Abstract
Long-term and widespread monitoring programs are essential to understanding the role of human-dominated landscapes in supporting wild bee populations. Urbanization results in increased impervious surfaces throughout the landscape, fragmentation of green space, and a loss of naturally occurring floral vegetation. All of these changes have a negative impact on pollinator diversity. The objective of this study was to assess the abundance and richness of wild bee species throughout a small city in northwest Pennsylvania and identify how management of land throughout the city may influence bee communities. Seventeen sites across a land use gradient, moving from areas with large open spaces and mainly permeable surfaces, to sites in the city center consisting of mainly impermeable surfaces, were sampled over a 2-year period. During this time, 106 known species were identified with four state records and 1 undescribed species. Bee species richness was greatest at sites with the largest amount of permeable surface and naturally-occurring, native vegetation. Richness decreased on the college campus and city center where landscapes were highly managed and impermeable surfaces were most abundant. While floral richness was not related to bee abundance and richness, the number of open blooms near traps did have a positive impact on bee species richness. Overall, this survey revealed considerable richness never before recorded for northwest Pennsylvania, suggesting the importance of conservation management in homeowner and community yard space.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Declines in Apis mellifera (the Western honeybee) amounting to an estimated 59% loss of colonies from 1947 to 2005 (NASS; Potts et al. 2010) have catalyzed research interest in wild bee populations. Wild bees, here understood as unmanaged bees, are represented by nearly 4000 species native to North America and 20,000 species globally (Mader et al. 2011). The contribution of these species to pollination requires further research and is likely far greater than previously recognized (Klein et al. 2007). Yet, like A. mellifera, populations of wild bees too are in decline, with models estimating a 23% loss in wild bee pollinators in the United States from 2008 to 2013 (Koh et al. 2015; Potts et al. 2010). Wild bee declines are attributed to a number of interactive factors including climate change; introduction of exotic, alien species of plants, pathogens, and pests; loss of forage; and land-use change including habitat loss and subsequent fragmentation from agricultural intensification and urbanization (Ellis et al. 2010; Goulson et al. 2015; Potts et al. 2010). Efforts focused on populations within intensified agricultural settings seek to quantify the economic value of wild bees in commercial pollination (Klein et al. 2007; Kremen et al. 2002; Losey and Vaughan 2006) and to determine ways in which wild bees can be utilized to bolster honeybee services in these areas (Garibaldi et al. 2013; Winfree et al. 2007). Additionally, there is also growing interest in how wild bee communities are altered within intensified urban settings, as human populations continue to skyrocket and urban areas expand (UN World Urbanization Prospects). In the United States, for example, 82% of residents live in urban areas, and this number is expected to grow (US Census Bureau, n.d.).
Urbanization is closely associated with habitat destruction, one of the leading causes of general species extinction (Czech et al. 2000; Pimm and Raven 2000). As the natural landscape is appropriated for urban development, expanses of impervious surfaces, such as roads, sidewalks, parking lots, and residential, commercial, and industrial buildings, replace the natural or semi-natural landscape and fragment what remains. Moreover, urbanization also disturbs natural vegetation by replacing native vegetation with exotic and ornamental flora in urban green spaces such as gardens and lawns (Smith et al. 2005). Together, these alterations of the landscape negatively affect bee abundance and richness (Potts et al. 2010). In an assessment of pollinator assemblages including bees and hoverflies on an urban–rural land-use gradient in the UK, Bates et al. (2011) found a decrease in species richness and abundance towards the urbanized landscape. Since its publication, others have observed a similar trend in various urban settings (Fortel et al. 2014; Geslin et al. 2016). In an analysis of 23 different studies, Ricketts et al. (2008) observed a general trend correlating declines in wild bee richness with distance and isolation from habitat. Winfree et al. (2009) in an analysis of 54 studies found that anthropogenic disturbance had a significant negative effect on unmanaged bee abundance and richness. Likewise, in a global assessment of 284 publications, Newbold et al. (2015) found that land-use change negatively affected bee diversity and abundance to varying degrees. Collectively, these findings corroborate concerns that wild bee abundance and diversity declines with habitat destruction and conversion (Potts et al. 2010).
Wild bees are particularly susceptible to habitat and land use change because they are dependent on nest substrate and proximal forage availability. O’Toole and Raw (2004) categorize bees into three main nesting guilds: belowground nesters, aboveground nesters, and cleptoparasites, bees that lay eggs in the nests of other bees (Fortel et al. 2016; Potts et al. 2003). The majority of wild bees are belowground nesters, which build their nests by excavating tunnels in the soil or occupying pre-existing soil cavities (e.g. abandoned rodent dens). Aboveground nesters, nest in cavities or structures above the soil, including cavities in rocks and wood, pithy plant stems, and abandoned tunnels and cavities created by other insects. With the exception of most bumblebees, 90% of wild bees are solitary within the spectrum of social behavior (Mader et al. 2011), and typical forage ranges usually remain within approximately 500 m of the nest site, although some estimates suggest that it can be greater (Gathmann and Tscharntke 2002; Greenleaf et al. 2007; Zurbuchen et al. 2010). When bees were sampled from urban parks and gardens in New York City, the majority of individuals collected were cavity-nesters while less than 25% of individuals were soil nesters (Matteson et al. 2008), contrasting comparative samples from rural, un-urbanized areas where soil-nesters predominated (Giles and Ascher 2006). These findings are attributed to the increased nesting site availability for cavity-nesters in urban areas, such as trees, wood piles, sheds, homes, and fences, coupled with a degradation of adequate soil substrate, through compaction and frequent disturbance from activities like mowing and traffic (Cane et al. 2006; Matteson et al. 2008).
