Abstract
Tetrakis(l,3-dihydrobenzoxazine) calix[4]resorcinarenes 4–6 were synthesized via the Mannich reaction from the corresponding resorcin[4]arenes 1–3 with S-(−)-α-methylbenzylamine or R-(+)-α-methylbenzylamine and formaldehyde (aq.). The products were well characterized by FT-IR, 1H NMR, 13C NMR spectroscopies and single crystal X-ray diffraction analysis. Molecular structures of compounds 4, 5 and 6 showed the same R/S configuration to the starting amines. Compounds 4–6 are stabilized by a collar of intramolecular hydrogen bonding networks between the hydroxy groups and the oxygens from the benzoxazine rings. Compounds 4 and 5 could encapsulate the guest molecules of acetone and dichloromethane, respectively. The UV and 1H NMR titration experiments were performed to study the host-guest chemistry between compound 4 and small acetone molecules, indicating that compound 4 exhibited encapsulation behavior towards acetone molecules through hydrogen bonding interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crown ethers and cyclodextrins, as a new research field of supramolecular chemistry, have become representatives of the first and second generation of supermolecule host compounds. After crown ethers and cyclodextrins, calixarenes are the third-generation supramolecular compounds, which have more modifiable sites than crown ethers and cyclodextrin. The top edge of the C-2 positions, hydroxyl and the bottom edge of the alkyl substituents are easy to introduce functional groups for derivatization. Since the pioneering work of Cram [1], calix[4]resorcinarene and its functionalized derivatives have become the focus of chemists because of its excellent molecular platform character and potential applications in various fields [2,3,4]. For example, Shoichi Shimizu and coworkers constructed a water-soluble calix[4]resorcinarene sulfonic acid as recoverable and reusable catalysts through the modification on the C-2 sites of calix[4]resorcinarene [5]. Ma and coworkers prepared several structure diverse organooxotin clusters with calix[4]resorcinarenes and organic protease through self-assembly [6].
Host-guest chemistry, as an important branch of supramolecular chemistry, has attracted great attention nowadays. Unique self-assembling properties of resorcinarenes have been used to prepare novel host–guest compounds and nanomaterials: coated gold and cobalt nanoparticles [7,8,9,10], dimeric [11, 12] and hexameric capsules [13] and molecular containers [14, 15]. Functionalization on the C-2 positions of calix[4]resorcinarenes by Mannich condensation have been extensively studied [16], which includes the use of primary amines [17, 18], secondary amines [19‒21] and amino acids [22, 23]. Previous research on the reaction of primary amines and calix[4]resorcinarenes have been reported and led to isolations of tetrabenzoxazine derivatives, which could include some small guest molecules in the crystalline state [24‒26] and the flexibility of the benzoxazine rings could result in fast uptake and release of the guest [17, 25]. Moreover, tetrabenzoxazine derivatives could form very stable complexes with the guest molecules by multiple C–H⋯π interactions with eight surrounding aromatic rings [27]. In this paper, three starting calix[4]resorcinarenes 1–3 were synthesized based on a literature method (Scheme 1) [1], and their derivatives with benzoxazine moieties 4–6 were synthesized via the Mannich reaction from 1 to 3, S-(−)-α-methylbenzylamine or R-(+)-α-methylbenzylamine, and formaldehyde (Scheme 2).
Experimental section
General
Methanol (99%), ethanol (99%), ethanol (95%) and hydrochloric acid (37%) were commercial products of high purity and used as received. Propionaldehyde, n-butyraldehyde, iso-valeraldehyde, resorcinol, S-(−)-α-methylbenzylamine and R-(+)-α-methylbenzylamine were purchased from Sinopharm Chemical Reagent Co., Ltd. Compounds 1–3 were prepared following the literature method [1]. NMR spectra were recorded on a Bruker ALX 400 Plus spectrometer operating at 400 MHz for 1H NMR and 101 MHz for 13C NMR. Electronic absorption spectra were obtained on a Shimadzu UV-2600 spectrophotometer. Infrared spectra were recorded on a PerkinElmer 16 PC FT-IR spectrophotometer with use of pressed KBr pellets in the region of 400‒4000 cm−1. PL spectra were measured with a Shimadzu RF-5301PC fluorescence spectrophotometer. Elemental analyses were carried out using a PerkinElmer 2400 CHN analyzer.
