, H., Liu, H., Zhao, L., Xiao, B., Fan, T., Jiang, Y.: Tetrahedron 75, 130710 (2019)
Jonaghani, M.Z., Zali-Boeini, H., Moradi, H.: Spectrochim. Acta. A Mol. Biomol. Spectrosc. 207, 16–22 (2019)
Wang, P., Wu, X., Wu, J.: J. Photochem. Photobiol. A Chem. 382, 111929 (2019)
Rani, B.K., John, S.A.: J. Photochem. Photobiol. A Chem. 418, 113372 (2021)
Wang, P., Xue, S.: X, Yang. Microchem. J. 158, 105147 (2020)
Hu, J.-H., Li, J.-B., Qi, J., Sun, Y.: Sens. Actuators B 208, 581–587 (2015)
Khatun, M., Mandal, J., Tamang, V.W., Ghosh Chowdhury, S., Karmakar, P., Saha, A.: J. Photochem. Photobiol. A Chem. 447, 115231 (2024)
Bumagina, N.A., Antina, E.V., Nikonova, A.Y., Berezin, M.B., Ksenofontov, A.A., Vyugin, A.I.: J. Fluoresc. 26, 1967–1974 (2016)
Yun, J.Y., Chae, J.B., Kim, M., Lim, M.H., Kim, C.: Photochem. Photobiol. Sci. 18, 166–176 (2019)
Mandal, J., Jana, N.C., Chowdhury, S.G., Karmakar, P., Saha, A.: Inorg. Chem. Commun. 156, 111217 (2023)
Behura, R., Dash, P.P., Mohanty, P., Behera, S., Mohanty, M., Dinda, R., Behera, S.K., Barick, A.K., Jali, B.R.: J. Mol. Struct. 1264, 133310 (2022)
Azadbakht, R., Hakimi, M., Khanabadi, J.: Spectrochim. Acta A 250, 119236–119241 (2021)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: GAUSSIAN 09, Revision D.01. Gaussian Inc, Wallingford (2009)
Becke, A.D.: J. Chem. Phys. 98, 5648–5652 (1993)
Lee, C., Yang, W., Parr, R.G.: Phys. Rev. B 37, 785–789 (1988)
Hay, P.J., Wadt, W.R.: J. Chem. Phys. 82, 270–283 (1985)
Wadt, W.R., Hay, P.J.: J. Chem. Phys. 82, 284–298 (1985)
Hay, P.J., Wadt, W.R.: J. Chem. Phys. 82, 299–310 (1985)
Barone, V., Cossi, M.: J. Phys. Chem. A 102, 1995–2001 (1998)
Cossi, M., Barone, V.: J. Chem. Phys. 115, 4708–4717 (2001)
Cossi, M., Rega, N., Scalmani, G., Barone, V.: J. Comput. Chem. 24, 669–681 (2003)
O’Boyle, N.M., Tenderholt, A.L., Langner, K.M.: J. Comput. Chem. 29, 839–845 (2008)
Mondal, P., Sarkar, R., Hens, A., Rajak, K.K.: RSC Adv. 4, 38769–38782 (2014)
Acknowledgements
The authors thankfully acknowledge the Faculty of Chemistry of Bu-Ali Sina University and Ministry of Science Research and Technology of Iran.
Funding
We would like to note that no funding was received for this study.
Author information
Authors and Affiliations
Contributions
Reza Azadbakht and Mostafa Koolivand contributed to the collection of emission spectra, data analysis, and writing the original draft. Hasti Moshiri played a key role in synthesizing the compounds.
Corresponding author
Ethics declarations
Competing interests
All authors, including Reza Azadbakht, Hasti Moshiri, and Mostafa Koolivand, affirm that they have no conflict of interest.
Ethical approval
This article does not involve any studies with human or animal subjects.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azadbakht, R., Moshiri, H. & Koolivand, M. Development of a highly selective and sensitive fluorescent chemosensory for zinc ion detection in aqueous ethanol solution: synthesis of a new aza-oxo macrocycle using high-dilution condition method. J Incl Phenom Macrocycl Chem 104, 371–381 (2024). https://doi.org/10.1007/s10847-024-01223-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-024-01223-7