Urbanization also contributes to local declines in pollinators by limiting floral resources (Winfree et al. 2011). Several studies have demonstrated that bee abundance is associated with origin of floral resources (i.e. native or non-native), with increased bee abundance where native vegetation is the predominant resource (Frankie et al. 2005; Heinrich 1975; Pardee and Philpott 2014). Native vegetation is also positively associated with the presence of native pollinators throughout an ecosystem (Frankie et al. 2005). Floral specialists, specifically, can be negatively impacted by the lack of native vegetation in urban environments, which have been replaced with non-native, ornamental species (Frankie et al. 2005, 2009; Hernandez et al. 2009). Together, the solitary lifestyle of most wild bees in conjunction with their limited forage ranges tie wild bees to their nesting sites and make them dependent on proximal forage availability.
The impact of land-use on the abundance and richness of bee communities is often captured by contrasting communities in adjacent rural or suburban areas at the landscape scale (Cane et al. 2006; Matteson et al. 2008) or across the landscape scale using the ratio of impervious and pervious surfaces as a proxy for urbanization (Fortel et al. 2014; Geslin et al. 2016). Within the small city of Meadville, Pennsylvania, four distinct management regimes exist making this an interesting case study for how wild bee communities vary across land use. The objectives of this study are to evaluate bee species abundance and richness across an urban–rural gradient (Meadville, Pennsylvania, Pop. 13,265, US Census data) from more natural areas dominated by native flora and pervious surfaces, to a city center where ornamental plantings and impervious surfaces are much more common. While the scale of this study is significantly smaller than previous studies identifying how land use impacts bee communities, (Ahrné et al. 2009; Banaszak-Cibicka and Zminorski 2012; Cane et al. 2006; Fortel et al. 2014, 2016; Matteson et al. 2008) the data provides an important understanding of how various land use in small towns may impact wild bees.
Methods and materials
Study sites
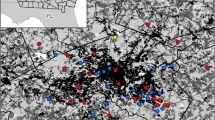
The study was conducted at 17 sites located across Meadville, Pennsylvania (41.6414°N 80.1514°W), a small city in Crawford County. Sites were placed at least 100 m apart and ran north to south across the study area in volunteered spaces. The entire study area spanned approximately 2.5 km, measured from the farthest two study sites. Sites were clustered into four distinct groups (fields, house yards, campus, and city center) based on qualitative assessment and quantitative metrics (Fig. 1). Qualitative assessment included geographic location and surrounding landscape descriptions while quantitative assessment employed GIS to evaluate land use cover at each of the site groupings.
Study region. a Individual sampling sites grouped together to form four discrete clusters: (a) fields, (b) house yards, (c) campus, (d) city center (moving from the top of the map to the bottom). b Land use assignments in sampling groups displaying impervious surfaces (red—roads and sidewalks, yellow—buildings) and impervious surfaces (dark green—trees and forests, light green—grass, dirt, and mulch). Scale is in km. (Color figure online)
Site descriptions and groupings
The sites hosted various habitat features, including flower beds, vegetable gardens, hedges, trees, and open lawn with varying degrees of shade. They were grouped by their land use characteristics and geographic location. Farthest north in the study region, four sites were clustered together to form the fields group (0.33 km2). These sites were located at Robertson Athletic complex, an Allegheny College owned plot of land that houses various athletic fields, including baseball diamonds, soccer and practice fields, and a synthetic turf football field. Sports fields are surrounded on all sides by a buffer of grass, which borders a temperate deciduous forest largely composed of red maple, sugar maple, red oak, ash, and beech (Rich Bowden, personal communication). Sampling arrays at these sites were placed in these grassy transitional spaces adjacent to the forests, which are managed minimally during the summer months. The second group, house yards (0.28 km2), located slightly southeast of the fields group, was situated in a residential area. The four sites in this cluster were placed in either the front or back yards of privately owned single-family housing units. Sampling arrays were placed along the borders of the yards to avoid disturbance. The third group, campus (0.32 km2), included four sites placed on the campus of Allegheny College and one site located on a college-owned house located in a residential area south of the main campus. These sites were distributed around flowerbeds and vegetable gardens. Finally, the southernmost site grouping consisted of four sites located in the city center of Meadville (city center 0.30 km2). Sampling arrays were placed in yards and flowerbeds of businesses and public spaces out of the way of heavy foot traffic. None of the sites in this study were sprayed with pesticides or had a history of pesticide application, and no area was watered throughout the course of the study.
