Abstract
There is increasing concern over the ecological impact of markedly increasing numbers of large herbivores (hereafter large herbivore overabundance) on forest ecosystems. To predict the ecological consequences of large herbivore overabundance, it is first necessary to understand how biological communities respond to large herbivore overabundance. Here, we examined the relationships between the life history traits of five insect taxonomic groups (moths, dung beetles, longicorn beetles, carabid beetles, and carrion beetles) and their responses to deer overabundance in Hokkaido, northern Japan. Insects were collected from three study sites: enclosure (20 deer/km2), control (10 deer/km2), and exclosure (0 deer/km2). The different taxonomic and functional insect species differed in their response to deer overabundance. The abundance (number of individuals) of longicorn beetles, dung beetles, and arbor-feeding moths was higher in the enclosure site than in the control site, whereas that of carabid beetles, carrion beetles, and herb- or shrub-feeding moths was higher in the control site than in the enclosure site. These results suggest that the type of food and the level of dependence on the understory are key traits determining insect sensitivity to deer overabundance. In addition, large or flightless species responded negatively to deer overabundance. Overall, this study demonstrated a significant change in insect communities following experimental deer overabundance, suggesting that large herbivore overabundance leads to the homogenization of biological communities. Unfortunately, because insects have diverse functions in forest ecosystems, such marked changes in both abundance and composition of insect communities will decrease ecosystem functions and resilience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is increasing concern over the ecological impact of markedly increasing numbers of large herbivores (large herbivore overabundance) on forest ecosystems (Fuller and Gill 2001; Côté et al. 2004; Martin et al. 2010). Since the late twentieth century, large mammalian herbivores have become overabundant in the northern hemisphere, including North America, Europe, and East Asia, which is largely due to the development of agriculture and forestry, predator loss, and reduced hunting (Rooney 2001; Côté et al. 2004; Uno et al. 2009). Large herbivore overabundance affects understory and tree regeneration through browsing and bark stripping (Akashi and Nakashizuka 1999; Vera et al. 2006; Rooney 2009). Indeed, many studies have reported that over-browsing by large herbivores alters the composition and abundance of understory species (Rooney 2009; Suzuki et al. 2013). Bark stripping by large herbivores also damages small trees, which in turn enhances tree mortality rates (Akashi and Nakashizuka 1999; Vospernik 2006). Furthermore, animal species that depend on forest environments are also indirectly influenced by the overabundance of large herbivores (invertebrates: Allombert et al. 2005; Minoshima et al. 2013; Teichman et al. 2013, birds: Chollet and Martin 2013; Teichman et al. 2013).
To manage and conserve forest ecosystems and ultimately reverse the ecological consequence of large herbivore overabundance, it is first necessary to understand and predict how biological communities respond to large herbivore overabundance (Côté et al. 2004). Previous research shows that different species and taxa respond differently to large herbivore overabundance (Melis et al. 2006; Suzuki et al. 2013; Bachand et al. 2014), which makes general predictions difficult. A life history trait-based approach that explores the relationships between life history traits and the sensitivity of species to environmental change enables us to gain insights into this issue. This approach is appropriate because life history traits are usually linked directly with various types of environmental change such as habitat loss and fragmentation and climate change (Jiguet et al. 2007; Ockinger et al. 2010; Soga and Koike 2012). Identifying the key traits that control species responses to large herbivore overabundance allows us not only to predict future species loss and change in community compositions following large herbivore overabundance (McKinney and Lockwood 1999) but also to predict changes in overall ecosystem function and resilience (Petchey and Gaston 2006; Mori et al. 2013). However, this approach has rarely been used in this field. To date, empirical studies that have investigated relationships between life history traits and the response of species to large herbivore overabundance are quite scarce and limited (Filazzola et al. 2014; Koike et al. 2014).
To investigate the relationships between species life history traits and their sensitivities, insects are excellent model organisms. This is because many insect species have species-specific life history traits, and they respond quickly to environmental change (Sumways 2007; Maleque et al. 2009). For insect species, types of food resources and the degree of dependence on plant species are expected to directly determine the sensitivity to large herbivore overabundance. Indeed, it is known that while the abundance of dung beetles increases with an increase in mammalian abundance (Stewart 2001; Nichols et al. 2009), herbivorous insects respond negatively (Den Herder et al. 2004; Teichman et al. 2013). Yet, among herbivorous insects, those that utilize tall trees as a food resource are less likely to respond negatively because the leaves of tall trees are located above the browsing height, yet sometimes they respond positively because of compensating growth due to browsing by the large herbivores (Gill 1992; Guillet and Bergström 2006). Furthermore, Allombert et al. (2005) and Mysterud et al. (2010) observed that while herbivorous insects, which typically inhabit understory vegetation, drastically responded to large herbivore overabundance, litter-dwelling arthropods showed a weaker response. Other researchers have observed a significant association between insect body size and sensitivity to large herbivore overabundance (Cole et al. 2006; Koike et al. 2014). It has often been reported that large species are vulnerable to various types of environmental change because they cannot adapt quickly to a disturbed environment due to their longer generation times (Ribera et al. 2001; Jelaska and Durbešic 2009). Besides body size, flight ability is also a key ecological trait for determining response to environmental change because flight ability directly links to dispersal ability (Den Boer 1990). Because flightless (apterous) species have lower dispersal ability, they are less likely to move quickly to suitable habitats (Den Boer 1990).