Synthesis of 1
Resorcinol (5.0 g, 45 mmol) was dissolved in 15 mL of 95% ethanol and 5.0 mL of 37% HCl under nitrogen. To this mixture stirred at 0 °C was added dropwise over a 30 min period 3.3 mL (45 mmol) of propionaldehyde. Under the condition of 0 °C, the reaction solution was maintained for 0.5 h. Then the clear reaction was allowed to warm to room temperature. The reaction mixture was refluxed for 6 h, in which a white product was precipitated. After cooling to room temperature, the solid was filtered, washed with cold 50% methanol-water and then dried in vacuo of an oil pump to a constant weight. Yield: 5.5 g (80%). 1 H NMR (400 MHz, DMSO-d6, ppm): δ 8.94 (s, 8 H, ArOH), 7.24 (s, 4 H, ArHupper), 6.16 (s, 4 H, ArHlower), 4.10 (t, J = 8.0 Hz, 4 H, ArCHAr), 2.13 − 2.10 (m, 8 H, CH2CH3), 0.80 (t, J = 8.0 Hz, 12 H, CH2CH3). IR spectrum (KBr, cm−1): ν(Ar ‒ OH) 3327, ν(C=CAr) 1617, 1435. Anal. Calc. for (C36H40O8): C 71.98; H 6.71%; found: C 72.02; H 6.69%.
Synthesis of 2
Compound 2 was prepared similarly from resorcinol (5.0 g, 45 mmol) and n-butyraldehyde (4.1 mL, 45 mmol). The white solid was filtered, washed with cold 50% methanol-water and then dried in vacuo of an oil pump to a constant weight. Yield: 6.3 g (85%). 1H NMR (400 MHz, DMSO-d6, ppm): δ 8.91 (s, 8 H, ArOH), 7.23 (s, 4 H, ArHupper), 6.13 (s, 4 H, ArHlower), 4.21 (t, J = 8.0 Hz, 4 H, ArCHAr), 2.09 − 2.04 (m, 8 H, CH2CH2CH3), 1.21 − 1.15 (m, 8 H, CH2CH2CH3), 0.88 (t, J = 8.0 Hz, 12 H, CH2CH3). IR spectrum (KBr, cm−1): ν(Ar‒OH) 3370, ν(C=CAr) 1614, 1435. Anal. Calc. for (C40H48O8): C 71.35; H 7.37%; found: C 71.38; H 7.35%.
Synthesis of 3
Compound 3 was prepared similarly from resorcinol (5.0 g, 45 mmol) and iso-valeraldehyde (4.9 mL, 45 mmol). The white solid was filtered, washed with cold 50% methanol-water and then dried in vacuo of an oil pump to a constant weight. Yield: 7.7 g (95%). 1H NMR (400 MHz, DMSO-d6, ppm): δ 8.87 (s, 8 H, ArOH), 7.09 (s, 4 H, ArHupper), 6.12 (s, 4 H, ArHlower), 4.34 (t, J = 8.0 Hz, 4 H, ArCHAr), 1.85 (t, J = 8.0 Hz, 8 H, CHCH2CH), 1.33 − 1.27 (m, 4 H, CH2CH(CH3)2), 0.85 (d, J = 4.0 Hz, 24 H, CH2CH(CH3)2). IR spectrum (KBr, cm−1): ν(Ar‒OH) 3372, ν(C=CAr) 1621, 1444. Anal. Calc. for (C44H56O8): C 74.13; H 7.92%; found: C 74.15; H 7.95%.