GIS analysis
GIS (acrGIS, version 10.4.1, Esri, Inc.) was used to quantify the ratio of impervious surfaces to pervious surfaces (Geslin et al. 2016). Using GPS, the exact location of each site was recorded and superimposed on satellite and aerial images of the study region. To delineate a border around each group, individual sites in the groups served as the vertices of the largest polygon that could be drawn using the sites in each group. The polygon was buffered at 100 m in which land use was digitized at a scale of 1:1000 (Fig. 1a). Remote sensing was used to categorize the land use into one of four land use types: impervious—building rooftops, impervious—roads and sidewalks, pervious—trees and forest, or pervious—grass, mulch, or dirt (Fig. 1b). These land use assignments were used to calculate the relative proportion of impervious and pervious surfaces that characterized each of the site groupings. In 2016, one of the campus sites was moved approximately 15 m; all land use analyses are based on the 2015 location, and no other sites were altered in placement from year to year.
Bee sampling methods
Bees were collected over two years during the months of June, July, and August in 2015 and May, June, July, August, and September in 2016. Collection methods included vane traps and bee bowls. A single blue vane trap containing 70% ethanol was suspended from a 1 m tall garden hook at the center of each sampling array. Bee bowls were placed within a 5 m radius from the vane trap. Bee bowls consisted of a small plastic bowl (3.25 oz, SOLO™, Urbana, IL) painted with fluorescent white or yellow paint (Sargent Art®, Hazleton, PA), filled with water containing a few drops of unscented dish soap (Dawn®, Cincinnati, OH). Bee bowls were affixed to wooden stakes and hammered into the ground at the bloom height of surrounding flowers, which varied by site and date. Two yellow and two white bee bowls were placed at each site (Leong and Thorp 1999). The traps were retrieved after 48 h.
In the lab, sample contents were poured through a mesh sieve, washed gently with soapy water (ECOS®, Addison, IL), rinsed with deionized water, and transferred to 70% ethanol. Bees were then sorted from non-bees (Triplehorn and Johnson 2005), those smaller than 5 mm in length from head to abdomen were disregarded, and remaining bees were dried (Metropolitan Vacuum Cleaner Co, Inc., Oakland, NJ), and pinned. Bees were identified to genus using taxonomic keys (Arnett 2000; Ascher and Pickering 2012) by P. Hickman, E. Moretti, and K. Moyer and were then identified to species where possible by E. Moretti and P. Hickman using the online taxonomic key DiscoverLife (Ascher and Pickering 2012). Sam Droege of the USGS Bee Inventory and Monitoring Lab assisted with species identification and confirmed species decisions. Hylaeus modestus and H. affinis (Family Colletidae) observations were grouped together as one species (Hylaeus affinis/modestus) because the two cannot currently be resolved decisively (Lerman and Milam 2016). Likewise, several individuals of the Nomada sp. were categorized as N. bidentate grp. because the genus is currently undergoing taxonomic revisions (S. Droege, personal communication).
Following Fortel et al. (2014), Lerman and Milam (2016) and Matteson et al. (2008), species were compiled in a list with respect to ecological characteristics. Characteristics were compiled from existing species lists, primary literature, and DiscoverLife (discoverlife.org). Bees were classified based on origin (native or exotic to North America) following Giles and Ascher (2006), pollen specificity (generalist or specialist) and nesting behavior (soil, cavity, soft or rotting wood, or pithy stems). For clarity in analysis, nesting behavior was further simplified to aboveground or belowground following O’Toole and Raw (2004). Cleptoparasitic species were grouped according to the nesting behavior of the species being parasitized.
Floral sampling
During each sample, angiosperms currently in bloom were identified to species within a 5 m radius of the center of the sampling array using the Peterson Field Guide to Wild Flowers of Northeastern/North-central North America (Peterson and McKenny 1968). Recorded angiosperms were categorized as being native, established alien, or unnatural based on Peterson and McKenny (1968) and the USDA Plants Database (https://plants.usda.gov/java/). Native and established alien species were considered naturally occurring if they can exist in nature without anthropogenic involvement. Unnatural species refer to those that were planted and would not be present in the environment otherwise; such species include ornamental hybrid plant varieties and garden herb cultivars. To determine if floral composition influenced bee abundance and richness, sites were categorized as predominantly natural or unnatural based on percentages of natural and unnatural species in bloom, or no blooms if none were present at that site. The total number of open blooms were counted at each site and collection date, if a species had greater than 100 blooms then the number was estimated.
Data analysis
To determine if our sampling efforts provided a sufficient measurement of species richness, a sample-based rarefaction curve was calculated (Gotelli and Colwell 2001) using the software EstimateS (Colwell 2013). Samples from all sites and years were randomized without replacement and the cumulative number of different species tabulated. This procedure was repeated for 100 randomizations. The software provided estimates of rarefied species richness (SMaoTao), which is the expected species accumulation curve.
Bee abundance was calculated as the total number of individual bees, and species richness was measured as the total number of species collected. “Singletons” were identified as species represented by only a single individual (Hinners et al. 2012). Individuals not identified to species were not included in richness analyses but were included in total abundance. For analysis of richness and abundance across the study region and analysis of nesting behavior, managed bees (i.e. Apis mellifera) were not included. To determine if mean bee abundance and richness, varied by year, site grouping, or the interaction (site grouping × year), 2-way ANOVAS were employed (JMP®, SAS Institute Inc., Version 12.0.1, 2015); p ≤ 0.05 was considered significant. Tukey’s HSD tests were used to separate means between sites and site groupings.