Here, we evaluate the effects of sika deer (Cervus nippon) overabundance on insect communities in a broad-leaved forest in Hokkaido, northern Japan. In Japan, deer populations have continuously increased since the late twentieth century (Uno et al. 2009), and the dramatic increase has degraded forest ecosystems across Japan, including soil erosion (Sakai et al. 2011) and declining vegetation (Takatsuki 2009). In this study, we investigated the abundance (number of individuals) of insects belonging to five different taxonomic groups (moths, dung beetles, longicorn beetles, carabid beetles, and carrion beetles). The aim of this study was to clarify the relationships between life history traits of insects and their responses to large herbivore overabundance. We predicted the following outcomes: (1) insect groups whose food resources increase with increasing deer density (dung and longicorn beetles) respond positively to deer overabundance, (2) groups that strongly depend on understory vegetation (herb or shrub feeders such as moths and ground beetles) respond negatively, and (3) large or flightless species respond negatively to deer overabundance.
Materials and methods
Study area and sampling sites
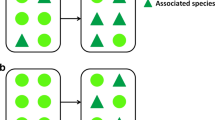
Our study area is located in the Tomakomai Experimental Forest (TOEF) of Hokkaido University (TOEF; 42°40′N, 141°36′E) in western Hokkaido, northern Japan. The mean annual temperature is 6.5 °C and the mean monthly temperature ranges from 19.1 to −3.2 °C. The annual precipitation is about 1200 mm, and the mean snow depth ranges from 20 to 50 cm. In TOEF, there are 2 fenced sites: a 16.4 ha enclosure and a 1.5 ha exclosure. At the enclosure and exclosure sites, the deer density has been manipulated since 2004 (Fig. 1). At the enclosure site, the deer density has been maintained at a high level (20 deer/km2). At the exclosure site, the deer have been excluded completely (0 deer/km2). The study was carried out in the 2 manipulated sites and in the surrounding area (hereafter called “control sites”). At the control site, deer densities estimated by light censuses were approximately 10 deer/km2 (TOEF, Unpublished results). The study area is situated in a secondary deciduous forest dominated by Japanese oak (Quercus crispula), painted maple (Acer mono), and Japanese linden (Tilia japonica). The understory vegetation is dominated by wood fern (Dryopteris crassirhizoma), Maianthemum dilatatum, and Japanese spurge (Pachysandra terminalis) (Hiura 2001).
Insect sampling
We surveyed insects from May to September 2014. Surveys were conducted under appropriate weather conditions (without rain). Accidentally, if there was rainy day during the sampling period, the sampling period was extended for 1 day. For each site, we set 6 sampling plots (three plots in two study periods; Fig. 1). These 6 plots were placed at least 50 m apart from each other and deer fences, following previous studies (Larsen 2005; Van Grunsven et al. 2014), at enclosure and control sites. Due to limited space at the exclosure site, sampling plots were placed as far apart as possible (ca 15 m from each other and ca 30 m from deer fences). We used 5 % acetic acid as a preservative solution for all types of traps (except the light trap), and the captured individuals were then dried, mounted, and identified to the level of species in the laboratory.
We sampled longicorn beetles using collision traps baited with benzyl acetate and ethanol. These traps consist of 2 crossed collision plates (approximately 210 mm high and 220 mm wide), a roof, and a bucket partially filled with preservative solution. These traps were set at 1.5 m from the ground. The species collected by the collision traps mainly consisted of flower-visiting species (Sayama et al. 2005). Therefore, to sample non-flower-visiting species, in addition to the collision trap, longicorn beetles were sampled by using malaise traps. Collision traps were set for 3 days in mid-June and mid-July, and malaise traps were set for 4 days in early June and from late July to early August. During these periods, the abundance of longicorn beetles is thought to be highest and flowers abundant (Hasegawa et al. 2007; Inari et al. 2012). To prevent interference between the two types of traps, we avoided overlapping their sampling periods.
We sampled moths using light traps (Okochi 2002). The light traps were constructed from two boxes made with nylon net illuminated by 4-W fluorescent light and 4-W UV light. One of the boxes was positioned over the lights and another box was positioned under the lights. The bottom of the box positioned over the lights was open, and the top of the box situated under the lights had a funnel shape to prevent the moths from escaping. Each light trap and light source were located 1.5 m from the ground and were operated for 1 night during 3 different periods (mid-June, mid-July, and mid-September) to avoid the effect of seasonal variation on the species composition (Yoshida 1980).
We sampled dung and ground beetles using pitfall traps baited with cattle dung and fermented milk drink. Pitfall traps were made from plastic containers (22.5 cm diameter and 26.6 cm deep) and plastic cups (8.3 cm diameter and 11.5 cm deep). The container of preservative solution and fermented milk drink was buried to its rim in the ground. Then, plastic cups with cattle dung were hung inside the container using wires. To prevent trap destruction by mammals or carnivores, the pitfall traps were covered with an iron fence, and chili powder was scattered around the trap. We also set plastic roofs on the traps to prevent interference from rain and fallen leaves. Pitfall traps were set for 3 days in mid-May, mid-June, and mid-September to cover the peaks of activity of these beetles (Sota 1987; Yusa and Nishiguchi 1989; Kanda et al. 2005).