Synthesis of 4
To a stirred solution of 1.0 g (1.7 mmol) of ethylcalix[4]resorcinarene (1) and 1.3 mL (13 mmol) of formaldehyde (37% aqueous solution) in ethanol (25 mL), 0.86 mL (6.7 mmol) of R-(+)-α-methylbenzylamine was added dropwise. The reaction mixture was refluxed under nitrogen atmosphere for 6 h, in which a light pink product was precipitated. After cooling to room temperature, the solid was filtered, washed with ethanol and then dried in vacuo of an oil pump to a constant weight. Yield: 1.6 g (80%). 1H NMR (400 MHz, CDCl3, ppm): δ 7.69 (s, 4 H, Ar‒OH), 7.18 (d, J = 8.0 Hz, 8 H, Ar‒H), 7.11 (s, 4 H, ArHlower), 7.05 (t, J = 8.0 Hz, 8 H, Ar‒H), 6.95 (t, J = 8.0 Hz, 4 H, Ar‒H), 5.15 (d, J = 8.0 Hz, 4 H, NCH2O), 4.95 (d, J = 12.0 Hz, 4 H, NCH2O), 4.11 (t, J = 8.0 Hz, 4 H, bridge CH), 3.97 (d, J = 16.0 Hz, 4 H, CHCH3), 3.82 − 3.76 (m, 8 H, ArCH2N), 2.23 − 2.20 (m, 8 H, CH2CH3), 1.30 (d, J = 4.0 Hz, 12 H, CHCH3), 0.95 (d, J = 4.0 Hz, 12 H, CH2CH3). 13C NMR (101 MHz, CDCl3, ppm): δ 149.7, 148.8, 144.5, 128.2, 127.0, 124.1, 123.4, 121.0, 109.0, 80.9 (NCH2O), 58.1, 44.6, 34.8, 26.7, 21.4, 12.7. IR spectrum (KBr, cm−1): ν(Ar‒OH) 3368, ν(C=CAr) 1601, 1471, ν(C‒N) 1348, ν(C‒O‒C) 1182, 1148. Anal. Calc. for (C76H84N4O8): C 77.26, H 7.17, N 4.74%; found: C 77.30, H 7.16, N 4.70%.
Synthesis of 5
To a stirred solution of 1.0 g (1.5 mmol) of n-propylcalix[4]resorcinarene (2) and 1.2 mL (12 mmol) of formaldehyde (37% water solution) in ethanol (25 mL), 0.79 mL (6.1 mmol) of S-(−)-α-methylbenzylamine was added dropwise. The reaction mixture was refluxed under nitrogen atmosphere for 6 h, in which a white product was precipitated. After cooling to room temperature, the solid was filtered, washed with ethanol and then dried in vacuo of an oil pump to a constant weight. Yield: 1.6 g (83%). 1H NMR (400 MHz, CDCl3, ppm): δ 7.65 (s, 4 H, Ar‒OH), 7.18 (d, J = 4.0 Hz, 8 H, Ar‒H), 7.12 (s, 4 H, ArHlower), 7.04 (t, J = 8.0 Hz, 8 H, Ar‒H), 6.95 (t, J = 8.0 Hz, 4 H, Ar‒H), 5.14 (d, J = 8.0 Hz, 4 H, NCH2O), 4.93 (d, J = 8.0 Hz, 4 H, NCH2O), 4.24 (t, J = 8.0 Hz, 4 H, bridge CH), 3.97 (d, J = 16.0 Hz, 4 H, CHCH3), 3.84 − 3.72 (m, 8 H, ArCH2N), 2.25 − 2.11 (m, 8 H, CHCH2), 1.34 − 1.32 (m, 8 H, CH2CH3), 1.30 (d, J = 8.0 Hz, 12 H, CHCH3), 1.00 (t, J = 4.0 Hz ,12 H, CH2CH3). 13C NMR (101 MHz, CDCl3, ppm): δ 149.6, 148.7, 144.5, 128.3, 127.0, 124.3, 123.4, 121.2, 108.9, 80.9 (NCH2O), 58.0, 44.6, 35.7, 32.3, 21.5, 21.1, 14.1. IR spectrum (KBr, cm−1): ν(Ar‒OH) 3367, ν(C=CAr) 1601, 1471, ν(C‒N) 1348, ν(C‒O‒C) 1182, 1147. Anal. Calc. for (C80H92N4O8): C 77.64, H 7.49, N 4.53%; found: C 77.60, H 7.45, N 4.55%.