For floral analysis, the bee species community was assessed by calculating the proportion of natives/exotics and pollen specialists/generalists at each site grouping. Linear regression was used to evaluate relationships between bee abundance and richness and floral richness. One-way ANOVAs were conducted to determine the variation in bee abundance and richness as influenced by bloom abundance and floral species composition (JMP®, SAS Institute Inc., Version 12.0.1, 2015); p ≤ 0.05 was considered significant. Tukey’s HSD tests were used to separate means between sites and site groupings.
Results
Bee fauna
Over the two sampling years, 1603 individuals were collected (350 bee specimens in 2015 and 1253 specimens from 2016; Table 1). The collection represented five families (Andrenidae, Apidae, Colletidae, Halictidae, and Megachilidae), 24 genera, and 106 known species. Halictids made up the majority of the collection, representing 1094 (68%) of the individuals collected and 38 (36%) species. This was followed by bees of the species Apidae, which made up 298 (19%) individuals and 25 (24%) species. Over the course of the study, the only managed bees (not considered wild) collected were Apis mellifera, which constituted 41 individuals (2.6% of the total collection). Fifty-three of the individuals collected could not be identified to species; however, nine genera were represented. The unknowns also included one Lasioglossum undescribed in the literature (S. Droege, personal communication). The majority of species collected (84%) were represented by fewer than 16 individuals and thus each constituted < 1% of the sample (Fig. 2). “Singletons,” made up 38% of the species collected. The species accumulation curve did not reach an asymptote indicating that all species in the environment were not collected (Fig. 3). The Chao2 species richness estimator predicts that 152 species are actually present in the sampled area, suggesting that 71% of the species were observed in the study.
The five most common bee species collected were L. ephialtum (17% of total), L. hitchensi (7%), Augochlora pura (6.6%), L. zonulum (5.6%), and Agapostemon virescens (4.4%). Lasioglossum ephialtum and L. zonulum were the only species found at all of the collection sites. The collection also included four state records for Pennsylvania, confirmed by S. Droege and J. Gibbs, Hylaeus pictipes, Lasioglossum michiganenense, Lasioglossum smilacinae and Chelostoma rapunculi.
Variation by site categories
Quantitative analysis of pervious to impervious surfaces demonstrate the transition from majority pervious surfaces found at the fields group (88% pervious, 12% impervious) to majority impervious surfaces found in the city center (15% pervious, 85% impervious) moving north to south across the study region (Fig. 4). The house yards group was also dominated by pervious surfaces (68% pervious, 32% impervious); while the campus group was almost equally pervious (47%) and impervious (53%). At each site, the majority of impervious surfaces were sidewalks and roads (Table 2). In the field sites and campus sites, tree/forests made up the majority of pervious surfaces, while in the house yards and city center, pervious surfaces were evenly split between trees/forests and grass, mulch, and dirt (Table 2).
Abundance
Excluding Apis mellifera, 549 individuals were collected from the fields sites (35% of all individuals collected), 332 from the house yards (21%), 320 from campus (20%), and 361 from city center (23%). A significantly greater number of bees were collected in 2016 than 2015 (F = 11.73, df = 1, p = 0.0008; Fig. 5). Abundance did not vary significantly between site groupings (F = 2.33, df = 3, p = 0.08) or site grouping*year (F = 0.70, df = 3, p = 0.55).
Mean abundance of wild bees at site groupings. Mean abundance of bees was calculated as the average number of individuals collected at each site during each sampling date per group (fields, house yards, and city center: n = 32; campus: n = 40; error bars = SEM). Average abundance differed by year (p = 0.0008) but not by sampling group
Species richness
A total of 106 species were collected during the 2-year survey. Seventy-one species were collected at the field sites, 51 species at the house yards, 66 at campus sites, and 53 at city center sites. Mean species richness per site per sampling date at the site groupings differed between years (F = 8.82, df = 1, p = 0.004) and site groupings (F = 4.39, df = 3, p = 0.006), with no year by site grouping interaction (Fig. 6). Fields sites had the greatest mean species richness per site per sampling date (7.8 ± 0.8) with campus (4.7 ± 0.5) and city center sites (4.8 ± 0.7; Fig. 6) having significantly fewer species.
Mean species richness. Mean species richness calculated as the average number of species collected at each site in each sampling month per group (fields, house yards, and city center: n = 32; campus: n = 40; error bars = SEM). Average richness differed significantly by group (p = 0.0056). Differences determined via Tukey HSD
Nesting behavior
Of the 1539 individuals identified to species that had a determined preferential nest substrate, 399 (26%) were aboveground nesters (31 species) and 1140 (74%) were belowground nesters (71 species) (Table 1). Average abundance of belowground nesters per site per sampling date did not differ between the site groupings (Fig. 7a), but richness did vary significantly (F = 4.27, df = 3, p = 0.007; Fig. 7b). Field sites had significantly greater mean richness of belowground nesters per site per sampling date (5.5 ± 0.5) than sites in the house yards (3.7 ± 0.6), campus (3.5 ± 0.4), and city center groups (3.3 ± 0.5). In total, 47 spp. of belowground nesters were collected from sites in the fields group, 35 spp. from the house yards, 41 spp. from campus, and 33 spp. from city center. Of the species collected from the house yards, campus, and city center, 80, 70, and 72% of species, respectively were also present in the fields. There were 10 spp. of belowground nesters only found at the fields sites, in contrast to 2 found exclusively at the house yards, 6 found only at campus sites, and 4 exclusive to the city center. Aboveground nester abundance (F = 3.72, df = 3, p = 0.013, Fig. 7a) and richness (F = 2.66, df = 3, p = 0.050, Fig. 7b) did differ across the sites. Both abundance and richness of aboveground nesters differed significantly between field sites and campus (Fig. 7a, b). Of species collected at campus sites 61% were also identified in the field sites.