Environmental conditions
We measured the understory layer (<1.5 m from the ground) cover and diversity, shrub layer (≥1.5 m, <3.0 m) and canopy layer (≥3.0 m) cover, tree species diversity, leaf litter depth, canopy openness, and dead wood volume (fallen trees and standing dead trees). These surveys were conducted in quadrats (20 × 20 m2) established at each site (4 quadrats each in the enclosure and control sites, 2 quadrats in the exclosure site). Leaf litter depth and the dead wood volume were measured in May, and the other parameters were measured in May, June, July, and September.
The understory layer cover was measured in 2 subquadrats (1 × 1 m2) established in each quadrat. To measure the α diversity of the understory vegetation, we calculated the Shannon–Wiener index based on the importance value (IV) score instead of simple abundance (Zou et al. 2013). The IV score of each species was calculated from the relative cover rank (1: <10 %; 2: ≥10 and <25 %; 3: ≥25 and <50 %; 4: ≥50 and <75 %; 5: ≥75 %) and height of each species in the subquadrats. To measure the α diversity of the tree species, the Shannon–Wiener index was also calculated based on the IV score using data from Hino et al. (Unpublished results). The IV score for each tree species was calculated from relative abundance and breast height area. The IV score for the ith species (IV i ) was calculated as:
where d is the cover rank of the ith species for the understory vegetation, but for the tree species, d is the number of individuals of the ith species; h is the height of ith species for understory vegetation, but for the tree species, d is the breast height area of the ith species; and S is the total number of species in a sample plot. Leaf litter depth, canopy openness, and dead wood volume were measured at the quadrat scale. The leaf litter depth was measured using a ruler inserted vertically and 5 times randomly in each quadrat. A hemispherical photograph was taken at the center of each quadrat using a digital camera equipped with a fish-eye lens. Canopy openness was calculated by CanopOn-2 software (Takenaka 2009). Fallen trees greater than 5-cm diameter at the point where it intersected a line were investigated in each quadrat (Ugawa et al. 2012). The length (l) and diameter (a i ) at 2-m intervals were measured for each fallen tree. Each fallen tree volume (V) was calculated using Huber’s formula:
where n is the distance from the base of each of fallen tree (n = 1, 3, …, n m from the base) and j is (n + 1) m from the base. To measure dead wood volume, we measured the diameter at breast height (DBH) and the height of whole dead stands greater than 5-cm DBH in each quadrat. Each dead wood volume was calculated using the formula for a circular cone (Hiura et al. 1998).
Species classifications
For each insect group except the longicorn beetles, the insect species observed in the field were divided into different functional groups by morphology or life history traits (Online Resource Tables 3–6). Moths were divided into herb- or shrub-feeding species or arbor-feeding species by their larval food habit. Based on this classification, 63 species (5450 individuals) that feed on both types of plants, 104 species (2107 individuals) that feed on other types of plants (i.e., litter), and 59 species (3060 individuals) with unknown larval food habits were not divided into any groups. Dung beetles were divided according to body size (S: <10 mm; L: ≥10 mm). Ground beetles and wingless (apterous or macropterous) insects were divided according to body size (S: <10 mm; M: ≥10 and <20 mm; and L: ≥20 mm). Species whose wing form varied significantly from individual to individual [carabid beetles: three species (2086 individuals), carrion beetles: one species (35 individuals)] were divided into neither apterous nor macropterous groups. In the latter analysis, however, small-sized carabid beetles were excluded because of the small sample size (total number of individuals <10). Longicorn beetles were not divided into any functional groups due to data limitation: 6 species (34 individuals) collected from the collision traps and 13 species (48 individuals) collected from the malaise traps.
Data analysis
To test for differences in environmental conditions between the three sites (enclosure, exclosure, and control sites), we performed a Kruskal–Wallis test, using the “kruskal.test” function followed by multiple pairwise comparison of the sites using the Wilcoxon rank sum test, using the “pairwise.wilcox.test” function (R version 2.15.2; R Core Team 2012). Our preliminary analysis demonstrated no evidence of spatial autocorrelation in the data across the 18 sampling sites. Therefore, we treated the data from each site as spatially independent samples in later analyses.
To test whether insect communities differed between the three sampling sites (enclosure, exclosure, and control sites), we used generalized linear models (GLMs), using the “glm” function (R version 2.15.2; R Core Team 2012). For GLMs, the abundance of each taxonomic or functional group was used as a response variable with a Poisson distribution and a log link function. Sampling sites were used as categorical explanatory variables (we used control sites as a reference). For carrion beetles, however, macropterous species were not captured at the enclosure site. Thus, the comparison of abundance of macropterous species was only conducted between the control and exclosure sites. In this study, the number of sampling days differed between the plots due to trap destruction by wind or animals. Thus, we used sampling period (days) as the offset term. We calculated estimated abundance using the estimates of parameters in GLMs. All statistical analyses were performed using R ver.2.15.2 (R Core Team 2012). In all of the analyses, we defined the p value as 0.05.