Synthesis of 6
To a stirred solution of 1.0 g (1.4 mmol) of iso-butylcalix[4]resorcinarene (3) and 1.1 mL (11 mmol) of formaldehyde (37% water solution) in ethanol (25 mL), 0.71 mL (5.6 mmol) of R-(+)-α-methylbenzylamine was added dropwise. The reaction mixture was refluxed under nitrogen atmosphere for 6 h, in which a white product was precipitated. After cooling to room temperature, the solid was filtered, washed with ethanol and then dried in vacuo of an oil pump to a constant weight. Yield: 1.5 g (85%). 1H NMR (400 MHz, CDCl3, ppm): δ 7.70 (s, 4 H, Ar‒OH), 7.19 (d, J = 8.0 Hz, 8 H, Ar‒H), 7.11 (s, 4 H, ArHlower), 7.07 (t, J = 8.0 Hz, 8 H, Ar‒H), 6.98 (t, J = 8.0 Hz, 4 H, Ar‒H), 5.14 (d, J = 8.0 Hz, 4 H, NCH2O), 4.93 (d, J = 16.0 Hz, 4 H, NCH2O), 4.35 (t, J = 8.0 Hz, 4 H, bridge CH), 3.97 (d, J = 16.0 Hz, 4 H, CHCH3), 3.81 − 3.72 (m, 8 H, ArCH2N), 2.15 − 1.99 (m, 8 H, CHCH2), 1.52 − 1.49 (m, 4 H, CH(CH3)2), 1.30 (d, J = 8.0 Hz, 12 H, CHCH3), 0.97 (d, J = 16.0 Hz, 24 H, CH(CH3)2). 13C NMR (101 MHz, CDCl3, ppm): δ 149.6, 148.7, 144.5, 128.3, 127.1, 124.3, 123.4, 121.4, 108.9, 81.0 (NCH2O), 58.0, 44.6, 42.7, 31.0, 30.2, 26.1, 23.0, 22.7, 21.5. IR spectrum (KBr, cm−1): ν(Ar‒OH) 3374, ν(C=CAr) 1601, 1467, ν(C‒N) 1348, ν(C‒O‒C) 1182, 1149. Anal. Calc. for (C84H100N4O8): C 77.98, H 7.79, N 4.33%; found: C 77.95, H 7.82, N 4.31%.
Single crystal X-Ray analysis
Crystallographic data and experimental detail for compounds 4, 5 and 6 are summarized in Table S1 (see Supporting Information). Suitable single crystals were selected and mounted on a Bruker SMART Apex 2000 CCD diffractometer using graphite-monochromated Mo-Kα (λ = 0.71073 Å) radiation at 296(2) K. The data was corrected for absorption using the program SADABS [28]. Cell parameters were retrieved using SMART software and refined using SAINT on all observed reflections [29]. Structures were solved by the direct methods and refined by full-matrix least-squares on F2 using the SHELXTL software package [30, 31]. All non-hydrogen atoms were refined anisotropically. The positions of all hydrogen atoms were generated geometrically (\({\mathrm C}_{\mathrm{sp}^3}\) –H = 0.96 Å, \({\mathrm C}_{\mathrm{sp}^2}\) –H = 0.93 Å, O‒H = 0.82 Å) and assigned isotropic displacement parameters.
