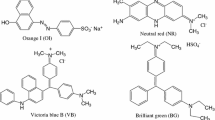
Abstract
By using β-cyclodextrin aromatic aldehyde derivatives 5 and 6 as starting materials, the novel β-cyclodextrin-[60]fullerene conjugate 7 and bis-β-cyclodextrin-[60]fullerene conjugate 8 were designed and conveniently synthesized via the Huisgen [2+3] cycloaddition for the first time. The UV–Vis complexation experiments, fluorescence titration spectra and complexation ESI–MS spectrum suggested that they possessed excellent binding abilities for dyes with 1:1 host–guest complexes. The highest association constant of compound 8 for Orange I was as high as 6.97 × 105.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs) are shaped like a truncated cone, rather than a perfect cylinder, as a consequence of the chair conformation of the glucopyranose units. The capacity to form inclusion complexes with a wide variety of guest molecules is one of the most interesting properties of CDs. Based on their unique properties, they exhibited various application prospective in the pharmaceutical, agrochemical, food and cosmetic fields [1–7]. For examples, some of β-CDs derivatives and bis-β-CDs derivatives were reported as good receptors for all kinds of organic dyes [8–12]. Given that the organic dyes are the aromatic molecules with large conjugated structures, it is reasonable that the β-CDs derivatives with large aromatic structures would exhibit excellent binding abilities for dyes based on the π-π interaction between aromatic conjugate groups and organic dyes.
On the other hand, [60]fullerene has been considered as a powerful building block in material sciences and supramolecular chemistry due to its unique aromatic conjugate structures. If C60 unit is introduced into β-cyclodextrin, the obtained β-CD-C60 conjugates might show excellent dyes binding abilities based on the the π-π intermolecular action. However, comparing with the extensive studies of the supramolecular complexations of β-CDs with C60, the β-CD-C60 conjugates are seldom reported up to now [13–16]. There were only two kinds of synthetic methods for β-CD-C60 conjugates in literatures so far. One was the 1,3-dipolar cycloaddition of β-CDs-N3 derivatives with C60 reported by Jiang [17], Wael [18] and Noto groups [19], respectively. Another method was via Hirsch–Bingel reaction described by Zhou and Liu [20, 21]. Also, the electrochemical behaviors or DNA cleavage performances of these β-CD-C60 conjugates were studied but no complexation behavior was investigated. In this paper, we firstly used the Huisgen [2+3] cycloaddition to prepare novel β-cyclodextrin-[60]fullerene conjugate 7 and bis-β-cyclodextrin-[60]fullerene conjugate 8. Also, the dyes complexation behaviors of β-cyclodextrin-[60]fullerene conjugates were investigated for the first time. The results suggested that novel β-cyclodextrin-[60]fullerene conjugates possess outstanding dyes complexation abilities as expected.
Experimental
General experimental information
IR spectra were recorded on a Perkin-Elmer PE-983 infrared spectrometer as KBr pellets with absorption in cm−1. 1H NMR spectra was recorded in DMSO-d6 on a Bruker-ARX 600 instrument at 30 °C. Chemical shifts are reported in ppm, using tetramethylsilane (TMS) as internal standard. ESI–MS spectra were obtained from DECAX-30000 LCQ Deca XP mass spectrometer (mobile phase: MeCN, flow: MeCN, electrospray temperature: 450 °C). Elemental analyses were performed at Vario EL III Elemental Analyzer. TLC analysis was performed using pre-coated silica gel glass plates. All chemicals were purchased from commercial suppliers and used without further purification. The other organic solvents and inorganic reagents were purified according to standard anhydrous methods before use. 4-(Prop-2-ynyloxy)benzaldehyde 3, 3, 4-bis-(prop-2-ynyloxy)benzaldehyde 4 and β-CD derivatives 5 and 6 were prepared according to published procedure [22].