Results
Environmental conditions
Results from the Kruskal–Wallis test revealed that the cover of the understory layer (p = 0.003) and leaf litter depth (p < 0.001) were significantly different between the 3 study sites (Online Resource Fig. 1a and h; Table 1). The cover of the shrub layer (p = 0.08) and dead wood volume (p = 0.06) were also marginally different between the 3 sites (Online Resource Fig. 1b and f; Table 1). The Shannon diversity of the understory (p = 0.60), tree species (p = 0.42), the cover of the canopy layer (p = 0.22), canopy openness (p = 0.73), and fallen tree volume (p = 0.16) did not significantly differ between the 3 sites (Online Resource Fig. 1; Table 1). Pairwise Wilcoxon rank sum tests showed a significant difference in understory cover between the exclosure and enclosure sites (p = 0.03), between the control and enclosure sites (p = 0.04), and a difference in leaf litter depth between the exclosure and enclosure sites (p = 0.002; Online Resource Fig. 1).
Insect communities
In total, 18,589 individuals belonging to 384 species were sampled. For longicorn beetles, 6 species (34 individuals) were collected by collision traps and 13 species (48 individuals) were collected by malaise traps (Online Resource Table 3). For moths, 339 species (14,084 individuals) were observed (Online Resource Table 4). These species included 122 arbor feeders (2621 individuals) and 54 herb or shrub feeders (856 individuals). For dung beetles, 3 small species (292 individuals) and 1 large species (1024 individuals) were sampled (Online Resource Table 5). For carabid beetles, 1 small (8 individuals), 8 medium-sized species (2880 individuals), and 7 large species (122 individuals) were captured (Online Resource Table 6). Carabid beetles were also grouped to 8 apterous species (588 individuals) and 5 macropterous species (336 individuals). For carrion beetles, 4 medium-sized (62 individuals) and 1 large (35 individuals), and 1 apterous species (52 individuals) and 3 macropterous species (10 individuals) were collected (Online Resource Table 7).
For longicorn beetles collected by malaise traps, abundance in the enclosure site was significantly higher than in the control site (p = 0.001; GLM; Fig. 2a; Online Resource Tables 1–3). For longicorn beetles collected by collision traps, significant differences in abundance between the 3 sites were not observed (GLM; Fig. 2b; Online Resource Tables 1–3).
Abundance of each insect taxonomic group at the three sampling sites estimated by generalized linear models: a longicorn beetles sampled by malaise traps, b longicorn beetles sampled by collision traps, c moths, d dung beetles, e carabid beetles, and f carrion beetles. Double asterisk indicates a significant difference compared with the control site (p < 0.05). Estimates are shown using the mean values (bold lines in squares) and associated 95 % CIs (vertical bars)
For moths, the total abundance was lower in the enclosure than in the control site (p < 0.001; GLM; Fig. 2c; Online Resource Tables 1, 2 and 4). Abundance of arbor feeders was lower in the exclosure than in the control site (p = 0.03), whereas abundance of herb or shrub feeders in the exclosure was significantly lower than in the control site (p = 0.02; GLM; Fig. 3a; Online Resource Table 1, 2 and 4).
Abundance of each insect functional group at the 3 sampling sites estimated by generalized linear models: a moths, b dung beetles, c carabid beetles, and e carrion beetles. Double and single asterisk indicate significant (p < 0.05) and marginally significant (p < 0.1) differences compared with the control site, respectively. Estimates are shown using the mean values (squares) and associated 95 % CIs (vertical bars). For moths, 167 species (10,607 individuals) that feed on both types of plants or other types of plants (i.e., litter) and whose larval food habits are unknown were not divided into any groups. For ground beetles, species whose wing form significantly varied from individual to individual [carabid beetles: 3 species (2086 individuals), carrion beetles: 1 species (35 individuals)] were divided into neither apterous nor macropterous groups. Longicorn beetles were not divided into functional groups because the sample size was too small and appropriate traits were not found
For dung beetles, the total abundance in the control site was significantly lower than in the enclosure (p = 0.002) and exclosure sites (p = 0.002; GLM; Fig. 2c; Online Resource Tables 1, 2 and 5). Abundance of large dung beetles in the control site was also significantly lower than in the enclosure (p < 0.001) and exclosure sites (p < 0.001; GLM; Fig. 3b; Online Resource Tables 1, 2 and 5). For small dung beetles, abundance did not differ between the 3 sites (GLM; Fig. 3b; Online Resource Tables 1, 2 and 5).
For carabid beetles, the total abundance in the control site was significantly higher than in the enclosure (p < 0.001) and exclosure sites (p < 0.001; GLM; Fig. 2d; Online Resource Tables 1, 2 and 6). Abundance of medium-sized carabid beetles in the control site was significantly higher than in the enclosure (p < 0.001) and exclosure sites (p < 0.001), whereas the abundance of large beetles did not differ between the 3 sites (GLM; Fig. 3c; Online Resource Tables 1, 6). Abundance of apterous species in the enclosure site was significantly lower than in the control site (p = 0.001; GLM; Fig. 3c; Online Resource Tables 1, 2 and 6). Abundance of macropterous species was significantly higher in the exclosure than in the control sites (p = 0.02; GLM; Fig. 3c; Online Resource Tables 1, 2 and 6).