a Mean abundance and b richness of belowground and aboveground nesters. Means calculated as the average number (a) and number of species (b) of belowground nesters and aboveground nesters, respectively, collected at each site in each sampling month per group (fields, house yards, and city center: n = 32; campus: n = 40; error bars = SEM). Mean abundance differed significantly across site groupings for aboveground nesters (p = 0.0132) but not for belowground nesters
Floral resources and bee fauna
Linear regression analyses did not indicate strong positive relationships between floral species richness and bee species richness (r2 = 0.031) or abundance (r2 = 0.020). Mean bee richness was significantly greater when > 100 open blooms were counted at the site (F = 5.80; df = 2; p = 0.004); however, the same pattern was not observed for bee abundance (F = 1.73; df = 2; p = 0.18). When sites were categorized based on the percentage of vegetation that was natural, non-natural or had no blooms, there was no significant difference in the abundance of native bee species (F = 0.91; df = 2; p = 0.41) or exotic bee species (F = 1.13; df = 2; p = 0.33), or the richness of native species (F = 0.55; df = 2; p = 0.58) or exotic species (F = 0.65; df = 2; p = 0.53) between these categories.
The most frequently collected genus, Lasioglossum spp., consists primarily of pollen generalists (Table 1). Ninety-one percent of species collected were pollen generalists and 9% pollen specialists. Eighty-nine percent of species collected were native, and 11% exotic. Field sites were mainly composed of native species (92%) where naturally occurring flora was most abundant. All other site groupings were composed of fewer native plant species with 82% of the vegetation in house yards being native, 84% on campus, and 87% in city center. Communities of bees in house yards had the greatest proportion of exotic species (18%) when compared to the bee communities in fields (8%), on campus (16%) and in city center (13%). Bee communities at the field sites had the greatest proportion of floral specialists (11%), which was not surprising considering that the host plants of these species, i.e. asters and goldenrod, occurred more frequently at those sites. Pollen-generalists comprised the majority of the wild bee community trapped in the city center (98%) with only 2% of that community deemed pollen-specialists. The most common pollen-specialist was Peponapis pruinosa (Say, 1837), which was most frequently collected on campus likely within range of gardens containing cucurbits, its host plant group.
Discussion
Abundance and richness
The most recent assessment of bees in PA suggests 371 known species recorded in the state and 60 species recorded for Crawford County (Donovall and vanEngelsdorp 2010). This current survey reports 106 species within a single town in Crawford County, demonstrating an important assessment of local bee fauna that will contribute to ongoing monitoring efforts in the US. Continued sampling throughout this area and increases in the sampling area are needed to appropriately estimate diversity.
Abundance is notoriously challenging to predict and is commonly variable in surveys of bees (Hinners et al. 2012; Williams et al. 2001). Bee abundance was significantly greater in 2016 than 2015. There was no dramatic difference in the average temperature or precipitation between the two sampling seasons. Cane and Payne (1993) posited that year-to-year variation could be impacted by climatic effects, such as winter severity, as well as nesting and forage success of the prior year, all of which influence brood survivorship. Potts et al. (2003) also observed a greater correlation between bee abundance and the floral resources available the year prior to sampling. The winter prior to summer 2016 was milder than the winter before summer 2015, which may account for the variation in abundance by year (nrcc.cornell.edu). Other studies have suggested that floral availability the year before may be an indicator of bee abundance (Cane and Payne 1993; Tepedino and Stanton 1981); however, we are unable to determine if that is the case in this study. Bee abundance in general fluctuates annually, regionally, and locally (Cane and Payne 1993) with bees emerging at different times of the year depending on their forage needs and overwintering habits (Wilson and Carill 2016). Likewise, throughout the summer months, species vary in when they are most active. As an example, in this collection Nomada were collected exclusively in the months of May and June. Bees of this genus are restricted to only a few months of early to late spring activity in contrast to others, like those of the genus Bombus, which are active throughout early spring into late summer (Wilson and Carill 2016).
Differences in species richness were identified across the study region. Mean richness was greater within the fields group than on campus and in the city center. In addition to having the greatest species richness, the fields group had the most species unique to those sites (16), compared with campus (9), house yards (5) and city center (4). Richness estimates suggest that additional species are likely present throughout the sampled area. Additionally, Bombus griseocollis, a species common to the area (S. Droege, personal communication), was absent from the collection both sampling years. Pan-trapping methods disproportionally attract Halictid bees (Cane et al. 2000), the most abundant group captured in the bee bowls, while under-collecting honey bees, Colletes, and bumble bees (Roulston et al. 2007). For future studies, sampling at floral resources using hand netting would be useful to capture additional species, specifically pollen-specialists (Roulston et al. 2007; Stephen and Rao 2005; Westphal et al. 2008; Williams et al. 2001; Wilson et al. 2008).