All UV–Vis experiments were performed in DMSO solution by adding aliquots stock solution of respective dyes. The wavelength of the measurement in UV–Vis spectra were 278 nm for OI(Orange I) and 266 nm for NR(Neutral red), respectively. Fluorescence spectra were measured in a conventional quartz cell (10 × 10 × 45 nm) at 25 °C on a Hitachi F-4500 spectrometer equipped with a constant-temperature water bath, with excitation and emission slits 10 nm wide. The excitation wavelengths were 310 nm. In the fluorescence titration experiments, the concentrations of hosts 7 and 8 were 1 × 10−5 M and the concentrations of dyes were 0, 0.2, 0.4, 0.6, 0.8, 1.0, 2.0 × 10−5 M, respectively, in the phosphate buffer (pH 7.20). The stoichiometry of the complexes was determined by the Job method of continuous variations. The association constant was calculated by Benesi–Hilderbrand formula with nonlinear curve fitting procedure [23, 24].
Synthesis of β-cyclodextrin-[60]fullerene conjugate 7
Under N2 atmosphere, a mixture of compound 5 (0.33 g, 0.25 mmol), C60 (0.18 g, 0.25 mol), and sarcosine (0.225 g, 2.5 mmol) in dry toluene (100 mL) was refluxed for 48 h. After the reaction mixture was cooled to room temperature, the solvent was removed under reduced pressure and the residue was further purified by column chromatography (SiO2 100–200 mesh, methanol/CH2Cl2 (1:4, V/V) as eluant). The compound 7 was obtained as brown solid in yield of 26 %. Compound 7: IR/cm−1: 2919, 2849, 1664, 1509, 1377, 576, 526; 1H NMR (400 MHz, DMSO-d6) δppm: 2.83 (s, 3H, NCH3), 3.25–4.02 (m, 42H, CH and CH2), 4.30 (bs, 1H, NCH2-(exo)), 4.41–4.51 (m, 6H, OH-6), 4.75–4.89 (m, 7H, CH), 5.05(s, 2H, CH2O), 5.22 [bs, 1H, NCH2-(endo)], 5.62–5.91 (m, 15H, CH and OH-2,3), 7.2–8.25 (m, 5H, ArH and NCH). 13C NMR (100 MHz, DMSO-d6) δppm: 163.2, 160.4, 155.2, 148.6, 147.1, 146.4, 145.5, 144.8, 144.3, 143.1, 142.8, 142.6, 141.9, 132.2, 131.8, 130.2, 126.0, 124.2, 115.9, 115.5, 103,8, 102.8, 101.9, 85.4, 84.1, 82.4, 82.0, 81.3, 73.6, 73.5, 73.2, 73.1, 72.8, 72.4, 72.1, 70.4, 68.3, 67.9, 61.7, 60.3, 59.9, 59.4, 50.4, 41.1. MS m/z (%): 2090.9 (MNa+, 100). Anal. Calcd. For C114H82N4O35: C 66.21, H 4.00, N 2.71; found C 66.25, H 4.04, N 2.67 %.
Synthesis of β-cyclodextrin-[60]fullerene conjugate 8
Under N2 atmosphere, a mixture of compound 6 (0.63 g, 0.25 mmol), C60 (0.18 g, 0.25 mol), and sarcosine (0.225 g, 2.5 mmol) in dry toluene (150 mL) was refluxed for 48 h. After the reaction mixture was cooled to room temperature, the solvent was removed under reduced pressure and the residue was further purified by column chromatography (SiO2 100–200 mesh, methanol/CH2Cl2 (1:3, V/V) as eluant). The compound 8 was obtained as brown solid in yield of 21 %. Compound 8: IR/cm−1: 2922, 1668, 1506, 1262, 1150, 576, 523; 1H NMR (400 MHz, DMSO-d6) δppm: 2.82 (s, 3H, NCH3), 3.19–3.95 (m, 84H, CH, CH2), 4.25–4.60 (m, 13H, NCH2-(exo) and OH-6), 4.75–4.98 (m, 14H, CH), 5.05 [bs, 1H, NCH2-(endo)], 5.33 (s, 4H, CH2O), 5.61–5.91 (m, 29H, CH and OH-2,3), 7.18–8.15 (m, 5H, ArH and NCH). 13C NMR (100 MHz, DMSO-d6) δppm: 163.8, 163.6, 160.8, 156.3, 147.2, 145.9, 145.5, 144.8, 142.7, 142.4, 141.6, 139.9, 139.5, 136.4, 135.3, 131.2, 130.5,126.4, 126.0, 119.6, 115.9, 102,8, 102.4, 102.1, 84.4, 82.1, 81.7, 81.5, 73.4, 73.2, 72.6, 72.4, 72.1, 70.3, 69.2, 68.9, 61.5, 60.4, 60.1, 59.8, 59.4, 50.3, 41.2. MS m/z (%):3304.2 (MNa+, 100). Anal. Calcd. For C159H153N7O70: C 58.19, H 4.70, N 2.99; found C58.12, H 4.75, N2.94 %.
