Abstract
Climate change is expected to drive patterns of extinction and colonisation that are correlated with geographic gradients in the climate, such as latitude and elevation. However, local population dynamics also depend on the fine-scale effects of vegetation and topography on resource availability and microclimate. Understanding how this fine-scale variation influences population survival in the face of changing climatic favourability could provide clues for adapting conservation to climate change. Here, we document a long-term decline of the butterfly Parnassius apollo in the Sierra de Guadarrama mountain range in central Spain, and examine recent population turnover and habitat use by the species to make inferences about its ecology and conservation. A decline since the 1960s throughout the elevation range suggests a regional deterioration in favourability for the species. Since 2006, local habitat quality has been the main correlate of population persistence, with populations that persisted from 2006 to 2012 associated with high availability of larval host plants. At a finer resolution, the larval distribution in a network of suitable habitat in 2011 and 2012 was most closely related to bare ground cover. Thus, although slope, aspect and elevation lead to considerable variation in microhabitat temperatures during the period of P. apollo larval development, vegetation structure appears to have been the most critical factor for local habitat use and population persistence. The results show that site selection and management retain key roles in conservation despite the broad-scale effects of environmental change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent climate change has been accompanied by shifts in species distributions towards higher latitudes and elevations (Parmesan and Yohe 2003; Chen et al. 2011a). The role of climate change in driving these range shifts is supported by evidence for colonisations at or beyond the high-latitude or elevation range limits (the “leading edge”), and local extinctions at low-latitude or elevation limits (the “trailing edge”) (Parmesan et al. 1999; Franco et al. 2006; Thomas 2010). However, there are fewer documented examples in terrestrial environments of contractions at the trailing edge of species distributions than of range expansions at the leading edge (Parmesan 2006; Sunday et al. 2012). In addition, the observed rates at which species have shifted their distributions uphill appear to lag behind rates of warming to a greater extent than poleward shifts in species distributions (Chen et al. 2011a), despite the shorter geographic distances involved, and hence the greater expected likelihood that dispersal can keep pace with patterns of warming over elevation gradients. Understanding these apparent delays to range contractions and elevation range shifts could be important to determine the mechanisms influencing species’ responses to climate change (Merrill et al. 2008; Chen et al. 2011b), and hence to plan adaptive measures for conservation.
In addition to changes in range limits, recent climate warming has stabilised population dynamics near the high-latitude range margins of several butterfly species in the UK, suggesting that regional climate conditions have shifted from “marginal” to “core” for the species concerned (Oliver et al. 2012). In contrast, climate warming may be causing regional conditions at lower latitudes to shift from core to marginal, with the consequence that rates of local population extinction increase, and the proportion of habitat that is occupied by species decreases (Hanski 1999). Under such circumstances, extinctions may first occur from sub-optimal habitats whose populations were formerly supported by immigration from populations nearby. As this process occurs, the effects of climate change on observed range shifts could be masked if coarse-scale spatial patterns of regional climate variation are less correlated with population dynamics than local, fine-scale variation in the microclimates created by topography and vegetation (Suggitt et al. 2011; Gillingham et al. 2012; Lawson et al. 2014; O’Connor et al. 2014). For example, despite a recent warming of average conditions, cool or wet summers may cause local extinctions of upper latitude range margin populations of the Silver-spotted skipper Hesperia comma in England, particularly in habitat patches whose topography or vegetation provide relatively cool microclimates (Lawson et al. 2012; Bennie et al. 2013).
The lowest latitude populations of many species are restricted to high-elevation mountain regions (e.g. García-Barros et al. 2004; Settele et al. 2008), where temperatures are cooler than at lower elevations. Ecological communities have responded to global warming by moving to higher elevations (Wilson and Gutiérrez 2012), but rates of elevation shifts vary among species and locations, with some species persisting at low elevations despite increased temperatures. One explanation is that some populations are able to persist because topographic and microhabitat structure provides microclimatic variation which individuals can exploit to meet their climatic requirements despite unfavourable ambient conditions. Fine-scale studies of population and distribution dynamics at species trailing margins are needed to understand how such local habitats mediate species responses to climate change, and thus to inform the types of management, vegetation structure or topography that may support persistent populations despite a deterioration in regional climatic conditions.
In this paper, we use three complementary approaches to detect factors influencing patterns of persistence by the butterfly Parnassius apollo over an elevation gradient near its low-latitude range margin. We document changes to the elevation range of the species over four decades, as well as recent patterns of population turnover and fine-scale habitat use in the Sierra de Guadarrama mountain range (central Spain), where mean annual temperature has increased by approximately 0.4 °C per decade since 1970 (Wilson et al. 2005). First, we test for longer-term changes to the elevation range of the species by comparing presence/absence data from transect sites in 2006–2008 and a historical dataset from 1967 to 1973 (Monserrat 1976). Second, we test for the factors influencing local population turnover in P. apollo’s remaining network of populations, using data from habitats where the species was recorded in 2006, and which were resurveyed in 2007, 2008 and 2012. Finally, we examine the influence of fine-scale variation in topography and vegetation on habitat use and the microclimates experienced by P. apollo larvae along a mountain ridge which represents a stronghold for the species in the region. Overall, we show how local vegetation and topography can have important effects on microclimate, habitat use and population persistence, over and above the effects of broad scale gradients in the climate.