Site groupings and nesting behavior
Several studies throughout the literature report a decrease in the abundance of belowground nesters and an increase in the number of aboveground nesters relative to the amount of impervious surfaces (Cane et al. 2006; Geslin et al. 2016; Matteson et al. 2008; Winfree et al. 2009); however, this pattern was not observed in our study. The close proximity of our sites is likely to fault for the inability to observe this pattern. Wild bees forage at around 500 m away from their nesting sites (Gathmann and Tscharntke 2002), so the locations of the collection sites where bees were captured while foraging may not necessarily reflect their nesting locations. Nevertheless, it remained important to evaluate this factor in relation to the land use groupings, as it is evaluated throughout the literature. Matteson et al. (2008) and Cane et al. (2006) suggested that landscapes dominated by impervious surfaces and structures, such as sheds, homes, fences, etc., and degradation of soil quantity and quality would provide nesting sites for cavity nesters. The field sites had the least amount of impervious surface and greater richness of belowground nesters. Aboveground nester abundance and richness was significantly greater at field sites than campus sites, but did not differ significantly from other site groupings. The decrease in aboveground nesters on campus may have been the result of management to remove potential substrates for aboveground nesters, such as pithy stems and rotting wood.
The need for further analysis of local features is best demonstrated by the field sites, which hosted the greatest richness of both aboveground and belowground nesters. This site was characterized by pervious surfaces (88%), including a majority of continuous forested areas (52.1%), as well as naturally-occurring vegetation and very little human management. Pardee and Philpott (2014) observed that bee abundance of all species, including belowground nesters, was positively correlated with woody vegetation. Woody vegetation is an excellent source of nesting substrate for aboveground nesters, which preference hollow and pithy stems as well as rotting wood with preexisting cavities. However, woody vegetation and herbaceous cover also imply a decrease in grass cover, which inhibits belowground nesters who preference mulch and open dirt substrates for nesting (Mader et al. 2011). For this reason, forest cover can also promote belowground nesting bees.
Floral resources and bee fauna
This survey did not reveal a strong correlation between floral species richness and bee richness and abundance. It is likely that the 5 m radius used for sampling flora was not great enough to provide a complete assessment of the floral resources available in these spatially heterogeneous sampling regions. The study did reveal an association between greater bee richness and greater bloom presence. Sites varied in the type of flora available to foraging bees, with naturally-occurring angiosperms being most common at the field sites. Bees collected from the field sites were represented by the greatest proportion of floral specialists compared to the other site groups, which was not surprising considering that the host plants of these species, including asters and goldenrod, occurred more frequently at the field sites. A correlation between naturally-occurring angiosperms and increased bee species richness has been reported in the literature (Lerman and Milam 2016; Pardee and Philpott 2014).
Native bee species composed the greatest proportion of the community at the field sites where native, naturally occurring vegetation was most common in the landscape. This finding supports the commonly observed pattern that native flora attracts native pollinators (Frankie et al. 2005; Heinrich 1975). Native bee species comprised most of the bee communities at all site groupings with exotic bees comprising 18% of the community at house yards, 16% of the community on campus, 13% in city center, and 8% at the fields. Several studies report a greater abundance of exotic bees in urbanized landscapes (Fetridge et al. 2008; Giles and Ascher 2006; Matteson et al. 2008) possibly due to the increased presence of non-native and ornamental vegetation. Similarly, in this study, pollen-generalists constituted the greatest proportion of bees collected in city center sites. Pollen-specialists are often found near their host plants (Cane and Payne 1993); however, for many specialists collected during this survey, the host species was not observed within a 5 m radius of the traps. For instance, cucurbits were not recorded at many of the sites were the most commonly collected floral specialist, Peponapis pruinosa, was collected. This may be the result of the sampling scheme, suggesting that the range of the floral assessments at each site was too limited (Minckley et al. 1999).
Conclusions
This survey of Meadville, PA bees suggests that human-dominated landscapes like small towns can host numerous and diverse bee species. The species richness and abundance seen across the study region demonstrates the importance and relevance of managing personal yards and municipal, communal green spaces for wild bees. While the scale of this study is significantly smaller than previous studies identifying the role of land management and impervious surfaces in structuring communities, it demonstrates the importance of various management throughout small towns in providing niches for a variety of bee species. These data provide a baseline for future studies of bee species richness throughout northwest Pennsylvania and provide an interesting view of bees in small towns, which are largely understudied.