Crystallographic data for compounds 4‒6 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC 2,215,954, 2,215,953, 2,215,955, respectively. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [fax: (+ 44)1233-336-033; e-mail: deposit@ccdc.cam.ac.uk].
Results and discussion
The 1H NMR and IR spectral data of calix[4]resorcinarenes 1‒3 matches those of literature reports (Figures S1‒6), and the yields of 1‒3 (80–95%) are similar to the literature-reported yields [1]. Compounds 1‒3 adopt a symmetrical (C4v) bowl-shaped conformation in solution based on their 1H NMR spectra [32]. As shown in Scheme 2, tetrakis(l,3-dihydrobenzoxazine) calix[4]resorcinarenes 4–6 were synthesized by condensation of 1‒3 with S-(−)-α-methylbenzylamine or R-(+)-α-methylbenzylamine in the presence of excess formaldehyde (37% water solution) in ethanol at reflux in relatively high yields (80–85%). IR spectra of compounds 4–6 show the peaks at about 1350 cm–1 which are the characteristic stretching vibration of C‒N single bond and the peaks at about 1180 cm−1 and 1150 cm−1 may related to C‒O‒C groups. Moreover, the intense absorption bands of stretching vibrations of the aromatic ring at about 1600 cm−1 and 1470 cm−1 are observed and the absorption band at about 3374 cm−1 are owning to hydroxyl groups (Figures S7, S11 and S14). The 1H NMR spectra of compounds 4–6 clearly exhibited two doublets at around 4.95 ppm and 5.15 ppm, owning to the NCH2O protons in the dihydrobenzoxazine rings [33, 34]. The 13C NMR showed the feature NCH2O carbons at about 80.9 ppm, indicative of the only structure of the product. Typical 1H and 13C NMR spectra of compound 4 are shown in Figs. 1 and 2. Moreover, the 2D NMR methods were employed to clarify the exact assignation of the signals, as shown in Figs. 3 and 4. In the 1H–1H NOSEY spectrum of compound 5, it could be found that there was a mutual coupling relationship between the hydrogen atoms in compound 5. For examples, the hydrogen atoms at positions 1 and 2, and the hydrogen at positions 3 and 4 have coupling correlations, respectively. While in the 1H–1H COSY spectrum of compound 5, coupling correlation between hydrogen atoms on adjacent carbon atoms in compound 5 is observed, such as hydrogen atoms at positions 1 and 2, and the hydrogen atoms at positions 3 and 4.
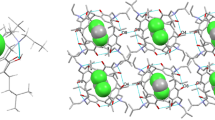
Slow crystallization of compounds 4, 5 and 6 from the mixture of dichloromethane and acetone afforded diffraction quality single crystals after a few days. Molecular structure of 4 was displayed in Fig. 5. Intramolecular hydrogen bonds come from adjacent resorcinol units, and the O⋯O distances is about 2.8 ± 0.1 Å. The C‒N‒C‒O torsion angles of adjacent oxazine rings are 62.1° and 65.9°, respectively. The C‒O‒C bond angles of adjacent oxazine rings are 113.2° and 114.4°, a little larger than the corresponding C‒N‒C bond angles (108.3° and 108.4°). Top view structure of compound 4 with lower rim ethyl substituents reveals that the acetone molecules are embedded in the macrocyclic hosts (Fig. 6). From the stacking diagram of 4, it could be seen that the regular spatial arrangement of benzoxazine rings and acetone molecules makes the structure more stable (Fig. 7).
Compound 5 crystallizes in the monoclinic space group P21. The unit cell contains one tetrabenzoxazine calix[4]resorcinarene molecule and one methylene chloride molecule (Fig. 8). Similar to compound 4, intramolecular hydrogen bonds between the hydroxy groups (O1, O4, O5, O6) and the oxygens (O2, O3, O7, O8) from the benzoxazine rings exist in compound 5 with a normal O⋯O distance of 2.8 ± 0.1 Å. The conformation of resorcinarene is slightly distorted with the dihedral angles of opposite resorcinol rings being about 65° and 75°, respectively. In order to reduce steric hindrance, the benzoxazine fragments are arranged in the same clockwise direction along the upper margin of resorcinarenes. All the nitrogen atoms of flexible benzoxazine rings point towards the cavity, and all the benzene rings show an angle of 40° relative to the z axis. The torsion angles of O‒C‒N‒C range from 63.6 to 66.8° in compound 5, slightly larger than in compound 4 (62.1−65.9°).