Methods
Study system
Parnassius apollo (L.) is a mountain butterfly in most of its European range (Tolman and Lewington 1997; Settele et al. 2008), and its larvae feed predominantly on Sedum spp (Crassulaceae) (Deschamps-Cottin et al. 1997). The butterfly has one generation per year (June–August), and hibernates as a small larva inside the egg (Tolman and Lewington 1997). P. apollo is estimated to have declined in Europe by nearly 30 % since 2000 (van Swaay et al. 2010), and the pattern of greatest declines at low elevations has been linked to climate change (Descimon et al. 2005). Land-use change and pollution may also be associated with the decline (Sánchez-Rodríguez and Baz 1996; Nieminen et al. 2001; Fred and Brommer 2005).
The Sierra de Guadarrama mountains run approximately 120 × 50 km in a South-West to North–East direction around 40°45′N 4°00′W in central Spain. The mountains divide plains with elevations of c. 700 m (to the north) and c. 500 m (to the south), with a peak elevation of 2,428 m (Fig. 1). The main regional host plant for P. apollo is Sedum amplexicaule (Sánchez-Rodríguez and Baz 1996), but larvae also feed on S. brevifolium, S. forsterianum and S. album (Ashton et al. 2009). Regional distribution surveys in 2006–2008 showed that habitats around 1,500–2,000 m elevation, with an intermediate ground cover of shrubs (c. 15–50 %), and widespread Sedum larval host plants were most likely to support P. apollo populations (Gutiérrez et al. 2013). These distribution patterns are believed to relate to a requirement for relatively cool climates at high elevations in Spain, and to habitat use and thermoregulation by P. apollo larvae, which alternate their foraging between Sedum plants growing on bare ground and in the shade of shrubs (Ashton et al. 2009; Gutiérrez et al. 2013).
Site distribution for P. apollo in 1967–1973 and 2006–2012. Triangles show 1967–1973 sites (n = 44), squares 2006–2008 random sites (n = 43) and circles additional 2006 sites with P. apollo presence (n = 21). Filled symbols are sites where P. apollo was observed, open symbols where absent; crosses indicate turnover, 2006–2012. Elevation bands are shown as 250 m increments from <0.75 km (pale grey) to >2 km (black). White frame shows location of larval transects (see Fig. 2). The inset map shows geographical context in Spain. Georeferencing units are in UTM (30T; ED50)
Changes to the elevation range of P. apollo
Parnassius apollo was recorded in 1967–1973 and 2006–2008 at grassland, scrub and woodland sites visited to record regional butterfly distributions (Fig. 1). 44 sites (elevation range 620–2,040 m) were visited at least once during the P. apollo flight period in 1967–1973 (554 visits in total in June–August) (Monserrat 1976). In 2006–2008, butterflies were counted on standardised 500 m long by 5 m wide transects (Pollard and Yates 1993) every 2 weeks at 40 sites (elevation range 550–2,250 m), with an additional three sites sampled in 2006 (246 visits in total over June–August 2006–2008). These “random sites” were selected as a stratified sample of elevations, without knowledge of whether suitable habitat was present for P. apollo. The butterfly was considered present in either time period at locations where one or more individuals were counted, and absent where no individuals were observed, an approach considered valid because the species is large, active during sunny weather, and easy to detect when present.
The location of sample sites in 1967–1973 was determined by inspection of 1:50,000 maps with V. Monserrat, who carried out the original surveys. In 2006–2008, Universal Transverse Mercator (UTM) coordinates were taken every 100 m along transects using a handheld Garmin GPS unit, and used to plot transects in ArcGIS (ESRI 2001). The average elevation of 100 m grid cells crossed by sample sites in both periods was estimated using a digital elevation model (Farr et al. 2007).
Because historical (1967–1973) and modern (2006–2008) sites did not match, we used an unpaired-site model (Tingley and Beissinger 2009) to infer any elevation range shift. We modelled the P. apollo distribution using a Generalised Linear Model (GLM) with binomial error structure and logit-link function for presence (1) or absence (0) against elevation (km), time (1967–1973 = 0, 2006–2008 = 1) and the interaction of time with elevation. The interaction term enabled testing of whether distribution changes had similar intensity across elevations (Moritz et al. 2008). For model selection, we followed the information-theoretic approach based on Akaike’s information criterion corrected for small sample size (AICc) (Burnham and Anderson 2002). The “best” model, with the lowest AICc, is the most parsimonious explanation of observations. We generated all potential models for each response variable, including the null model with only the intercept (five models for P. apollo distribution), and subsequently calculated AICc and AICc differences (ΔAICc) for other candidate models.