For carrion beetles, the abundance in the control site was significantly higher than in the enclosure (p < 0.001) and exclosure sites (p = 0.03; GLM; Fig. 2e; Online Resource Tables 1, 2 and 7). Abundance of medium-sized species was significantly lower in the enclosure than in the control sites (p < 0.001; GLM; Fig. 3d; Online Resource Tables 1, 2 and 7). Abundance of large species did not differ between the 3 sites (GLM; Fig. 3d; Online Resource Tables 1, 2 and 7). Abundance of apterous species in the enclosure site was significantly lower than in the control site (p = 0.003), whereas abundance of macropterous species did not differ between the 3 sites (GLM; Fig. 3d; Online Resource Tables 1, 2 and 7).
Discussion
To predict the ecological consequences of large herbivore overabundance, it is first necessary to understand how biological communities respond to large herbivore overabundance. This study is an attempt to investigate the response of various taxonomic groups of insect species to deer overabundance. We found that the sensitivity of response to large herbivore overabundance differed greatly between the different taxonomic or functional groups.
In our study, carabid beetles, carrion beetles, and herb- or shrub-feeding moths responded negatively to deer overabundance (Figs. 2, 3; Online Resource Table 1). As carabid and carrion beetles use the understory as their habitat, a declining understory layer would have a negative effect on these species (Latty et al. 2006; Taboada et al. 2008). Indeed, we observed that the understory layer cover declined with increasing deer density (Online Resource Fig. 1a; Table 1), supporting the above explanation. Because herb- or shrub-feeding moths typically use understory plants as food resources, these species are likely to compete with deer for food resources (Teichman et al. 2013; Schweitzer et al. 2014). In contrast, arbor-feeding moths did not respond negatively to deer overabundance (Fig. 3). Arbor feeders do not use the understory vegetation as a food resource; therefore, they would be less dependent on the understory than the herb or shrub feeders. Therefore, our results suggest that dependence on the understory is one of the key factors determining the response of insects to deer overabundance (Allombert et al. 2005; Mysterud et al. 2010). Because plants are fundamental components of survival and reproduction, the trends seen in our results are likely to apply to other taxonomic species including birds and small mammals (Flowerdew and Ellwood 2001; Chollet and Martin 2013).
In contrast to the above-mentioned insect groups, arbor-feeding moths, longicorn beetles, and dung beetles responded positively to deer overabundance (Figs. 2, 3; Online Resource Table 1). Surprisingly, we observed that the number of arbor-feeding moths was higher in the enclosure than in the control site (Fig. 3). Although deciphering the mechanism behind this pattern is difficult, it is possible that the quality of food for the arbor-feeders increased with increasing deer density. Lucas et al. (2013) indicates that deer overabundance enhances the growth rate of mature trees because deer defecation accelerates nutrient cycling and deer browsing weakens competition between trees and understory plants. Longicorn beetles also responded positively to deer increases (Fig. 2). As the majority of species observed in this study use dead wood as a larval food resource, the increase in dead wood volume in the enclosure site (Online Resource Fig. 1f; Table 1) is likely to enhance their food resource. Indeed, it has been reported that bark stripping by deer increases the dead wood volume (Akashi and Nakashizuka 1999; Côté et al. 2004). In this study, however, longicorn beetles collected in the collision traps were not abundant in the enclosure site (Fig. 2). A reason for this could be that the longicorn beetles captured by collision traps consisted mainly of flower-visiting species (Online Resource Table 3). For dung beetles, it is well known that abundance of dung beetles and mammals is closely linked to each other because dung beetles depend on mammal dung as an adult and larval food resource (Andresen and Laurance 2007; Nichols et al. 2009). Although we did not measure the amount of deer dung, the increased abundance of dung beetles in the enclosure site is likely due to increasing deer dung (Fig. 2). Therefore, our results suggest that the type of food is also a key factor determining the response of insects to deer overabundance.
We observed that body size and wing form were also associated with the response of insect species to deer overabundance. In this study, apterous species tended to respond negatively to deer overabundance (Fig. 3). This result concurs with previous studies that demonstrate that flightless species are more susceptible to disturbance (Jelaska and Durbešic 2009; Pakeman and Stockan 2014). In addition, smaller species responded negatively to deer overabundance (Fig. 3). For dung beetles, it has been reported that smaller species are more vulnerable to understory decline by deer over browsing because smaller species favor more structurally complex habitats (Koike et al. 2014). For carabid beetles, our results contrasted with previous studies, which found that large species are more vulnerable to disturbance (Rainio and Niemelä 2003; Brose 2003; Cole et al. 2006). One reason for this is that, unlike these previous studies, the majority of the smaller species observed in the field were flightless species (e.g., Pterostichus thunbergii and Silpha perforata venatoria).
Although the results were clear, this study inevitably had some limitations. First, our data were collected at Hokkaido from only three study sites, and the scale was relatively small. Therefore, caution is warranted for any generalizations of the findings. Second, pitfall and light traps were the most common methods used for measuring abundance and species richness of insect species; however, such passive trapping methods can be affected by several factors (e.g., habitat condition and trap size) (Koivula et al. 2003). Thus, more detailed assessments may be needed. Third, this study employed a cross-sectional design (comparing three study sites comprising different deer densities); therefore, we could not directly assess the effect of a deer population increase on insect communities. Further longitudinal studies could clarify this issue. Finally, a function describing the threshold at which insect communities sharply respond to a deer population increase remains to be determined, thereby posing a challenge for future studies.
