Abstract
Habitat degradation poses a great threat to biodiversity conservation. Abundance and diversity of butterflies is an indicator of good environmental health. Understanding how different butterfly species respond to habitat degradation is a necessary step towards the development of effective measures to enhance environmental protection. This study investigated the impact of land use patterns on the diversity, abundance, and conservation status of butterflies in the Kisii highlands; a densely-populated region in Kenya that has received little attention in ecological studies. Sampling was done through a line transect of 300 m. A total of 2799 individual butterflies comprising 67 species were recorded across seven land cover types; secondary forest, grasslands, riverine, human settlement, mixed farmlands, monoculture, and mining areas. The secondary forest, riverine and mixed farmlands recorded more butterflies (37.0, 26.0, and 15.5 %, respectively), followed by grasslands (12.5 %), while monoculture, human settlements, and mines had the least number of butterflies (3.0 % each). Moreover, the secondary forest, riverine and mixed farmland land cover types were the most species rich. Nymphalidae were the most abundant (38 species) whereas, Papilionidae the least (3 species) in the region. Junonia sophia was the dominant species. Butterflies were most diverse in the secondary forest (Shannon–Weaver diversity index, H′ = 2.89), while the human settlement had the least (H′ = 1.25). One-way ANOVA analysis indicated a higher species similarity between secondary forest, mixed farming and riverine land cover types compared to a low species similarity between secondary forest and mining, grassland, monoculture and human settlement. Butterfly abundance and distribution was different between the dry and wet season among the land cover types. Therefore, land use patterns had effects on butterfly abundance and diversity and their conservation is threatened if proper management practices are not put in place. Planning of land use activities should thus encourage agro-forests/secondary forests interspaced with other land use activities to enhance environmental health and improve on biodiversity conservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The combined effect of landscape degradation caused by agricultural development, urbanization, forestry practices together with accelerated climate change is the greatest current threat to biodiversity (Hole et al. 2011; Millennium Ecosystem Assessment 2005; Lee and Jetz 2008). Furthermore, these landscape modifications have been considered as a leading cause of species endangerment (Pimm and Raven 2000; Sala et al. 2000). In particular, Afrotropical forests, which host some of the world’s richest biodiversity hotspots, are rapidly diminishing due to anthropogenic disturbances resulting in loss of many unique habitats, as well as the extinction of many species, native biodiversity, and habitats needed to support unique or valued biodiversity (Laurance et al. 2012; Raina et al. 2011). Therefore, understanding the way various land uses affect biodiversity is key in identifying effective and efficient conservation strategies. However, the disturbed Afrotropical habitats are largely unknown in terms of their contribution to biodiversity conservation (Waltert et al. 2004), yet, these can be crucial for conserving tropical biodiversity including insects (Hughes et al. 2002; Waltert et al. 2005).
In Kenya, forest habitats have already been drastically altered by human activities and most of the natural forests have disappeared. The Kisii highlands in particular, are one of the most densely populated regions in Kenya with ecosystems highly threatened by human activities including small-scale farming, monoculture, urbanization, and mining. However, the consequences of land use patterns on the diversity and conservation of various species in the Kisii highlands are not well understood, yet the ability of different species to survive and reproduce in disturbed/modified areas is of great importance (Namu et al. 2008; Akite 2008). Recent studies have demonstrated the importance of conservation outside protected areas including agricultural regions and secondary forests, helping sustain important ecosystem services such as pollination, pest control, and water purification (Milder et al. 2010; Chazdon et al. 2009). It has been shown that if agriculture is conducted with moderate intensity and emphasis put on management of biodiversity and agrobiodiversity, it can play a substantial role in conservation efforts (Scherr and McNeely 2008). Moreover, the secondary forests have also been demonstrated to be important for the persistence of forest species in tropical, human-modified landscapes (Chazdon et al. 2009), hence their importance in biodiversity conservation need to be characterized.