References
Ahrné K, Bengtsson J, Elmqvist T (2009) Bumble bees (Bombus spp) along a gradient of increasing urbanization. PLoS ONE 4:e5574. https://doi.org/10.1371/journal.pone.0005574.t001
Arnett RH Jr (2000) American insects: a handbook of the insects of America north of Mexico, 2nd edn. CRC Press, Boca Raton
Ascher JS, Pickering J (2012) Discover Life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila). http://www.discoverlife.org. Accessed Dec 2016
Banaszak-Cibicka W, Zminorski M (2012) Wild bees along an urban gradient: winners and losers. J Insect Conserv 8:562–572. https://doi.org/10.1007/s10841-011-9419-2
Bates AJ, Sadler JP, Fairbrass AJ, Falk SJ, Hale JD, Matthews TJ (2011) Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE 6:e23459. https://doi.org/10.1371/journal.pone.0023459
Cane JH, Payne JA (1993) Regional, annual, and seasonal variation in pollinator guilds: intrinsic traits of bees (Hymenoptera: Apoidea) underlie their patterns of abundance at Vaccinium ashei (Ericaceae). Ann Entomol Soc Am 86(5):577–588. https://doi.org/10.1093/aesa/86.5.577
Cane JH, Minckley RL, Kervin LJ (2000) Sampling Bees (Hymenoptera: Apiformes) for pollinator community studies: pitfalls of pan-trapping. J Kans Entomol Soc 73(4):225–231
Cane JH, Minckley RL, Kervin LJ, Rouston TH, Williams NM (2006) Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol Appl 16(2):632–644
Colwell RK (2013) Estimate S: statistical estimation of species richness and shared species from samples. Version 9
Czech B, Krausman PR, Devers PK (2000) Economic associations among causes of species endangerment in the United States. Bioscience 50:593–601
Donovall LR, vanEngelsdorp D (2010) A checklist of the bees (Hymenoptera: Apoidea) of Pennsylvania. J Kans Entomol Soc 83(1):7–24. https://doi.org/10.2317/JKES808.29.1
Ellis JD, Evans JD, Pettis J (2010) Colony losses, managed colony population decline, and colony collapse disorder in the United States. J Apic Res 49(1):134–136. https://doi.org/10.3896/IBRA.1.49.1.30
Fetridge ED, Ashcher JS, Langellotto GA (2008) The bee fauna of residential gardens in a suburb of New York City (Hymenoptera: Apoidea). Ann Entomol Soc Am 101:1067–1077. https://doi.org/10.1603/0013-8746-101.6.1067
Fortel L, Henry M, Guilbaud L, Guirao AL, Kuhlmann M, Mouret H, Rollin O, Vaissiére BE (2014) Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS ONE 9(8):e104679. https://doi.org/10.1371/journal.pone.0104679
Fortel L, Guilbaud HM, Mauret H, Vaissiére BE (2016) Use of human-made nesting structures by wild bees in an urban environment. J Insect Conserv 20:239–253. https://doi.org/10.1007/s10841-016-9857-y
Frankie GW, Thorp RW, Schindler M, Hernandez J, Ertter B, Rizzardi M (2005) Ecological patterns of bees and their host ornamental flowers in two northern California cities. J Kans Entomol Soc 78:227–246
Frankie GW, Thorp RW, Hernandez J, Rizzardi M, Ertter B, Pawelek JC, Witt SL, Schindler M, Coville R, Wojcik VA (2009) Native bees are a rich natural resource in urban California gardens. Calif Agric 63:113–120. https://doi.org/10.3733/ca.v063n03p113
Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Klein AM (2013) Wild pollinators enhance fruit set of crops regardless of honeybee abundance. Science 339(6127):1608–1611. https://doi.org/10.1126/science.1230200
Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. J Anim Ecol 71(5):757–764. https://doi.org/10.1046/j.1365-2656.2002.00641.x
Geslin B, Le Féon V, Folschweiller M, Flacher F, Carmignac D, Motard E, Perret S, Dajoz I (2016) The proportion of impervious surfaces at the landscape scale structures wild bee assemblages in a densely populated region. Ecol Evol 6(18):6599–6615. https://doi.org/10.1002/ece3.2374
Giles V, Ascher JS (2006) A survey of the bees of the Black Rock Forest Preserve, New York (Hymenoptera: Apoidea). J Hymenopt Res 15:208–231
Gotelli N, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Goulson D, Nicholls E, Botías C, Rotneray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. https://doi.org/10.1126/science.1255957
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153:589–596. https://doi.org/10.1007/s00442-007-0752-9
Heinrich B (1975) Bee flowers: a hypothesis on flower variety and blooming times. Evolution 29:325–334
Hernandez JL, Frankie GW, Thorp RW (2009) Ecology of urban bees: a review of current knowledge and directions for future studies. Cities Environ 2:3
Hinners SJ, Kearns CA, Wessman CA (2012) Roles of scale, matrix, and native habitat in supporting a diverse suburban pollinator assemblage. Ecol Appl 22(7):1923–2935
Klein AM, Vaissiére BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc Lond B 274(1608):303–313. https://doi.org/10.1098/rspb.2006.3721
Koh I, Lonsdorf EV, Williams NM, Brittain C, Isaacs R, Gibbs J, Ricketts TH (2015) Modeling the status, trends, and impacts of wild bee abundance in the United States. PNAS 113(1):140–145. https://doi.org/10.1073/pnas.1517685113
Kremen C, Williams NM, Thorp RW (2002) Crop pollination from native bees at risk from agricultural intensification. PNAS 99(26):16812–16816. https://doi.org/10.1073/pnas.262413599
Leong JM, Thorp RW (1999) Colour-coded sampling: the pan trap colour preferences of oligolectic and nonoligolectic bees associated with a vernal pool plant. Ecol Entomol 24:329–335. https://doi.org/10.1046/j.1365-2311.1999.00196.x