Compound 6 crystallizes in the orthorhombic space group C2221. Molecular structure of compound 6 with intramolecular O‒H⋯··∙O hydrogen bonds is shown in Fig. 9. Top view structures of compounds 5 and 6 show that the cavity of compound 6 is almost square (Fig. 10B), but the cavity of compound 5 is almost rectangular (Fig. 10A). This is probably due to the presence of the guest molecules in compound 5, which changes its cavity to better accommodate the guest dichloromethane molecules. Unlike the structures of compounds 4 and 5, there is a big difference of the torsion angles of O‒C‒N‒C in compound 6, which are 58.4°, 66.0°, 66.3° and 68.9°, respectively, suggesting the flexibility of the oxazine ring. Stacking diagrams of compounds 5 and 6 exhibit that each pair of adjacent calixarene units is arranged in reverse order to stabilize the structures and reduce steric hindrance (Fig. 11A, B).
In light of the host-guest structure of compound 4 in the solid state, UV titration experiments were performed to study its host-guest behavior in solution. Different equivalents of acetone molecules were added into the trichloromethane solution of compound 4, and rigorously stirred for 2 h, affording dynamic host-guest complexes. Several parallel and independent measurements were conducted and the host-guest behavior was tested by the UV absorption spectra. As is shown in Fig. 12, UV absorption was enhanced and a red shift occurred with the addition of guest acetone molecules, suggesting that the guest molecules were captured into the cavity of compound 4 in solution.
In order to further study the host-guest system, the host-guest behavior was estimated through 1H NMR titration experiments. All titration experiments were conducted at the same concentration. Different equivalents of guest acetone molecules were added dropwise to a fixed concentration of the host compound 4 to study this host-guest interaction in solution. As is shown in Fig. 13, the chemical shift of the characteristic hydroxyl protons in compound 4 have changed significantly. When the molar ratio of guest acetone molecules and 4 increased from 0 to 1, the chemical shift of –OH hydrogen moved up field (7.70 ppm vs. 7.45 ppm), possibly due to formation of hydrogen bonds between –OH groups and acetone, indicating that the guest acetone molecules could be encapsulated by the cavity of compound 4 on the NMR time scale as fast exchange in solution.
In summary, three tetrakis(l,3-dihydrobenzoxazine) calix[4]resorcinarenes 4‒6 with different lower rim alkyl substituents were synthesized in relatively high yields (80–85%). The molecular structures of 4‒6 are confirmed by single crystal X-ray diffraction analysis, indicating that the tetrakis(l,3-dihydrobenzoxazine) calix[4]resorcinarenes are stabilized by intramolecular hydrogen bonding between hydroxyl groups and benzoxazine oxygen atoms with similar O⋯O distances being about 2.8 Å. Compounds 4‒6 bearing eight surrounding aromatic rings have a deep cavity, which could encapsulate small molecules of acetone and dichloromethane. Such host-guest chemistry has been further studied by UV titration experiments and 1H NMR titration experiment between compound 4 and acetone in solution. The changes of UV absorption behavior and chemical shift of hydroxyl protons in the 1H NMR spectra showed that the electron-rich macrocyclic cavity could encapsulate acetone molecules through hydrogen bonding interactions. Next, we will modify the synthesis of other types of organic amin and amino acid compounds for resorcinol calixarene, so as to further expand its cavity and explore its recognition process of organic small molecules.