Several methods involving ΔAICc are available to select confidence sets of models whose constituent terms should not be ignored (Burnham and Anderson 2002; Richards 2005, 2008). For analyses shown in this paper, we selected models with ΔAICc < 2. Our model confidence set included the model with the lowest AICc; and other models within 2 AICc units, as long as they did not add variables to simpler models with lower AICc (“nested” in the simpler models). We conducted model-averaging for all models in the confidence set to estimate the effects of variables or interactions that were included, using the MuMIn package (Bartoń 2013) in R version 3.0.3 (R Development Core Team 2014). Model-averaged coefficients were calculated by weighting using Akaike weights, and then averaging coefficients over all models in the confidence set (those included in Table 1), with the coefficient set to zero where a given variable was absent from a constituent model.
Population persistence and turnover
In 2006, in addition to the 43 random transects, a further 47 sites were visited where Sedum host plants were known to occur (Gutiérrez et al. 2013). At these additional sites, a 500 m transect was walked twice (1–2 weeks apart, weather permitting) on dates when P. apollo was expected to be close to its peak flight period based on elevation. P. apollo was observed at 26 sites in total of the 90 sampled in 2006 (Gutiérrez et al. 2013), and these 26 sites were revisited using the same protocol in 2007, 2008 and 2012. Flight period over the elevation gradient was estimated for each year using weekly transects from early June to mid-August in at least four populations (Ashton et al. 2009).
Habitat data were collected in 2006/2008 and again in 2012 for the 26 sites with P. apollo presence in 2006. In both periods, twenty 50 × 50 cm quadrats were carried out at each site, located every 25 m along the 500 m transect. The proportion of quadrats containing one of the four Sedum species known to be used as host plants in the region (“Host plant Sedum frequency”; recorded in 2006) and the percentage cover of shrubs (recorded in 2008) were shown to be most strongly related to presence or abundance of P. apollo across the total of 90 sites in 2006–2008 (Gutiérrez et al. 2013), so we recorded these variables again in 2012. Vegetation height (in 2006) and bare ground cover (in 2008) were also recorded for each quadrat in the earlier survey period (see Gutiérrez et al. 2013).
For the 26 sites occupied in 2006, we used a digital elevation model (Farr et al. 2007) to estimate mean elevation (as above), and mean insolation. Insolation was estimated as the total direct solar radiation per 100 m grid cell per year (kWh m−2), using the Solar Analyst 1.0 extension for ArcView GIS (Fu and Rich 2000), based on latitude, slope, aspect, and effects of shading from the elevations of surrounding cells.
The 26 sites were classified according to whether P. apollo was observed in all of the years that the site was visited (“Persistence”) or whether the species was not found by sampling in at least 1 year (local population “Turnover”). It was not possible to conduct transects near the estimated peak flight period at three sites in 2012, but adult individuals were observed, so all three populations were recorded as persistent. In addition, two sites where the butterfly had been recorded as absent in 2007 were not visited in 2008: the lack of a 2008 visit did not affect the classification of these sites as showing turnover. Hence, slight changes to overall annual sampling do not influence inferred turnover or persistence, but do mean that abundance data are not analysed here (see Gutiérrez et al. 2013 for models of abundance in 2006–2008).
Generalised Linear Models were performed with a binomial error term and logit-link, for Persistence (1) or Turnover (0) against (1) mean elevation, mean insolation, host plant Sedum frequency, and shrub cover, with the latter two variables recorded in 2006/08; and (2) the same variables, but with Sedum frequency and shrub cover measured in 2012, to test for changes in habitat or its effects on occupancy. Possible quadratic terms were included for all variables except Sedum, for which we had no expectation that high frequency would reduce persistence (Gutiérrez et al. 2013). The main aim of our analyses was to determine how habitat quality influenced population persistence; although it should be noted that the restriction of our analyses to sites where P. apollo was present in 2006 will underestimate absolute levels of population turnover by neglecting colonisations. Nevertheless, two-weekly butterfly transects from March to October were continued at 24 of the random sites from 2009 to 2012 (see Gutiérrez and Wilson 2014), providing us with information on the wider occurrence of colonisations by P. apollo in the region.
Fine-scale habitat associations
Effects of topography on microclimate, and the fine-scale habitat associations of P. apollo, were characterised for an approximately 8 × 5 km mountain ridge (elevation range, 1,198–1,886 m; Fig. 2) on the border of the provinces of Madrid and Ávila.