Lerman SB, Milam J (2016) Bee fauna and floral abundance within lawn-dominated suburban yards in Springfield, MA. Ann Entomol Soc Am 109(5):713–723. https://doi.org/10.1093/aesa/saw043
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Bioscience 56:311–323
Mader E, Shepherd M, Vaughan M, Black SH, LeBuhn G (2011) The xerces society guide attracting native pollinators. Storey Publishing, North Adams
Matteson KC, Ascher JS, Langellotto GA (2008) Bee richness and abundance in New York City urban gardens. Ann Entomol Soc Am 101(1):140–150
Minckley RL, Cane JH, Kervin L, Roulston TH (1999) Spatial predictability and resource specialization of bees (Hymenoptera: Apoidea) at a superabundant, widespread resource. Biol J Linn Soc 67:119–147
Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko I, Senior RA, Kleyer M (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50. https://doi.org/10.1038/nature14324
O’Toole C, Raw A (2004) Bees of the world. Facts On File, New York
Pardee GL, Philpott SM (2014) Native plants are the bee’s knees: local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosyst 17:641–659. https://doi.org/10.1007/s11252-014-0349-0
Peterson RT, McKenny M (1968) A field guide to wildflowers: northeastern and north-central North America. Houghton Mifflin Harcourt, Boston
Pimm SL, Raven P (2000) Biodiversity: extinction by numbers. Nature 403:843–845. https://doi.org/10.1038/35002708
Potts SG, Vulliamy B, Dafni A, Ne’eman G, Willmer P (2003) Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84(10):2628–2642. https://doi.org/10.1890/02-0136
Potts SG, Beismeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts, and drivers. Trends Ecol Evolut 25(6):345–353. https://doi.org/10.1016/j.tree.2010.01.007
Ricketts TH, Regetz J, Steffan-Dewenter I, Cunningham SA, Kremen C, Bogdanski A, Gemmill-Herren B, Greenleaf SS, Klein AM, Mayfield MM, Morandin LA, Ochieng A, Vianna BF (2008) Landscape effects on crop pollination services: are there general patterns? Ecol Lett 11:499–515
Roulston TH, Smith SA, Brewster AL (2007) A comparison of pan trap and intensive net sampling techniques for documenting a bee (Hymenoptera: Apiformes) fauna. J Kans Entomol Soc 80:179–181
Smith RM, Thompson K, Hodgson JG, Warren PH, Gaston KJ (2005) Urban domestic gardens (IX): composition and richness of the vascular plant flora, and implications for native biodiversity. Biol Cons 129(3):312–322. https://doi.org/10.1016/j.biocon.2005.10.045
Stephen WP, Rao S (2005) Unscented color traps for non-Apis bees (Hymenoptera: Apiformes). J Kans Entomol Soc 78:373–380
Tepedino VJ, Stanton NL (1981) Diversity and competition in bee-plant communities on short-grass prairie. Oikos 36:35–44
Triplehorn CA, Johnson NF (2005) Borror and Delong’s introduction to the study of insects, 7th edn. Thomson Brooks, Belmont
US Census Bureau (n.d.) US and World Population Clock. http://www.census.gov/popclock/. Accessed Feb 2017
Westphal C, Bommarco R, Carré G, Lamborn E, Morison N, Petanidou T, Vaissière BE (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78:653–671. https://doi.org/10.1890/07-1292.1
Williams NM, Minckley RL, Silveira FA (2001) Variation in native bee faunas and its implications for detecting community changes. Conserv Ecol 5(1):7
Wilson JS, Carill OM (2016) The bees in your backyard: a guide to North America’s bees. Princeton University Press, New Jersey
Wilson JS, Griswold T, Messinger OJ (2008) Sampling bee communities (Hymenoptera: Apiformes) in a desert landscape: are pan traps sufficient? J Kans Entomol Soc 81(3):288–300. https://doi.org/10.2317/JKES-802.06.1
Winfree R, Williams NM, Dushoff J, Kremen C (2007) Native bees provide insurance against ongoing honey bee losses. Ecol Lett 10:1105–1113. https://doi.org/10.1111/j.1461-0248.2007.01110.x
Winfree R, Aguilar R, Vazquez DP, LeBuhn G, Aizen MA (2009) A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90(8):2068–2076. https://doi.org/10.1890/08-1245.1
Winfree R, Bartomeus I, Cariveau DP (2011) Native pollinator in anthropogenic habitats. Annu Rev Ecol Evol Syst 42:1–22. https://doi.org/10.1146/annurev-ecolsys-102710-145042
Zurbuchen A, Landert L, Kaiber J, Müller A, Hein S, Dorn S (2010) Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol Conserv 143:669–676. https://doi.org/10.1016/j.biocon.2009.12.003
Acknowledgements
The authors would like to thank Sam Droege of the USGS Bee Inventory and Monitoring Lab for invaluable assistance in bee identification and advice on methodology. Kaye Moyer for helping with the collection, processing and identification of bees during the second field season. Special thanks to all of the funding sources provided through Allegheny College: Class of 1939 Senior Research Fund, Edward David Class of 1961 Faculty Support Fund, Christine Scott Nelson Faculty Support Fund, McCune Foundation Charitable Trust, and Dr. Barbara Lotze Research Fellowship Fund.
Funding
This study was funded by contributions to Allegheny College’s undergraduate research fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Choate, B.A., Hickman, P.L. & Moretti, E.A. Wild bee species abundance and richness across an urban–rural gradient. J Insect Conserv 22, 391–403 (2018). https://doi.org/10.1007/s10841-018-0068-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-018-0068-6