In assessing the threats of land use patterns on biodiversity, butterflies have emerged as model organisms to indicate the ecological integrity of habitats due to their sensitive nature to environmental changes (Kremen 1992). Butterflies are the most studied group of insects that play a significant ecological role in agricultural landscapes, in particular, recycling of nutrients as well as components of the food chain and they are suspected to play a role in wild and cultivated food crops. In Kenya, many researchers have contributed to the understanding of butterfly diversity and abundance of aspects such as effects of plant structure on butterfly diversity, the butterflies of Kenya and their natural history, butterfly species composition and abundance in an old, middle-age, and young secondary forest (Nyamweya and Gichuki 2010; Larsen 1996; Namu et al. 2008). In addition, some studies have examined the effect of forest disturbance on butterfly diversity (Namu 2005). However, the red list status on butterflies in Kenya is largely underdeveloped (Critical Ecosystem Partnership Fund 2005). This is mainly because the invertebrate taxa have not been subjected to rigorous International Union for Conservation of Nature (IUCN) evaluation and as such, most species are still data-deficient. Further comprehensive conservation assessments have been made on three vertebrate groups only (mammals, birds, and amphibians) (Borghesio 2008; Rodríguez et al. 2010; Western et al. 2009) although invertebrates face extinction risk (Dunn 2005). Moreover, previous studies on butterflies showed that much of the research has been done in the temperate regions and data in sub-Saharan Africa is deficient yet the tropics account for approximately 90 % of butterfly species in the world (Fox 2013; Munyuli 2012). Thus, most regions in the sub-Saharan Africa including the Kisii highlands are data-deficient.
Therefore in understanding the threat of land use patterns on biodiversity in the Kisii highlands, Kenya, the assessment of butterfly diversity and abundance is crucial. This could contribute to their conservation and protection. Pursuant to that, this study examined the effects of land use patterns on the diversity and abundance of butterflies in the Kisii highlands, Kenya. Herein, the relationship between land use patterns and butterfly diversity and the conservation status in the Kisii highlands and the effect of seasons on the abundance and diversity of butterfly species in the Kisii highlands are reported.
Materials and methods
Study area
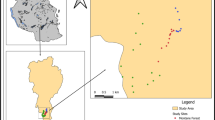
This study was conducted in the Kisii highlands, South Western Kenya (0°41′N, 34°46′E), covering an area of 2 230 km2 and lies at an altitude of 1280–2100 m above sea level (Fig. 1). The Kisii highlands have a humid climate and the local average annual rainfall is 1500 mm/year. The dry season occurs between December and February and the rainy season between March and May. The average daily temperature is approximately 20 °C. The area is densely populated (800 persons/km2) with a human population of 2.2 million (Kenya National Bureau of Statistics 2009). The economy of the area is based on commerce and agriculture. Farm sizes are small (average is 0.5 ha), accommodating an average of six persons (Kisii County Government 2013). Most of the original vegetation in these highlands has been cleared and the area is under intensive cultivation with no nationally/internationally recognized protected areas. The largest patch of forest is the Nyangweta forest, a secondary forest, which is approximately 2.58 km2. Its secondary growth is relatively young (8 years old) and is often heavily affected by farming.
Study site; the Kisii highlands in Kenya. The Kisii highlands are enlarged on the right side map and the sampling sites are highlighted; Manga (grasslands), Kisii town (human settlement/urban), Kemera (mixed farming), Tabaka (mining), Ogembo (riverine), Nyansiongo (monoculture), Nyangweta forest (forest)
Habitat characterization
In the study area, there were seven distinct land cover types that are described in Table 1.
Sampling methods
Our field sampling was conducted in 35 sampling plots stratified across seven land covers; secondary forest, human settlement, riverine, grasslands, mining areas, mixed farmlands, and monoculture (tea estates). Five plots were thus sampled for each of the land cover types. Transects were used as the basic units for all butterflies and land cover types. In each sampling period, the sampling was conducted by two surveyors. All the sampling was done in both seasons, that is, the dry season (November, 2012–March, 2013) and the rainy (wet) season (March–July, 2013). Sampling was done under good weather conditions during sunny and calm days from 08.00 to 12.00 and 14.00–17.00 hours. All sites were sampled for ten consecutive days in each of the seasons.