Results and discussion
Synthesis and characterization
The synthetic routes were shown in Scheme 1. Although Huisgen [2+3] cycloaddition was extensively applied in preparing fullerene derivatives, it was not used in synthesizing β-cyclodextrin-[60]fullerene conjugate due to the absence of β-CD aromatic aldehyde derivative. Lately, we reported the synthesis of several β-cyclodextrin aromatic aldehydes by click chemistry in good yields [22]. Thus, the β-cyclodextrin-[60]fullerene conjugate 7 and bis-β-cyclodextrin-[60]fullerene conjugate 8 were designed and synthesized by using these β-cyclodextrin aromatic aldehydes as reactants. As shown in Scheme 1, the mono-6-azido-β-CD 2 was obtained from the readily available mono-6-tosyl-β-CD according to references [22]. Subsequently, by reacting compound 2 with 4-(prop-2-ynyloxy)benzaldehyde 3 or 3, 4-bis-(prop-2-ynyloxy)benzaldehyde 4, β-cyclodextrin aromatic aldehyde 5 and bis-β-cyclodextrin aromatic aldehyde 6 were smoothly obtained by click reaction in yields of 60–75 %. Finally, by treating compound 5 or 6 with sarcosine and C60 in refluxing toluene, the novel β-cyclodextrin-[60]fullerene conjugate 7 and bis-β-cyclodextrin-[60]fullerene conjugate 8 were obtained via 1,3-dipolar cycloaddition of azomethine ylides to C60 in isolated yields of 21 and 26 % after column chromatography, respectively. To the best of our knowledge, compounds 7 and 8 were the first examples of β-cyclodextrin-[60]fullerene conjugates via Huisgen [2+3] cycloaddition.
The structures of new compounds 7 and 8 were characterized by elemental analyses, UV, IR, ESI–MS, 1H NMR and 13C NMR spectra, etc. Their UV spectra exhibited the typical absorption features of mono-fulleropyrrolidine at 433 nm as shown in Fig. 1. The ESI–MS spectra of compounds 7 and 8 possessed corresponding pseudomolecular ion peak (MNa+) at 2090.9 and 3304.2, indicating the accomplishment of Huisgen [2+3] cycloaddition. The 1H NMR spectrum of compounds 7 and 8 also presented the corresponding signals of protons. On the other hand, as generally observed for substituted CDs, the proton signals at sugar units were hardly exploitable due to overlapping and broadening [25–27]. This phenomenon suggested a modification of the conical CD structures leading to non-equivalent glucopyranose units [25–27]. Thus, the complete signal assignment was difficult for these CD derivatives. By contrast, in 13C NMR spectra, the characteristic signals for triazole cycle, NCH2 and NCH3 were observed obviously for compounds 7 and 8. All these typical characterization of spectra were similar to structures of mono-fulleropyrrolidine in literatures [28, 29].