Location of larval transects for P. apollo. Filled symbols indicate larval presence, and open symbols larval absence, for 2011 (squares) and 2012 (circles). Elevation is shown in 100 m increments from <1.2 km (pale grey) to >1.7 km (black); pixel size 100 × 100 m. Area corresponds to white frame in Fig. 1. Georeferencing units are in UTM (30T, ED50)
Transects for the habitat associations of larvae were conducted in 2011 (13–28 May) and 2012 (21–30 April), the differences in dates related to availability of field researchers. A low-intensity transect procedure was established to allow widespread sampling for P. apollo larvae despite a relatively remote site and changeable spring weather. Transects were carried out when broadly suitable open habitat with Sedum, bare ground and shrubs was encountered. Transects were walked only under dry weather conditions without low cloud, between 1000 and 1900 hours Central European Summer Time, with the earliest transect on which larvae were observed beginning at 1040 hours, and the latest at 1807 hours. In each transect location, two parallel routes of 100 paces (5 m apart) were carried out through the habitat. Time, weather and GPS grid references (estimated to the nearest m) were recorded at the beginning and end of each transect. During the transect, on every second pace (whenever the surveyor’s right foot touched the ground), it was recorded whether the front of the foot was in contact with shrub, bare ground, or neither. Contacts with shrub or bare ground were tallied for each pair of transects to give a percentage frequency. For every two paces, the presence or absence (not cover) of each Sedum species was also recorded within a 5 metre wide strip (2.5 metres either side of the walked route). All larvae observed during the transect were recorded, with their length (in millimetres, to the nearest 5 mm) and the perpendicular distance to the transect route (in cm, to the nearest 10 cm). The central point of each transect was calculated as the average of start and end grid references, and it was a proviso for inclusion in analysis that transects in the same or separate years were at least 50 m apart. 50 m was adjudged to represent a distance beyond which larvae would be relatively unlikely to move, so that, although egg laying females could move between some transect locations, the observation of larvae provided evidence for successful overwintering and larval foraging in spring.
To record the microclimate conditions that larvae were exposed to, miniature Hobo TidBit dataloggers were placed in shaded positions beneath the edges of dwarf shrubs (5–10 cm vegetation height; mean = 7.5 cm) to cover the range of elevations and aspects over which P. apollo habitat occurred. 21 loggers recorded temperature hourly in the field in March–May 2012, and were used to calculate mean daily maximum and mean daily average temperature (°C) throughout this period.
To model larval habitat associations, we used a Generalised Linear Model with binomial error term and logit-link, for larval presence (1) or absence (0) against frequency of Sedum, bare ground and shrub cover, elevation and modelled insolation for March–May. Year (2011 or 2012) was included as a factor, and quadratic terms were tested for variables other than Sedum frequency. To model the determinants of spring microclimate conditions, we used General Linear Models with a Gaussian error term for temperature against elevation (km), modelled insolation for March–May (kWh m−2), and vegetation height (cm). We analysed both overall mean temperature, and mean daily maximum temperature (logged to remove model overdispersion). Mean daily maximum temperature is likely to influence whether threshold temperatures are exceeded for larval emergence and activity. To illustrate how topography influenced spring microclimate temperatures on the mountain ridge (Fig. 2), we estimated March–May temperatures for the insolation levels estimated for the 100 m grid cells in the landscape with the minimum, 10th percentile, 90th percentile and maximum modelled insolation.
Results
Changes to the elevation range of P. apollo
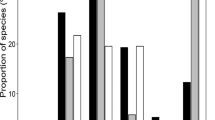
The prevalence of P. apollo decreased, and the elevation range contracted between 1967–1973 and 2006–2008 (Fig. 3). The lowest elevation presences in the random sites were at 980 m in 1967–1973, and 1,450 m in 2006–2008, while the species was observed at the highest elevation sampled in both periods (2,040 and 2,250 m respectively). The average elevation of P. apollo presences was 1,517 m in 1967–1973 (290 m above the average elevation sampled), versus 1,822 m in 2006–2008 (477 m above the average sampled). In both periods, P. apollo was more frequent at high elevations, but in 1967–1973 it was virtually ubiquitous above 1,500 m, whereas in 2006–2008 it was rare at all elevations (Fig. 3). P. apollo was present at 20 (45 %) of 44 sample locations in 1967–1973, with 252 individuals recorded, versus 5 (12 %) of 43 random sites in 2006–2008, with only 10 individuals recorded from the random transect sites over the 3 years period.
Proportion of occupied sites in 250 m elevation bands for P. apollo in 1967–1973 (n = 44 sites) (black) and 2006–2008 (n = 43 sites) (white). Number of sites per elevation band shown above each bar (n for 1967–1973 and 2006–2008 separated by hyphens). Lines fitted based on equation in Table 1a), for 1967–1973 (solid) and 2006–2008 (dashed)
The best model for P. apollo’s elevation range included the effects of elevation, time period, and an interaction between the two (Table 1), and indicated that relative occupancy by P. apollo increased with elevation, and decreased from 1967–1973 to 2006–2008 (Fig. 3). The negative interaction term between elevation and time indicated that incidence decreased more markedly at high elevations over time, suggesting a distribution shift to lower elevations (i.e. opposite to that expected under warming). However, the model containing separate effects of elevation (positive) and time (negative) performed similarly well (ΔAICc = 1.66), such that overall there was little evidence for an elevation shift. ΔAICc exceeded 29 in all simpler models.