In conclusion, our study demonstrated a significant change in insect communities following experimental deer overabundance in Japan. Some taxonomic or functional groups whose food increases with deer overabundance responded positively to deer overabundance (arbor-feeding moths, longicorn beetles, and dung beetles), although others who depend on understory vegetation responded negatively (herb- or shrub-feeding moths, carabid beetles, and carrion beetles). These results suggest that large herbivore overabundance in forest ecosystems leads to the homogenization of biological communities (McKinney and Lockwood 1999). Our study also suggests that the type of food and the level of dependence on the understory are key factors determining the response to large herbivore overabundance. Our results also imply that as the deer density increases, the biological communities will become largely dominated by small or highly mobile generalist species. Our results would help not only to predict the effect of large herbivore overabundance but also to understand the appropriate density of large herbivores to conserve forest ecosystems. Further research should attempt to detect the thresholds of these changes (Côté et al. 2004). Importantly, insects perform a wide variety of functions in forest ecosystems, such as the decomposition of dung and carcass (Nichols et al. 2008; Sugiura et al. 2013), pollination (Matsuki et al. 2008), and nutrient cycling (Cigan et al. 2015). Therefore, unfortunately, drastic changes in both abundance and composition of insect communities will also decrease ecosystem function and resilience (Hooper et al. 2005).
References
Akashi N, Nakashizuka T (1999) Effects of bark-stripping by Sika deer (Cervus nippon) on population dynamics of a mixed forest in Japan. For Ecol Manage 113:75–82. doi:10.1016/S0378-1127(98)00415-0
Allombert S, Stockton S, Martin J-L (2005) A natural experiment on the impact of overabundant deer on forest invertebrates. Conserv Biol 19:1917–1929. doi:10.1111/j.1523-1739.2005.00280.x
Andresen E, Laurance SGW (2007) Possible indirect effects of mammal hunting on dung beetle assemblages in Panama. Biotropica 39:141–146. doi:10.1111/j.1744-7429.2006.00239.x
Bachand M, Pellerin S, Moretti M et al (2014) Functional responses and resilience of boreal forest ecosystem after reduction of deer density. PLoS ONE 9:e90437. doi:10.1371/journal.pone.0090437
Brose U (2003) Bottom-up control of carabid beetle communities in early successional wetlands: mediated by vegetation structure or plant diversity? Oecologia 135:407–413. doi:10.1007/s00442-003-1222-7
Chollet S, Martin J-L (2013) Declining woodland birds in North America: should we blame Bambi? Divers Distrib 19:481–483. doi:10.1111/ddi.12003
Cigan PW, Karst J, Cahill JF et al (2015) Influence of bark beetle outbreaks on nutrient cycling in native pine stands in western Canada. Plant Soil. doi:10.1007/s11104-014-2378-0
Cole LJ, Pollock ML, Duncan R et al (2006) Carabid (Coleoptera) assemblages in the Scottish uplands: the influence of sheep grazing on ecological structure. Entomol Fenn 17:229–240
Côté SD, Rooney TP, Tremblay J-P et al (2004) Ecological impacts of deer overabundance. Annu Rev Ecol Evol Syst 35:113–147. doi:10.1146/annurev.ecolsys.35.021103.105725
Den Boer PJ (1990) The survival value of dispersal in terrestrial arthropods. Biol Conserv 54:175–192. doi:10.1016/0006-3207(90)90050-Y
Den Herder M, Virtanen R, Roininen H (2004) Effects of reindeer browsing on tundra willow and its associated insect herbivores. J Appl Ecol 41:870–879. doi:10.1111/j.0021-8901.2004.00952.x
Filazzola A, Tanentzap AJ, Bazely DR (2014) Estimating the impacts of browsers on forest understories using a modified index of community composition. For Ecol Manage 313:10–16. doi:10.1016/j.foreco.2013.10.040
Flowerdew JR, Ellwood SA (2001) Impacts of woodland deer on small mammal ecology. Forestry 74:277–287. doi:10.1093/forestry/74.3.277
Fuller R, Gill RMA (2001) Ecological impacts of increasing numbers of deer in British woodland. Forestry 74:193–199. doi:10.1093/forestry/74.3.193
Gill RMA (1992) A review of damage by mammals in north temperate forests: 3. Impact on trees and forests. Forestry 65:363–388. doi:10.1093/forestry/65.4.363-a
Guillet C, Bergström R (2006) Compensatory growth of fast-growing willow (Salix) coppice in response to simulated large herbivore browsing. Oikos 113:33–42. doi:10.1111/j.0030-1299.2006.13545.x
Hasegawa M, Kurihara T, Makihara H et al (2007) Longicorn beetles of Japan, 1st edn. Tokai University Press, Kanagawa
Hiura T (2001) Stochasticity of species assemblage of canopy trees and understorey plants in a temperate secondary forest created by major disturbances. Ecol Res 16:887–893. doi:10.1046/j.1440-1703.2001.00449.x