Complexation Studies for dyes
The inclusion complexes of compounds 7 and 8 were investigated by titration of UV–Vis spectroscopy in the presence of two normal dyes [Orange I (OI) and Neutral red (NR)] with various concentrations (0.0, 0.2, 0.4, 0.6, 0.8, 1.0, 2.0 × 10−5 M). The results were shown in Figs. 2, 3, 4, 5 and 6. Moreover, based on the absorbance at maximal absorption wavelength, the association constants and correlation coefficients were calculated by Benesi–Hildebrand equation, which was usually used to study the complexation behaviors of host–guest [23, 24]. The calculated formula was as follows:
where H is host; G is guest; n is the ratio of complexation; [H] and [G] are the concentration of host and guest, respectively; Ks is association constant; Δε is molar absorption coefficient; ΔA is the change of absorbance at maximal absorption wavelength.
The calculated results of correlation coefficients (R) and associations constants were summarized in Table 1. One can see that the values of correlation coefficients were close to 1 when the ratios of complexation (n) were set as 1. These results suggested the formation of 1:1 host–guest complexes for compounds 7 and 8 with dyes. The associations constants of compound 7 were higher than 104 but that of compound 8 were higher than 105. The highest association constant of compound 8 with OI was as high as 6.97 × 105. These results indicated that the more β-CD units were favorable for dyes complexation based on the cooperative action of polytopic β-CD units, which were in accordance with other reports [25–27]. Moreover, comparing with their precursors 5 and 6 [22], compound 7 showed similar association constants to precursor 5, but compound 8 exhibited far higher values of constants than precursor 6. The better complexation abilities of compound 8 could be attributed to cooperative complexation of C60 unit and β-CD unit by comparing the structures of compound 8 and precursor 6. Compound 7 did not show the similar changes, indicating that the cooperative action was restrained. These phenomena might be ascribed to the self-inclusion of C60 unit in cavity of β-CDs for compound 7, which was usually observed in C60-CDs derivatives complexation systems [13–16]. This kind of self-inclusion was disadvantageous for binding dyes. However, it was difficult for bis-β-cyclodextrin-[60]fullerene conjugate 8 to form self-inclusion due to the steric hindrance of more CD units. So compound 8 presented excellent dyes complexation abilities based on the cooperative intermolecular action of β-CD units and C60 unit. Based on the complexation results, it was reasonable to deduce that the dye was binded in the cavity composed of two β-CD units and C60 unit.
Also, the fluorescence titration spectra were employed to test the complexation behaviors of compound 7 and 8 for OI and NR. The results were exhibited in Figs. 7 and 8. After calculation according to Benesi-Hildebrand equation, the association constants and correlation coefficients were summarized in Table 2. These results suggested the 1:1 host–guest complexes for compounds 7 and 8 with dyes. Moreover, the K s values for compound 7 and 8 with dyes showed similar results to the UV titration spectra i.e. the K s values for compound 8 were at 105, which were higher than that for compound 7 (order of magnitude at 104). Some slight deviation for two sets of values in UV spectra and fluorescence might be attributed to the operating skills and the stability of apparatus. The highest association constant of compound 8 with OI was as high as 5.51 × 105, which was also in accordance with the UV titration results approximately.
Furthermore, the ESI–MS spectrum was used to investigate the complexation behavior of compound 8 before and after with excess Neutral red (mol ratio = 1:4) in DMSO solution. The ESI–MS spectrum after complexation was exhibited in Fig. 9. Two peaks were observed for compound 8 with Na+ (MNa+) at 3304.3 and the ion of the complex formed between compound 8 and Neutral red (M+NR+) at 3557.4, respectively. It was interesting that although four times of neutral red was added, only the 1:1 complexation peak appeared and the relative abundance attained 100. This result indicated that the strong complexation action existed between host and guest, and preferred to 1:1 binding ratio for complexation, which was also in agreement with the result of UV–Vis spectra. In a word, all the complexation experiments suggested that compounds 7 and 8 exhibited excellent complexation abilities for dyes with 1:1 stoichiometric ratio in DMSO solution.