Population persistence and turnover
In addition to the five random sites where P. apollo was recorded in 2006, targeted surveys in 2006 found the species in 21 locations. In 17 of this total of 26 occupied sites, adults were observed in every one of the following survey years (2007, 2008 and 2012), suggesting the existence of persistent populations during the survey period. The remaining nine sites showed evidence of population turnover after 2006, with adults absent in two or three of the survey years at six sites, and one of the survey years at three sites. Between 2009 and 2012, P. apollo was only observed at one of the 19 random transect sites from which the species had been absent in 2006–2008, with a single individual seen at 1,220 m elevation in 2011.
Confidence sets of models for persistence versus turnover included positive effects of Sedum host plant frequency using habitat data from either 2006 (Table 1b) or 2012 (Table 1c). Analysis using the 2012 data also showed a positive effect of shrub cover (Table 1c). However, these models were relatively weakly supported relative to the null model (ΔAICc = 2.6–2.9). Average Sedum host plant frequency at the 26 sites was 0.48 in 2006, and 0.43 in 2012; while average shrub cover was 26 % in 2008, and 23 % in 2012, but neither host plant frequency nor shrub cover at the sites changed significantly between 2006/08 and 2012 (Wilcoxon signed-rank tests, P = 0.09 for Sedum frequency, P = 0.32 for shrub cover). There was strong consistency between the two surveys in the relative Sedum host plant frequency and shrub cover per site (n = 26 sites, Pearson’s r = 0.80, P < 0.001 for Sedum; r = 0.63, P < 0.001 for shrubs). Sedum host plant frequency and shrub cover were not significantly correlated with each other in either period. We found no evidence that elevation or modelled insolation were related to population turnover.
Fine-scale habitat associations
Parnassius apollo was recorded on 23 out of 68 larval habitat transects (12 out of 31 in 2011, and 11 out of 37 in 2012; Fig. 2). The model with the lowest AICc for larval presence versus absence showed an association of larvae with intermediate bare ground cover [Logit probability of presence = −12.03 (±SE 4.00) + 0.65 Bare ground (±SE 0.23) − 0.008 Bare ground2 (±SE 0.003)] (Fig. 4). Two further models had ΔAICc of <2 (one showed a positive effect of Sedum frequency, and the other a negative effect of modelled insolation in March–May), but each of these included the bare ground cover effect (i.e. they were nested in the simpler model), so the additional variables were excluded from the final model. The highest ranked model which did not include the effect of intermediate bare ground cover instead had a positive effect of shrub cover and a negative effect of shrub cover2, with ΔAICc of 7.8; while the null model had ΔAICc of 9.49. The best model predicted a greater than 50 % probability of larval presence for 30–45 % bare ground cover, with a maximum at 39 % bare ground cover (Fig. 4).
Probability of presence of larvae as a function of bare ground cover on transects. Outline bars show proportion of transects where larvae were present for 10 % increments of bare ground cover (minimum 10–20 %; maximum 60–70 %; sample sizes above bars). Smooth curve shows best-fitting model for probability of larval presence (Logit probability = − 12.03 + 0.65 bare ground − 0.008 bare ground2)
The best models for March–May daily mean temperature, and for log mean daily maximum temperature, included a negative effect of elevation, and a positive effect of modelled March–May insolation (Table 2). In both cases the next best model also included a negative effect of vegetation height (ΔAICc = 3.22 for daily mean; ΔAICc = 1.77 for daily maximum), but these models were nested within the simpler models of altitude and modelled insolation. AICc differences of the best model from the null model were 25.19 for mean, and 15.22 for maximum temperatures. The 10 % of grid cells in the landscape with the lowest modelled insolation during March–May had aspects of 274°–86°, whereas the 10 % of grid cells with highest modelled insolation had aspects of 112°–254°. At equivalent elevations, estimated average temperatures during March–May were 1.7–4.2 °C warmer for the top tenth percentile of modelled insolation cells than for the lowest tenth percentile, and mean daily maxima were 3.1–14.9 °C warmer (Fig. 5).
Effects of topography on March–May microclimate in P. apollo larval habitat. a Mean temperature and b mean daily maximum (both °C) against elevation for 21 dataloggers located beneath 5–10 cm dwarf shrub vegetation. Lines show modelled temperature based on the equations in Table 2 applied to the 100 m grid cells shown in Fig. 2. Solid lines show maximum (upper line) to 90th percentile (lower line) of modelled insolation in March–May, corresponding to south-facing slopes. Dotted lines show minimum (lower line) to 10th percentile (upper line) of modelled insolation, corresponding to north-facing slopes
Discussion
The elevation range of Parnassius apollo contracted between 1967–1973 and 2006–2008 in the Sierra de Guadarrama, as regional temperatures warmed. Declines occurred throughout the elevation range, with some evidence for greater decline at higher elevations, suggesting an overall decrease in regional favourability for the species. Patterns of population turnover since 2006 indicate the importance of local habitat quality for population persistence, in this case related to host plant frequency and shrub cover. At a fine resolution, habitat structure and topography are likely to play important roles in determining both resource availability and the microclimate conditions experienced by larvae, potentially influencing population dynamics and the future conservation prospects of the species.