All the butterflies were recorded using complementary methods (Pollard walks, sweep nets, and bait traps) (Liley et al. 2004; Pollard and Yates 1993), both along permanent 300 m transects in each study plot. Six traps were exposed at 50 m intervals along the 300 m transect, 10–15 cm above the ground. Sweep nets were also used on each sampling day to collect butterflies along the transects during the Pollard walks. Each transect was surveyed by walking its length at a slow, constant pace for 30 min and recording all the butterflies and land cover characteristics. All the recording was conducted about 5 m either side of the transect line. Voucher specimens were kept when it was found necessary for further classification analysis (Caldas and Robbins 2003; Ramesh et al. 2010). Butterflies were then identified up to the species level using various taxonomic treatises (D’Abrera 2001; Hecq 2002; Larsen 2005).
Data analysis
For the basic biodiversity analysis, the data from the three sampling methods were pooled to obtain total butterfly and species per land cover. Species dominance (D) was calculated according to Buschini and Woiski (2008): D = (abundance of a species/total abundances recorded) × 100. If D was >5 %, the species was considered dominant, if 2.5 % < D < 5 %, the species was considered an accessory species/species of intermediate abundance, and if D < 2.5 %, the species was considered an incidental species. Rare species were the ones that had less than 5 individual butterflies and/or sampled from only one land cover type. The unique species were the ones occurring with one individual: singleton, or with two individuals: doubleton).
To compare diversity among different land use patterns and seasons, ANOVA (one way) was done using the general linear model in SAS version 9.1.3 (SAS Institute, Inc.; Cary, NC). Comparison of means were done using least significant differences (LSD) at the 95 % confidence level. The correlation (Pearson’s correlation) between species richness and abundance of butterflies in the different land cover types was calculated using Microsoft Excel 2010. The collected data were also analyzed using the ‘Biodiversity Pro’ software to calculate the Shannon–Weaver Diversity Index (H) (Shannon and Weaver 1949) and the Simpson Diversity Index (D) (Simpson 1949) of the land cover types. The two indices were used since any single diversity index may not provide sufficient information. The Shannon diversity index, which combines the number of species within a site with the relative abundance of each species, was used to calculate diversity between various habitats (Clarke and Warwick 2001). Differences in the distribution of butterfly species between the dry and wet seasons were tested using Levene’s test for homogeneity of variances (Palmer 1994).
Results
Butterfly abundance and diversity
A total of 2799 butterfly individuals belonging to 5 families and 67 species were sampled across the seven land cover types in the Kisii highlands, Kenya (Tables 2 and 3). There were 5 unique species (Fig. 2), 8 rare species (Fig. 2), 42 incidental species, 6 species with intermediate dominance, and 6 dominant species from all the land cover types (Table S1). The Nymphalidae family was dominant (66 % of the individuals recorded), whereas Papilionidae had the least number of individuals (1 % of the individuals recorded) (Table 2). Across the land cover types, a large number of individuals were abundant in the secondary forest and riverine land cover types (37 and 26 % of total individuals, respectively), followed by mixed farming and grasslands whereas human settlement, monoculture, and mining land cover types had the least number of butterflies (3 % each) (Table S1).
Different butterfly species showed variation in abundance among the land cover types. Overall, the most abundant/dominant species was Junonia sophia (D = 12.08 %) followed by Metisella orientalis (D = 11.50 %), Amauris albimaculata (D = 11.04 %), Eurema brigitta (D = 8.11 %), Catopsilia florella (D = 5.32 %) and Junonia terea (D = 5.22 %) (Table S1). For the forest land cover type, Amauris albimaculata (22.51 %), Junonia sophia (9.56 %) and Amauris tartarea (9.47 %), were the dominant species (Table S1). The most abundant species in the mixed farming land cover type were Junonia sophia (34.10 %) and Junonia terea (20.51 %). In the riverine land cover, Metisella orientalis (44.17 %) followed by Ypthima asterope (9.33 %) and Bicyclus vulgaris (7.82 %), were the abundant species. The grasslands had Eurema brigitta (37.82 %) followed by Junonia oenone (21.20 %), and Amauris albimaculata (11.75 %) as the dominant species. The mining land cover was dominated by Junonia sophia (18.42 %), Danaus chrysippus (18.42 %) and Junonia oenone (17.10 %) whereas human settlement land cover type was dominated by Catopsilia florella (29.67 %), Danaus chrysippus (27.47 %) and Junonia sophia (25.27 %). The most abundant species in the monoculture land cover type was Junonia sophia (35.29 %) followed by Catopsilia florella (22.35 %) and Ypthima asterope (15.29 %) (Table S1).