Conclusions
In conclusion, the novel β-cyclodextrin-[60]fullerene conjugate 7 and bis-β-cyclodextrin-[60]fullerene conjugate 8 were synthesized by reacting the β-cyclodextrin aromatic aldehyde derivatives 5 and 6 with C60 via the Huisgen [2+3] cycloaddition for the first time. Their structures were confirmed by elemental analysis, FT-IR, ESI–MS and NMR spectra. The UV–Vis and fluorescence complexation experiments for Orange I and Neutral red indicated that compound 7 showed similar complexation abilities to its precursor 5 but bis-β-CD derivative 8 exhibited far better complexation abilities than its precursor 6. The highest association constant of compound 8 with OI was as high as 6.97 × 105. Both UV–Vis titration spectroscopy, fluorescence titration spectra and complexation ESI–MS spectrum indicated 1:1 complexes in DMSO solution. The excellent dyes complexation abilities could be attributed to the cooperative binding action of β-CD units and C60 unit, which supplied a new idea for design and synthesis of cyclodextrin derivatives with effective complexation abilities for dyes.
References
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Villalonga, R., Cao, R., Fragoso, A.: Supramolecular chemistry of cyclodextrins in enzyme technology. Chem. Rev. 107, 3088–3116 (2007)
Zhou, Y.H., Zhao, M., Mao, Z.W., Ji, L.N.: Ester hydrolysis by a cyclodextrin dimer catalyst with a metallophenanthroline linking group. Chem. Eur. J. 14, 7193–7201 (2008)
Zhang, L., Wu, Y., Brunsveld, L.: A synthetic supramolecular construct modulating protein assembly in cells. Angew. Chem. Int. Ed. 46, 1798–1802 (2007)
Mura, P.: Analytical techniques for characterization of cyclodextrin complexes in aqueous soluteon: a review. J. Pharm. Biomed. Anal. 101, 238–250 (2014)
Sun, T., Guo, Q., Zhang, C., Hao, J., Xing, P., Su, J., Li, S., Hao, A., Liu, G.: Self-assembled vesicles prepared from amphiphilic cyclodextrins as drug carriers. Langmuir 28, 8625–8636 (2012)
Ma, M.F., Guan, Y., Zhang, C., Hao, J.C., Xing, P.Y., Su, J., Li, S.Y., Chu, X.X., Hao, A.Y.: Stimulus-responsive supramolecular vesicles with effective anticancer activity prepared by cyclodextrin and ftorafur. Colloids Surf. A 454, 38–45 (2014)
Shaikh, M., Swamy, Y.M., Pal, H.: Supramolecular host–guest interaction of acridine dye with cyclodextrin macrocycles: photophysical, pKa shift and quenching study. J. Photochem. Photobiol. A 285, 41–50 (2013)
Liu, Y., Kang, S.Z., Zhang, H.Y.: Synthesis of cyclodextrin derivative bearing a cyclohexylamino moiety and its inclusion complexation with organic dye molecules. Microchem. J. 70, 115–121 (2001)
Arun, K.T., Jayaram, D.T., Avirah, R.R., Ramaiah, D.: β-Cyclodextrin as a photosensitizer carrier: effect on photophysical properties and chemical reactivity of squaraine dyes. J. Phys. Chem. B 115, 7122–7128 (2011)
Liu, Y., Chen, Y.: Cooperative binding and multiple recognition by bridged bis(β-cyclodextrin)s with functional linkers. Acc. Chem. Res. 39, 681–699 (2006)
Liu, Y., Li, B., You, C.C., Wada, T., Inoue, Y.: Molecular recognition studies on supramolecular systems. 32. Molecular recognition of dyes by organoselenium-bridged bis(β-cyclodextrin)s. J. Org. Chem. 66, 225–232 (2001)
Ikeda, A., Ishikawa, M., Aono, R., Kikuchi, J., Akiyama, M., Shinoda, W.: Regioselective recognition of a [60]fullerene-bisadduct by cyclodextrin. J. Org. Chem. 78, 2534–2541 (2013)