Changes to the elevation range of Parnassius apollo
In 1967–1973 and 2006–2008, P. apollo had an upper range limit apparently established by the maximum elevation of the mountains, and a lower limit well above the minimum elevation sampled (Fig. 3). In both periods, low elevations were very patchily occupied (900–1,400 m in 1967–1973; 1,300–2,000 m in 2006–2008). The increase in minimum elevation by 470 m (based on comparable sampling in 1967–1973 and 2006–2008) greatly exceeds the average uphill shift for montane butterflies in the region (212 m), or that crudely expected based on a 1.3 °C increase in mean annual temperatures (c. 225 m in isotherms) (Wilson et al. 2005).
However, we found no indication of greater loss from low elevations, despite expectations that climate warming may first extirpate low-elevation parts of species ranges (Franco et al. 2006; Thomas et al. 2006; Thomas 2010). Indeed, the best supported model included an interaction term suggesting greater relative loss of populations from high elevations. For P. apollo, the fact that populations were sparsely distributed below c. 1,500 m in 1967–1973 means that relatively few extinctions could lead to the observed uphill contraction in the low-elevation limit. In contrast, at higher elevations in 1967–1973 the relatively abundant and widespread populations may have produced sufficient numbers of dispersing individuals to support some populations by immigration into sub-optimal habitats. The marked decline at high elevations could stem partly from a decline in favourability throughout the region (possibly related to climate), that has reduced population sizes even at high elevations: as a result, populations in sub-optimal habitats would be less likely to be bolstered by immigration, leading to local extinctions. In a comparable example, Beever et al. (2003) found greater recent population loss for the mountain pika Ochotona princeps at high than low latitudes, and suggested that low-latitude populations in marginal habitats may have been extirpated long ago, whereas recent changes to the climate have increased the extinction risk of high-latitude populations.
In relatively restricted mountain regions, unfavourable weather may have the potential to act broadly over population networks, leading to regionally correlated declines (e.g. Roland and Matter 2013 for the butterfly Parnassius smintheus). Hence, even though regional distributions of species may be related to broad-scale environmental correlates of the climate (e.g. elevation, Gutiérrez Illán et al. 2010), changes to occupancy patterns during periods of climate change may be mediated by effects of local habitat or microclimate on population dynamics, together with the effects of relative connectivity on rates of population rescue or recolonisation (Lawson et al. 2012).
Population persistence and turnover
In areas with sufficiently widespread larval host plants and available shrub cover, P. apollo populations have been able to persist since 2006 throughout the elevation range of the species. The associations of the species with high larval host plant frequency and shrub cover seem robust (see also Gutiérrez et al. 2013), and are probably related to observations that eggs are not laid directly on Sedum leaves, so larvae must locate sufficiently widespread suitable plants; and shrubs can act both as egg-laying substrates, and to provide shelter for larvae (Ashton et al. 2009).
Without an exhaustive survey of populations in the region, we have not been able to quantify connectivity for P. apollo, but the increasing restriction of the species to isolated ridges and peaks has probably reduced connectivity between populations, leading both to reduced chances of rescue or recolonisation following local declines or extinctions, and potentially an increased risk of local inbreeding depression (see Schmeller et al. 2011). Recent increases in tree cover in the Sierra de Guadarrama (de las Heras et al. 2011) might also contribute to reduced connectivity for the species, such as has been found among populations of P. smintheus in the Rocky Mountains (Roland and Matter 2007).
Declines in local population density, potentially linked to climate conditions, are also likely to contribute to reduced population connectivity. One of the few random transects to record P. apollo in 2006 (at 1,450 m elevation: four individuals were seen in total from 2004 to 2006) corresponds to an area where previous researchers sighted 27–61 P. apollo each year between 1994 and 1997 (Sánchez-Rodríguez and Baz 1996; Baz 2002). Regular two-weekly transects throughout 2007–2013 (D. Gutiérrez and R.J. Wilson unpublished data) provide robust evidence that P. apollo is now extinct from this location. Sites such as this that contain favourable quantities of host plants and shrubs but are small and isolated from the core parts of the species distribution probably stand little long-term chance of persistence. Single individuals of P. apollo have been seen sporadically at one 1,220 m elevation site from among the random transect sites that were not occupied in 2006, suggesting dispersal either from known populations 5–7 km away, or from unknown populations shorter distances away. The lack of observations of P. apollo in other regularly-sampled sites, combined with the evidence of turnover from known populations, suggest that local extinctions are more frequent than new colonisations in the region.