References
Cram, D.J., Karbach, S., Kim, H.E., Knobler, C.B., Maverick, E.F., Ericson, J.L., Helgeson, R.C.: Cavitands as open molecular vessels form solvates. J. Am. Chem. Soc. 110, 2229–2237 (1988)
Yamanaka, M., Kobayashi, K.: Capsular assemblies of calix[4]resorcinarene-based cavitands. Asian J. Org. Chem. 2, 276–289 (2013)
Kobayashi, K., Yamanaka, M.: Self-assembled capsules based on tetrafunctionalized calix[4]resorcinarene cavitands. Chem. Soc. Rev. 44, 449–466 (2015)
Timmerman, P., Verboom, W., Reinhoudt, D.N.: Resorcinarenes. Tetrahedron 52, 2663–2704 (1996)
Shimizu, S., Shimada, N., Sasaki, Y.: Mannich-type reactions in water using anionic water-soluble calixarenes as recoverable and reusable catalysts. Green. Chem. 8, 608–614 (2006)
Dong, Y.B., Shi, H.Y., Yang, J., Liu, Y.Y., Ma, J.F.: Molecular dumbbell, sandwich, and paddle-wheel assembled with methylresorcin[4]arene cavitands and organooxotin clusters. Cryst. Growth Des. 15, 1546–1551 (2015)
Kim, B., Tripp, L.S., Wei, A.: Self-organization of large gold nanoparticle arrays. J. Am. Chem. Soc. 123, 7955–7956 (2001)
Wei, A., Kim, B., Pusztay, S.V., Tripp, S.L., Balasubramanian, R.: Resorcinarene-encapsulated nanoparticles: building blocks for self-assembled nanostructures. J. Incl. Phenom. Macro. 41, 83–86 (2001)
Wei, A.: Calixarene-encapsulated nanoparticles: self-assembly into functional nanomaterials. Chem. Commun. 15, 1581–1591 (2006)
Liu, J., Wei, A.: Prenucleation and coalescence of cobalt nanoclusters mediated by multivalent calixarene complexes. Chem. Commun. 28, 4254–4256 (2009)
Pei, W.-Y., Xu, G.-H., Yang, J., Wu, H., Chen, B.-L., Zhou, W., Ma, J.-F.: Versatile assembly of metal-coordinated calix[4]resorcinarene cavitands and cages through ancillary linker tuning. J. Am. Chem. Soc. 139, 7648–7656 (2017)
Coleman, A.W., Jebors, S., Shahgaldian, P., Ananchenko, G.S., Ripmeester, J.A.: Para-acylcalix[n]arenes: from molecular to macroscopic assemblies. Chem. Commun. 20, 2291–2303 (2008)
Shivanyuk, A.: Nanoencapsulation of calix[4]arene inclusion complexes. J. Am. Chem. Soc. 129, 14196–14199 (2007)
Beyeh, N.K., Pan, F., Rissanen, K.: Hierarchical ordering in ternary co-crystals of c60, n–benzyl ammonium resorcinarene bromide and solvent molecules. Cryst. Growth Des. 14, 6161–6165 (2014)
Puttreddy, R., Beyeh, N.K., Taimoory, S.M., Trant, M.D., Rissanen, J.F.: Host–guest complexes of conformationally flexible C-hexyl-2-bromoresorcinarene and aromatic N-oxides: solid-state, solution and computational studies. J. Org. Chem. 14, 1723–1733 (2018)
Kashapov, R.R., Razuvayeva, Y.S., Ziganshina, A.Y., Mukhitova, R.K., Sapunova, A.S., Voloshina, A.D., Nizameev, I.R., Marsil, K., Kadirov, M.K., Zakharova, L.Y.: Design of N–methyl–D–glucamine-based resorcin[4]arene nanoparticles for enhanced apoptosis effects. Mol. Pharm. 17, 40–49 (2020)
Chwastek, M., Szumna, A.: Higher analogues of resorcinarenes and pyrogallolarenes: bricks for supramolecular chemistry. Org. Lett. 22, 6838–6841 (2020)