Gangadhar, T., Bhoi, V.I., Kumar, B.S., Murthy, C.N.: Supramolecular self-assembly and nanoencapsulation of [60]fullerene by bis-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 79, 215–223 (2014)
Bhoi, V.I., Kumar, S., Murthy, C.N.: The self-assembly and aqueous solubilization of [60]fullerene with disaccharides. Carbohydr. Res. 359, 120–127 (2012)
Bhoi, V.I., Murthy, C.N.: Aqueous solubilization of [60]Fullerene by selectively modified cyclodextrin. Fuller. Nanotubes. Carbon Nanostruct. 19, 668–676 (2011)
Wang, J., Zhang, Z., Wu, W., Jiang, X.: Synthesis of β-cyclodextrin-[60]fullerene conjugate and Its DNA cleavage performance. Chin. J. Chem. 32, 78–84 (2014)
Rather, J.A., Debnath, P., Wael, K.D.: Fullerene-β-cyclodextrin conjugate based electrochemical sensing device for ultrasensitive detection of p-nitrophenol. Electroanalysis 25, 2145–2150 (2013)
Giacalone, F., D’Anna, F., Giacalone, R., Gruttadauria, M., Noto, R.: Cyclodextrin-[60]fullerene conjugates: synthesis, characterization, and electrochemical behavior. Tetrahedron Lett. 47, 8105–8108 (2006)
Xiao, S., Wang, Q., Yu, F., Peng, Y., Yang, M., Sollogoub, M., Sinaÿ, P., Zhang, Y., Zahng, L., Zhou, D.: Conjugation of cyclodextrin with fullerene as a new class of HCV entry inhibitors. Bioorg. Med. Chem. 20, 5616–5622 (2012)
Zhang, Y.M., Chen, Y., Yang, Y., Liu, P., Liu, Y.: Supramolecular architectures by fullerene-bridged bis(permethyl-bcyclodextrin)s with porphyrins. Chem. Eur. J. 15, 11333–11340 (2009)
Guo, H.Y., Yang, F.F., Zhang, Y.M., Di, X.D.: Facile synthesis of mono- and polytopic β-cyclodextrin aromatic aldehydes by click chemistry. Synth. Commun. 3, 338–347 (2015)
Yang, F.F., Hong, B.Q., Chai, X.F., Yin, F.J., Chen, Y.Y.: Excellent Ag+ selective receptors: syntheses and complexation properties of novel biscalix[4]arene with benzalazine groups. Supramol. Chem. 21, 691–698 (2009)
Connors, K.A.: Binding Constants. Wiley, New York (1987)
Mourer, M., Hapiot, F., Tilloy, S., Monflier, E., Menuel, S.: Easily accessible mono- and polytopic beta-cyclodextrin hosts by click chemistry. Eur. J. Org. Chem. 32, 5723–5730 (2008)
Mourer, M., Hapiot, F., Monflier, E.: Click chemistry as an efficient tool to access beta-cyclodextrin dimers. Tetrahedron 64, 7159–7163 (2008)
Leung, D.K., Atkins, J.H., Breslow, R.: Synthesis and binding properties of cyclodextrin trimers. Tetrahedron Lett. 42, 6255–6258 (2001)
Bushby, R., Hamley, I., Liu, Q., Lozman, O., Lydon, J.: Self-assembled columns of fullerene. J. Mater. Chem. 15, 4429–4435 (2005)
Di, X.D., Yang, F.F., Ye, J.Q., Guo, H.Y., Yan, X.Y.: Design, synthesis and properties of novel benzo-15-crown-5/C60 dyads by 1,3-dipolar cycloaddition. Chem. Res. Chin. Univ. 30, 242–244 (2014)
Acknowledgments
This work was supported by National Natural Science Foundation of China (No: 21406036), Fujian Natural Science Foundation of China (No. 2014J01034), Fujian province science and technology key project (2014N0025) and the Program for Innovative Research Team in Science and Technology in Fujian Province University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, H., Fang, X., Yang, F. et al. Novel β-cyclodextrin-[60]fullerene conjugates based on Huisgen [2+3] cycloaddition: synthesis and dyes complexation properties. J Incl Phenom Macrocycl Chem 84, 79–86 (2016). https://doi.org/10.1007/s10847-015-0585-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0585-9