Fine-scale habitat associations
In an earlier study, we found that larvae use both bare ground and shrub cover for thermoregulating and foraging, with apparently stronger associations with shrubs at lower elevations (Ashton et al. 2009). The larval transects conducted here did not have the statistical power to detect variation in habitat use over local climate gradients, but did indicate an association of P. apollo larvae with bare ground cover of 30–45 % (Fig. 4). This intermediate to high cover of bare ground may provide opportunities for larvae to thermoregulate by moving relatively short distances between bare ground and shrubs. Springtime temperatures on bare ground are on average 2–3 °C warmer than temperatures beneath vegetation (Ashton et al. 2009), such that variation in habitat structure could have a comparable effect on the average temperatures experienced by larvae to that caused by differences between shallow south-facing versus north-facing slopes (Fig. 5a).
Nevertheless, variation in habitat structure is unlikely to compensate for the 3–15 °C warmer daily maxima experienced on south-facing versus north-facing slopes. We found a weak indication that larvae were negatively associated with modelled insolation, which might be expected if larvae whose springtime emergence is advanced by warm temperatures on steep south-facing slopes are at greater risk of mortality if cold conditions return. Such “false-spring” events have been linked with P. apollo declines elsewhere in Europe (e.g. Descimon et al. 2005; Schmeller et al. 2011). For other montane butterflies, earlier snowmelt dates have been implicated in increased larval mortality, and in declining larval host plant or nectar plant quality (Boggs and Inouye 2012; Roland and Matter 2013). Whilst we do not have evidence to link snowmelt date to the declines observed in P. apollo in the Sierra de Guadarrama, we note that the annual period of snow cover has decreased by 20–30 days since P. apollo was widespread in the region in 1967–1973 (Giménez-Benavides et al. 2007). In this context, the protection of topographically heterogeneous habitats that include some sheltered north-facing locations where cool conditions or snow cover remain more reliably until later in the year, as well as warm south-facing slopes where larvae are able to develop relatively quickly in cool years, could act as an insurance for P. apollo against climate variability (see Weiss et al. 1988).
Conclusion
Despite a widespread recent decline by P. apollo in the Sierra de Guadarrama, suitable local habitats can enable populations to survive throughout the remnant elevation range occupied by the species. The results show that identifying and maintaining suitable habitat, and monitoring population responses, remain fundamental for conservation in the face of climate change. In the case of P. apollo, regional conservation in the short term will depend on maintenance of open areas with bare ground, shrubs, and larval host plants, probably through continued low-intensity livestock grazing. Determining the longer term prospects of this species and others at low-latitude range margins requires continued research into how resource availability, local microclimate and population connectivity interact to determine population and metapopulation persistence in a warming climate.
References
Ashton S, Gutiérrez D, Wilson RJ (2009) Effects of temperature and elevation on habitat use by a rare mountain butterfly: implications for species responses to climate change. Ecol Entomol 34:437–446
Bartoń K (2013) Package MuMIn: Multi-model inference. R package version 1.9.13. http://CRAN.R-project.org/package=MuMIn. Accessed 25 April 2014
Baz A (2002) Nectar plants for the threatened Apollo butterfly (Parnassius apollo L. 1758) in populations of central Spain. Biol Conserv 103:277–282
Beever EA, Brussard PF, Berger J (2003) Patterns of apparent extirpation among isolated populations of pikas (Ochotona princeps) in the Great Basin. J Mammal 84:37–54
Bennie J, Hodgson JA, Lawson CR, Holloway CTR, Roy DB, Brereton T, Thomas CD, Wilson RJ (2013) Range expansion through fragmented landscapes under a variable climate. Ecol Lett 16:921–929
Boggs CL, Inouye DW (2012) A single climate driver has direct and indirect effects on insect population dynamics. Ecol Lett 15:502–508
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Chen I, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011a) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026
Chen I, Hill JK, Shiu H, Holloway JD, Benedick S, Chey VK, Barlow HS, Thomas CD (2011b) Asymmetric boundary shifts of tropical montane Lepidoptera over four decades of climate warming. Glob Ecol Biogeogr 20:34–45
de las Heras P, Fernández-Sañudo P, López-Estébanez N, Roldán MJ (2011) Territorial and boundary effects in a protected area of the Central Iberian Peninsula. Cent Eur J Geosci 3:1–11
Deschamps-Cottin M, Roux M, Descimon H (1997) Valeur trophique des plantes nourricières et préférence de ponte chez Parnassius apollo L. (Lepidoptera, Papilionidae). CR Acad Sci III-Vie 320:399–406