Alver, C.A., Mauricio, M.: Preparation of methacrylate-based polymers modified with chiral resorcinarenes and their evaluation as sorbents in norepinephrine microextraction. Polymers 11, 1428 (2019)
Ligimol Louis, L., Alexander, V., Kumar, D.S., Senthan, S.A., Viveke, A.A.: Photoluminescence and electrochemical studies of tetranuclear ruthenium(II) polypyridyl complexes of benzimidazolyl functionalised pyrenylcalix[4]resorcinarene. Inorg. Chim. Acta 486, 245–251 (2019)
Liu, J.-L., Liu, X.-L., Jia, A.-Q., Shi, H.-T., Zhang, Q.-F.: Supramolecular structures and crystal stability of diisobutylaminomethylated calix[4]resorcinarenes. J. Incl. Phenom. Macro. 98, 49–56 (2020)
Ngodwana, L., Bout, W., Nqaba, Z., Motlokoa, T., Vatsha, B.: Methodologies for the derivatization of resorcin[4]arenes at the upper rim ortho-positions. Eur. J. Org. Chem. 2022, 1–13 (2022)
Betty, A.V.-S., Alver, C.-A., Zuly, J.R.-M., Mauricio, M.: Aminomethylated calix[4]resorcinarenes as modifying agents for glycidyl methacrylate (GMA) rigid copolymers surface. Polymers 11, 1427 (2019)
Sakowicz, A.M., Szumna, A.: Chiral water-soluble molecular capsules with amphiphilic interiors. Front. Chem. 10, 883093 (2022)
Amecke, R., Bohmer, V., Paulus, E.F., Vogt, W.: Regioselective formation of dissymmetric resorcarene derivatives with C4-symmetry. J. Am. Chem. Soc. 117, 3286–3287 (1995)
Atwood, J.L., Szumna, A.: Anion-sealed single-molecule capsules. Chem. Commun. (2003). https://doi.org/10.1039/B301511D
Lenz, K., Alexander, S., David, B.G., Julius, R.: Spin labeling monitors weak host–guest interactions. Chem. Commun. 3, 272–273 (2004)
Atwood, J.L., Szumna, A.: Hydrogen bonds seal single-molecule capsules. J. Am. Chem. Soc. 124, 10646–10647 (2002)
Sheldrick, G.M.: Sadabs: University of Göttingen. Göttingen, Germany (1996)
SMART and SAINT + for, Windows, N.T.: Version 6.02a, Bruker Analytical X-ray Instruments Inc., Madison (1998)
Sheldrick, G.M.: Shelxtl Software Reference Manual (Version 5.1). Bruker AXS Inc, Madison (1997)
Sheldrick, G.M.: A short history of SHELXTL. Acta Crystallogr. A64, 112–122 (2008)
Högberg, A.G.S.: Stereoselective synthesis and DNMR Study of two 1,8,15,22-Tetraphenyl [14]metacyclophan-3,5,10,12,17,19,24,26-octols. J. Am. Chem. Soc. 102, 6046–6050 (1980)
Moharem, T., El, G., Harry, H., Alexandra, M.Z.S.: Highly diastereoselective functionalisation of calix[4]resorcinarene derivatives and acid catalysed epimerisation reactions. Tetrahedron Lett. 36, 4905–4909 (1995)
Szumna, A.: Cyclochiral conformers of resorcin[4]arenes stabilized by hydrogen bonds. Org. Biomol. Chem. 5, 1358–1368 (2007)
Acknowledgements
This project was supported by National Natural Science Foundation of China (90922008).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, XM., Wang, Q., Sun, M. et al. Tetrakis(benzoxazine) calix[4]resorcinarenes as hosts for small molecules. J Incl Phenom Macrocycl Chem 103, 289–299 (2023). https://doi.org/10.1007/s10847-023-01195-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-023-01195-0