In general, butterfly species diversity and abundance were well correlated (r = 0.98, df = 6, P < 0.05). There were no unique species in disturbed areas compared to those of less-disturbed areas (forests and riverine) (Table S1). To determine species distribution among the seven land cover types, the data was analyzed by one-way ANOVA. It was found that there was no significant difference in butterfly species distribution between forest and riverine (F 25, 41 = 0.145, P > 0.05), mixed farming and riverine (F 14, 52 = 0.04, P > 0.05), mining and riverine (F 7, 59 = 0.05, P > 0.05), and riverine and human settlement (F 19, 47 = 1.73, P > 0.05) at 95 % confidence level (Table 3). However, there were significant differences in the distribution of butterfly species between the other land cover types as shown in Table 3.
Regarding species diversity, the secondary forest land cover had the most number of species/were most diverse (Shannon diversity index (H’) of 2.89), followed by riverine and mixed farmlands (Table 4). On the contrary, monoculture and human settlement habitats had the least number of species/were least diverse (Table 4). The Simpson diversity index showed the same pattern as the H’ with minor variations (Table 4). These indices revealed that individuals were not evenly distributed, indicating that some species were more abundant than others in particular land cover types. This reflects the difference in the efficiency of different butterfly species to adapt to a particular habitat. The abundance of individuals of a species at any given point on a temporal scale is, again dependent on various biotic and abiotic environmental factors (Ramesh et al. 2010).
Seasonal variation of butterflies
In the study, butterfly abundance and species distribution significantly varied seasonally, with more species and higher densities being recorded in the dry season compared to the rainy season (Table 5). Regarding species distribution, the Levene’s test for equality of variances (equal variances assumed) was significant at the 0.05 probability level (Table 6). This means that the variance between the land cover types was significantly different and thus the values under ‘equal variances not assumed’ were used. The t values were significant at 0.001 probability level hence there was a significant difference in the distribution of species in the two seasons in all the land cover types (Table 6).
Discussion
Butterfly abundance and diversity
The abundance and diversity of butterflies in the Kisii highlands, Kenya for different land cover types was not similar. The highest number of species and individuals were recorded in the forest and riverine land cover types. Moreover, it was found that the secondary forest, riverine and mixed farming habitats were the ones with the most unique and rare species. This can be attributed to the land cover characteristics and availability of diverse plants and access to host plants such as Lantana camara, Ricinus communis, and ornamental flowering plants that promote butterfly richness and density. Most of these plants provide rich nectar sources for adult butterflies. It has been demonstrated that plant species that act as a rich source of nectar do influence the occurrence of butterfly species (Tiple et al. 2007). Additionally, some studies have shown that habitat heterogeneity plays a significant role in insect diversity including butterflies (Ngongolo and Mtoko 2013; Fitzherbert et al. 2006). Therefore, the forest, riverine and mixed farmland land cover types which had a variety of plants could support a high number and diverse group of butterflies. On the other hand, species diversity and abundance were low in grasslands, human settlement, monoculture, and mining. This could be attributed to the high anthropogenic activities in the areas such as forest clearance, farming, forest fires, and urbanization, which reduces the tree density and habitat heterogeneity. This is in tandem with results obtained by Akite (2008), where it was demonstrated that there is a marked decrease in butterfly species and abundance by anthropogenic disturbances including charcoal burning, grazing, cultivation, and logging in Sango Bay and Iriiri areas of Uganda. Differences in the butterfly diversity and abundance in the seven land use cover types/habitat indicates that the microhabitat conditions preferred by different species are not the same. This was demonstrated by some butterflies occurring in only a specific habitat or being abundant in a particular habitat such as Amauris albimaculata in the secondary forest, Junonia sophia in mixed farms, Metisella orientalis in the riverine, and Eurema brigitta in the grassland land cover types. This could also be as a result of some butterflies being diet specialists/generalists, which could affect distribution and richness depending on the host plants available (Franzén et al. 2013). Overall, butterfly abundance and diversity varied inversely with the intensity of habitat destruction, suggesting that insect conservation efforts should be directed towards habitat restoration.