Hiura T, Fujito E, Ishii T et al (1998) Stand structure of a deciduous broad-leavd forest in Tomakomai experimental Forest, based on a large-plot data. Res Bull Hokkaido Univ For 55:1–10 (in Japanese)
Hooper DU, Chapin FS, Ewel JJ et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35. doi:10.1890/04-0922
Inari N, Hiura T, Toda MJ, Kudo G (2012) Pollination linkage between canopy flowering, bumble bee abundance and seed production of understorey plants in a cool temperate forest. J Ecol 100:1534–1543. doi:10.1111/j.1365-2745.2012.02021.x
Jelaska LS, Durbešić P (2009) Comparison of the body size and wing form of carabid species (Coleoptera: Carabidae) between isolated and continuous forest habitats. Ann la Société Entomol Fr 45:327–338. doi:10.1080/00379271.2009.10697618
Jiguet F, Gadot AS, Julliard R et al (2007) Climate envelope, life history traits and the resilience of birds facing global change. Glob Chang Biol 13:1672–1684. doi:10.1111/j.1365-2486.2007.01386.x
Kanda N, Yokota T, Shibata E, Sato H (2005) Diversity of dung-beetle community in declining Japanese subalpine forest caused by an increasing sika deer population. Ecol Res 20:135–141. doi:10.1007/s11284-004-0033-6
Koike S, Soga M, Nemoto Y, Kozakai C (2014) How are dung beetle species affected by deer population increases in a cool temperate forest ecosystem? J Zool 293:227–233. doi:10.1111/jzo.12138
Koivula M, Kotze DJ, Hiisivuori L, Rita H (2003) Pitfall trap efficiency: do trap size, collecting fluid and vegetation structure matter? Entomol Fenn 14:1–14. doi:10.1603/EN13145
Larsen TH (2005) Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 37:322–325
Latty EF, Werner SM, Mladenoff DJ et al (2006) Response of ground beetle (Carabidae) assemblages to logging history in northern hardwood–hemlock forests. For Ecol Manage 222:335–347. doi:10.1016/j.foreco.2005.10.028
Lucas RW, Salguero-Gómez R, Cobb DB, et al (2013) White-tailed deer (Odocoileus virginianus) positively affect the growth of mature northern red oak (Quercus rubra) trees. Ecosphere 4:art84. doi:10.1890/ES13-00036.1
Maleque MA, Maeto K, Ishii HT (2009) Arthropods as bioindicators of sustainable forest management, with a focus on plantation forests. Appl Entomol Zool 44:1–11. doi:10.1303/aez.2009.1
Martin J-L, Stockton SA, Allombert S, Gaston AJ (2010) Top-down and bottom-up consequences of unchecked ungulate browsing on plant and animal diversity in temperate forests: lessons from a deer introduction. Biol Invasions 12:353–371. doi:10.1007/s10530-009-9628-8
Matsuki Y, Tateno R, Shibata M, Isagi Y (2008) Pollination efficiencies of flower-visiting insects as determined by direct genetic analysis of pollen origin. Am J Bot 95:925–930. doi:10.3732/ajb.0800036
McKinney M, Lockwood J (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 5347:450–453
Melis C, Buset A, Aarrestad PA et al (2006) Impact of red deer Cervus elaphus grazing on bilberry Vaccinium myrtillus and composition of ground beetle (Coleoptera, Carabidae) assemblage. Biodivers Conserv 15:2049–2059. doi:10.1007/s10531-005-2005-8
Minoshima M, Takada MB, Agetsuma N, Hiura T (2013) Sika deer browsing differentially affects web-building spider densities in high and low productivity forest understories. Ecoscience 20:55–64. doi:10.2980/20-1-3580
Mori AS, Furukawa T, Sasaki T (2013) Response diversity determines the resilience of ecosystems to environmental change. Biol Rev 88:349–364. doi:10.1111/brv.12004
Mysterud A, Aaserud R, Hansen LO et al (2010) Large herbivore grazing and invertebrates in an alpine ecosystem. Basic Appl Ecol 11:320–328. doi:10.1016/j.baae.2010.02.009
Nichols E, Spector S, Louzada J et al (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474. doi:10.1016/j.biocon.2008.04.011
Nichols E, Gardner TA, Peres CA, Spector S (2009) Co-declining mammals and dung beetles: an impending ecological cascade. Oikos 118:481–487. doi:10.1111/j.1600-0706.2009.17268.x
Ockinger E, Schweiger O, Crist TO et al (2010) Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol Lett 13:969–979. doi:10.1111/j.1461-0248.2010.01487.x
Okochi I (2002) A new portable light trap for moth collection. Bull FFPRI 1:231–234 (in Japanese)
Pakeman RJ, Stockan JA (2014) Drivers of carabid functional diversity: abiotic environment, plant functional traits, or plant functional diversity? Ecology 95:1213–1224. doi:10.1890/13-1059.1
Petchey OL, Gaston KJ (2006) Functional diversity: back to basics and looking forward. Ecol Lett 9:741–758. doi:10.1111/j.1461-0248.2006.00924.x
Rainio J, Niemelä J (2003) Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers Conserv 12:487–506. doi:10.1023/A:1022412617568
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ribera I, Doledec S, Downie IS, Foster GN (2001) Effect of land disturbance and stress on species traits of ground beetle assemblages. Ecology 82:1112–1129. doi:10.2307/2679907