Seasonal variation of butterflies
Riverine and forest areas had the highest number of shared species in both the wet and dry seasons. This can be due to habitat similarity between the two land cover types, and due to low anthropogenic activities. In all the seven land use patterns/habitats, there were differences in the distribution of species and abundance in the two seasons (dry and wet). However, in the farmlands, the number of butterflies declined during the wet season probably due to cultivation of lands for planting crops. During this season, the cleared weed species on which the butterflies depend on as their source of nectar could affect butterfly numbers. In the riverine land cover, the dry season differed significantly with the wet season by having a higher number of butterfly species, indicating that weather conditions may have played a role in influencing the composition and abundance of butterfly species. In the riverine land cover, the butterfly abundance was also more, maybe due to the water sources which extend the nectaring season. Secondly, butterfly distribution is strongly influenced by temperature and humidity levels; during the dry season, many species retreat to river bottoms or other moist locations (DeVries 1987). This behavior could be the reason for the richer butterfly fauna observed in the riverine strips during the dry season.
Many researchers (Murphy et al. 1990; Spitzer et al. 1997; Dale and Beyler 2001) have suggested that butterflies are suited to serve as indirect measures of environmental variation. The findings of this study support this idea. The butterfly community was found to be sensitive to anthropogenic changes, and demonstrated an increasing loss of species from sites that were more intensively developed/disturbed. The assemblage of species, as a whole, demonstrated this with a reduction in total abundance of butterflies across the seven land cover types.
Conservation and management implications
From this study it can be concluded that, butterfly communities varied significantly among different land cover types. In a heterogeneous ecosystem like the Nyangweta secondary forest and riverine land cover type along river Gucha, vegetation type played a major role in diversity and abundance of butterfly patterns. It was also shown that the forest, riverine and mixed farming land covers should be given high priority in future conservation plans and monitoring schemes.
As discussed in the introduction, conservationists have begun to focus more on disturbed areas especially on agricultural landscapes in their effort to conserve the remaining biodiversity at local, regional and global scales. Forests and vegetation type play a significant role in the diversity and abundance of butterflies. The small forest patches are able to support butterfly populations but intensification of small-scale farming due to the increasing population pressure of people in Kisii highlands have eliminated primary forests. There is also increased grazing and resource extraction in the secondary forests, and reduced tree cover. These practices are quite likely a major reason why butterfly communities in most land cover types in the study area are small relative to those of forests and riverine, where anthropogenic activities were lower. Given high rates of population increase and economic constraints in the study area, it is evident that biodiversity conservation is a low priority for most people. The conservation and livelihood needs may make it difficult to increase substantially the quantity of forests in the Kisii highlands. However, there are many other management practices that can provide both conservation and livelihood benefits for instance, re-forestation, agroforestry and sustainable consumptive use.
Predicting how land-use changes affect biodiversity and the vulnerability of biodiversity requires a good understanding of the dynamic human-environment interactions associated with land-use change. For conservation planning in the Kisii highlands, it is critical to evaluate the effectiveness of different land use patterns in maintaining the diversity of native species in a human dominated landscape.
References
Akite P (2008) Effects of anthropogenic disturbances on the diversity and composition of the butterfly fauna of sites in the Sango Bay and Iriiri areas, Uganda: implications for conservation. Afr J Ecol 46:3–13
Borghesio L (2008) Effects of human subsistence activities on forest birds in Northern Kenya. Conserv Biol 22(2):384–394
Buschini MLT, Woiski TD (2008) Alpha-beta diversity in trap-nesting wasps (Hymenoptera: aculeata) in Southern Brazil. Acta Zool 89(4):351–358
Caldas A, Robbins RK (2003) Modified pollard transects for assessing tropical butterfly abundance and diversity. Biol Conserv 110:211–219
Chazdon RL, Peres CA, Dent D, Sheil D, Lugo AE, Lamb D, Stork NE, Miller S (2009) The potential for species conservation in tropical secondary forests. Conserv Biol. doi:10.1111/j.1523-1739.2009.01338.x
Clarke KR, Warwick RM (2001) A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar Ecol Prog Ser 216:265–278
Critical Ecosystem Partnership Fund (2005) Eastern arc mountains and coastal forests of Tanzania and Kenya. ICIPE, Kenya
D’Abrera B (2001) Butterflies of the Afrotropical region. Melbourne, Australia
Dale VH, Beyler SC (2001) Challenges in the development and use of ecological indicators. Ecol Indic 1:3–10
DeVries PJ (1987) Ecological aspects of ant association and host plant use in a riodinid butterfly. Ph.D. Dissertation, University of Texas, Austin, USA
Dunn RR (2005) Modern insect extinctions, the neglect majority. Conserv Biol 19:1030–1036
Fitzherbert E, Gardner T, Davenport TRB, Caro T (2006) Butterfly species richness and abundance in the Katavi ecosystem of Western Tanzania. Afr J Ecol 44(3):353–362
Fox R (2013) The decline of moths in Great Britain: a review of possible causes. Insect Conserv Diver 6(1):5–19
Franzén M, Nilsson SG, Johansson V, Ranius T (2013) Population fluctuations and synchrony of grassland butterflies in relation to species traits. PLoS One 8(10):e78233. doi:10.1371/journal.pone.0078233
Hecq J (2002) Euriphene. In: Bauer E, Frankenbach T (eds) Butterflies of the world, Part 15, Nymphalidae VI. Goeke and Evers, Keltern, Germany
Hole DG, Huntley B, Arinaitwe J, Butchart SHM, Collingham YC, Fishpool LDC, Pain DJ, Willis SG (2011) Toward a management framework for networks of protected areas in the face of climate change. Conserv Biol 25(2):305–315
Hughes JB, Daily GC, Ehrlich PR (2002) Conservation of tropical forest birds in countryside habitats. J Ecol 5:121–129
Kenya National Bureau of Statistics (2009) Population and Housing census 2009. Nairobi, Kenya
Kisii County Government (2013) The first county integrated development plan 2013–2017. Kisii, Kenya
Kremen C (1992) Assessing the indicator properties of species assemblages for natural areas monitoring. Ecol Applic 2(2):203–217
Larsen TB (1996) The Butterflies of Kenya and their natural history. Oxford University Press, Oxford
Larsen TB (2005) Butterflies of West Africa. Apollo books, Svendborg, Denmark 1–595, 1–270
Laurance WF, Useche DC, Rendeiro J et al (2012) Averting biodiversity collapse in tropical forest protected areas. Nature 489:290–294
Lee TM, Jetz W (2008) Future battlegrounds for conservation under global change. Proc R Soc 275:1261–1270
Liley D, Brereton T, Roy D (2004) The current level of butterfly monitoring in UK woodlands. Report to the forestry commission, Report No. SO4–35
Milder JC, Scherr SJ, Bracer C (2010) Trends and future potential of payment for ecosystem services to alleviate rural poverty in developing countries. Ecol Soc 15(2):4
Millennium Ecosystem Assessment (2005) Ecosystem and human well-being: biodiversity synthesis. Island press, Washington