Rooney TP (2001) Deer impacts on forest ecosystems: a North American perspective. Forestry 74:201–208. doi:10.1093/forestry/74.3.201
Rooney TP (2009) High white-tailed deer densities benefit graminoids and contribute to biotic homogenization of forest ground-layer vegetation. Plant Ecol 202:103–111. doi:10.1007/s11258-008-9489-8
Sakai M, Natuhara Y, Imanishi A et al (2011) Indirect effects of excessive deer browsing through understory vegetation on stream insect assemblages. Popul Ecol 54:65–74. doi:10.1007/s10144-011-0278-1
Sayama K, Makihara H, Inoue T, Okochi I (2005) Monitoring longicorn beetles in different forest types using collision traps baited with chemical attractants. Bull FFPRI 4:189–199 (in Japanese)
Schweitzer D, Garris JR, McBride AE, Smith JAM (2014) The current status of forest Macrolepidoptera in northern New Jersey: evidence for the decline of understory specialists. J Insect Conserv 18:561–571. doi:10.1007/s10841-014-9658-0
Soga M, Koike S (2012) Life-history traits affect vulnerability of butterflies to habitat fragmentation in urban remnant forests. Ecoscience 19:11–20. doi:10.2980/19-1-3455
Sota T (1987) Mortality pattern and age structure in two carabid populations with different seasonal life cycles. Res Popul Ecol 29:237–254. doi:10.1007/BF02538889
Stewart AJA (2001) The impact of deer on lowland woodland invertebrates: a review of the evidence and priorities for future research. Forestry 74:259–270. doi:10.1093/forestry/74.3.259
Sugiura S, Tanaka R, Taki H, Kanzaki N (2013) Differential responses of scavenging arthropods and vertebrates to forest loss maintain ecosystem function in a heterogeneous landscape. Biol Conserv 159:206–213. doi:10.1016/j.biocon.2012.11.003
Sumways MJ (2007) Insect conservation biology, 1st edn. Springer, Netherlands
Suzuki M, Miyashita T, Kabaya H et al (2013) Deer herbivory as an important driver of divergence of ground vegetation communities in temperate forests. Oikos 122:104–110. doi:10.1111/j.1600-0706.2012.20431.x
Taboada Á, Tárrega R, Calvo L et al (2008) Plant and carabid beetle species diversity in relation to forest type and structural heterogeneity. Eur J For Res 129:31–45. doi:10.1007/s10342-008-0245-3
Takatsuki S (2009) Effects of sika deer on vegetation in Japan: a review. Biol Conserv 142:1922–1929. doi:10.1016/j.biocon.2009.02.011
Takenaka A (2009) CanopOn 2. http://takenaka-akio.org/etc/canopon2/. Accessed 4 Dec 2014
Teichman KJ, Nielsen SE, Roland J (2013) Trophic cascades: linking ungulates to shrub-dependent birds and butterflies. J Anim Ecol 82:1288–1299. doi:10.1111/1365-2656.12094
Ugawa S, Takahashi M, Morisada K et al (2012) Carbon stocks of dead wood, litter, and soil in the forest sector of Japan: general description of the National Forest Soil Carbon Inventory. Bull FFPRI 11:207–221
Uno H, Kaji K, Tamada K (2009) Sika deer population irruptions and their management on Hokkaido Island, Japan. In: McCullough DR, Takatsuki S, Kaji K (eds) Sika deer. Springer, Tokyo, pp 405–419
Van Grunsven RHA, Lham D, Van Geffen KG, Veenendaal EM (2014) Range of attraction of a 6-W moth light trap. Entomol Exp Appl 152:87–90. doi:10.1111/eea.12196
Vera FWM, Bakker ES, Olff H (2006) Large herbivores: missing partners of western European light-demanding tree and shrub species? In: Danell K, Bergström R, Duncan P, Pastor J (eds) Large herbivore ecology, ecosystem dynamics and conservation. Cambridge University Press, Cambridge, pp 203–231
Vospernik S (2006) Probability of bark stripping damage by red deer (Cervus elaphus) in Austria. Silva Fenn 40:589–601. doi:10.14214/sf.316
Yoshida K (1980) Seasonal fluctuation of moth community in Tomakomai Experiment Forest of Hokkaido University. Res Bull Coll Exp For Hokkaido Univ 37:675–685
Yusa H, Nishiguchi C (1989) Seasonal changes in the number and species of dung beetles in Kawatabi farm (Tohoku University). Bull Exp Farm Tohoku Univ 5:49–53
Zou Y, Sang W, Bai F, Axmacher JC (2013) Relationships between plant diversity and the abundance and α-diversity of predatory ground beetles (Coleoptera: Carabidae) in a mature Asian temperate forest ecosystem. PLoS ONE 8:e82792. doi:10.1371/journal.pone.0082792
Acknowledgments
The field survey was supported by Tomakomai Experimental Forest of Hokkaido University. This study was partly funded by JSPS no. 25292085, by the Ministry of Environment (SG-3), by 26th Pro-Natura Fund in Japan, and by Hokkaido University no. 2015_04_D.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iida, T., Soga, M., Hiura, T. et al. Life history traits predict insect species responses to large herbivore overabundance: a multitaxonomic approach. J Insect Conserv 20, 295–304 (2016). https://doi.org/10.1007/s10841-016-9866-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-016-9866-x