Munyuli MBT (2012) Butterfly diversity from Farmlands of Central Uganda. Hindawi Publishing Corporation 2012:481509
Murphy DD, Freas KE, Weiss SB (1990) An environment-metapopulation approach to population viability analysis for a threatened invertebrate. Conserv Biol 4:41–51
Namu NF (2005) Effects of forest disturbances on butterfly diversity in Kakamega Forest National Reserve (K. F.H.R.) Western, Kenya. M. Sc. Thesis, University of Nairobi, Kenya
Namu FN, Githaiga JM, Kioko EN, Ndegwa PN, Hauser CL (2008) Butterfly species, composition, and abundance in an old, middle-aged, and young secondary forest. In: Kuhne L (ed) Butterflies and moth diversity of Kakamega Forest (Kenya). Brandenburgische Universitätsdruckerei und Verlagsgesellschaft, Germany, pp 47–61
Ngongolo K, Mtoko S (2013) Using butterflies to measure biodiversity health in Wazo hill restored quarry. Entomol Zool Stud 1(4):81–86
Nyamweya NH, Gichuki NN (2010) Effects of plant structure on butterfly diversity in Mt. Marsabit forest—Northern Kenya. Afric J Ecol 48:304–312
Palmer AR (1994) Fluctuating asymmetry analyses: a primer. In: Markow TA (ed) Developmental instability: its origins and evolutionary implications. Kluwer Academic Publishers, Dordrecht, pp 335–364
Pimm SL, Raven P (2000) Biodiversity: extinction by numbers. Nature 403:843–845
Pollard E, Yates TG (1993) Monitoring butterflies for ecology and conservation, vol 1. Chapman and Hall, London
Raina SK, Kioko E, Zethner O, Wren S (2011) Forest habitat conservation in Africa using commercially important insects. Annu Rev Entomol 56:465–485
Ramesh T, Hussain JK, Selvanayagam M, Satpathy KK, Prasad MVR (2010) Patterns of diversity, abundance and habitat associations of butterfly communities in heterogeneous landscapes of the department of atomic energy (DAE) campus at Kalpakkam South India. Int J Biodiver Conserv 2(4):75–85
Rodríguez JP, Rodríguez-Clark KM, Baille JEM et al (2010) Establishing IUCN red list criteria for threatened ecosystems. Conserv Biol 25(1):21–29
Sala OE, Chapin FS III, Armesto JJ et al (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Scherr SJ, McNeely JA (2008) Biodiversity conservation and agricultural sustainability: towards a new paradigm of ‘ecoagriculture’ landscapes. Phil Trans R Soc B 363:477–494
Shannon CF, Weaver W (1949) The Mathematical theory of communication. University of Illinois Press, Urbana
Simpson EH (1949) Measurement of diversity. Nature 163:688
Spitzer K, Joras J, Havelka J (1997) Effects of Small-scale disturbance on butterfly communities of an Indochinese montane rainforest. Biol Conserv 80:9–15
Tiple AD, Khurad AM, Dennis RLH (2007) Butterfly diversity in relation to a human-impact gradient on an Indian university campus. Nota Lepidopteral 30(1):179–188
Waltert M, Mardiastuti A, Muhlenberg M (2004) Effects of land use on bird species richness in Sulawesi, Indonesia. Conserv Biol 18:1339–1346
Waltert M, Bobo KS, Sainge MN, Fermon H, Muhlenberg M (2005) From forest to farmland: habitat effects on Afrotropical forest bird diversity. Ecol Appl 15:1351–1366
Western D, Russell S, Cuthill I (2009) The status of wildlife in protected areas compared to non-protected areas of Kenya. PLoS One 4(7):e6140
Acknowledgments
We thank Dr. Esther Kioko and Mr. Mugambi of the National Museums of Kenya for their assistance in the identification of butterflies and for their helpful discussions and comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sagwe, R.N., Muya, S.M. & Maranga, R. Effects of land use patterns on the diversity and conservation status of butterflies in Kisii highlands, Kenya. J Insect Conserv 19, 1119–1127 (2015). https://doi.org/10.1007/s10841-015-9826-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-015-9826-x