Descimon H, Bachelard P, Boitier E, Pierrat V (2005) Decline and extinction of Parnassius apollo populations in France-continued. In: Kühn E, Feldmann R, Thomas JA, Settele J (eds) Studies on the ecology and conservation of butterflies in Europe, vol 1., General concepts and case studiesPensoft, Sofia pp, pp 114–115
ESRI (2001) ArcGIS 8.1. Environmental Systems Research Institute Inc, Redlands
Farr TG, Rosen PA, Caro E et al (2007) The shuttle radar topography mission. Rev Geophys 45:RG2004
Franco AMA, Hill JK, Kitschke C, Collingham YC, Roy DB, Fox R, Huntley B, Thomas CD (2006) Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Glob Chang Biol 12:1545–1553
Fred MS, Brommer JE (2005) The decline and current distribution of Parnassius apollo (Linnaeus) in Finland: the role of Cd. Ann Zool Fenn 42:69–79
Fu P, Rich PM (2000) The solar analyst 1.0 user manual. Helios Environmental Modelling Institute, Lawrence
García-Barros E, Munguira ML, Cano JM, Romo H, Garcia-Pereira P, Maravalhas ES (2004) Atlas of the butterflies of the Iberian Peninsula and Balearic Islands (Lepidoptera: Papilionoidea and Hesperioidea). Sociedad Entomológica Aragonesa, Zaragoza
Gillingham PK, Palmer SCF, Huntley B, Kunin WE, Chipperfield JD, Thomas CD (2012) The relative importance of climate and habitat in determining the distributions of species at different spatial scales: a case study with ground beetles in Great Britain. Ecography 35:831–838
Giménez-Benavides L, Escudero A, Iriondo JM (2007) Reproductive limits of a late-flowering high-mountain Mediterranean plant along an elevational climate gradient. New Phytol 173:367–382
Gutiérrez Illán J, Gutiérrez D, Wilson RJ (2010) The contributions of topoclimate and land cover to species distributions and abundance: fine resolution tests for a mountain butterfly fauna. Glob Ecol Biogeogr 19:159–173
Gutiérrez D, Wilson RJ (2014) Climate conditions and resource availability drive return elevational migrations in a single-brooded insect. Oecologia 175:861–873
Gutiérrez D, Harcourt J, Díez SB, Gutiérrez Illán J, Wilson RJ (2013) Models of presence–absence estimate abundance as well as (or even better than) models of abundance: the case of the butterfly Parnassius apollo. Landsc Ecol 28:401–413
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Lawson CR, Bennie J, Thomas CD, Hodgson JA, Wilson RJ (2012) Local and landscape management of an expanding range margin under climate change. J Appl Ecol 49:552–561
Lawson CR, Bennie J, Hodgson JA, Thomas CD, Wilson RJ (2014) Topographic microclimates drive microhabitat associations at the range margin of a butterfly. Ecography 37:732–740
Merrill RM, Gutiérrez D, Lewis OT, Gutiérrez J, Díez SB, Wilson RJ (2008) Combined effects of climate and biotic interactions on the elevational range of a phytophagous insect. J Anim Ecol 77:145–155
Monserrat VJ (1976) La distribución ecológica de las mariposas diurnas del Guadarrama. Tesis, Universidad Complutense de Madrid, Madrid
Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR (2008) Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322:261–264
Nieminen M, Nuorteva P, Tulisalo E (2001) The effect of metals on the mortality of Parnassius apollo larvae (Lepidoptera: Papilionidae). J Insect Conserv 5:1–7
O’Connor RS, Hails RS, Thomas JA (2014) Accounting for habitat when considering climate: has the niche of the Adonis blue butterfly changed in the UK? Oecologia 174:1463–1472
Oliver TH, Roy DB, Brereton T, Thomas JA (2012) Reduced variability in range-edge butterfly populations over three decades of climate warming. Glob Chang Biol 18:1531–1539
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Parmesan C, Ryrholm N, Stefanescu C et al (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation. Chapman and Hall, London
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 17 March 2014
Richards SA (2005) Testing ecological theory using the information-theoretic approach: examples and cautionary results. Ecology 86:2805–2814
Richards SA (2008) Dealing with overdispersed count data in applied ecology. J Appl Ecol 45:218–227
Roland J, Matter SF (2007) Encroaching forests decouple alpine butterfly population dynamics. Proc Natl Acad Sci USA 104:13702–13704
Roland J, Matter SF (2013) Variability in winter climate and winter extremes reduces population growth of an alpine butterfly. Ecology 94:190–199
Sánchez-Rodríguez JF, Baz A (1996) Decline of Parnassius apollo in the Sierra de Guadarrama, Central Spain (Lepidoptera: Papilionidae). Holarct Lepid 3:31–36
Schmeller DS, Dolek M, Geyer A, Settele J, Brandl R (2011) The effect of conservation efforts on morphological asymmetry in a butterfly population. J Natl Conserv 19:161–165
Settele J, Kudrna O, Harpke A et al (2008) Climatic risk atlas of European butterflies. BioRisk 1 special issue. Pensoft, Sofia-Moscow
Suggitt AJ, Gillingham PK, Hill JK, Huntley B, Kunin WE, Roy DB, Thomas CD (2011) Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 120:1–8
Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nat Clim Chang 2:686–690
Thomas CD (2010) Climate, climate change and range boundaries. Divers Distrib 16:488–495
Thomas CD, Franco A, Hill JK (2006) Range retractions and extinction in the face of climate warming. Trends Ecol Evol 21:415–416
Tingley MW, Beissinger SR (2009) Detecting range shifts from historical species occurrences: new perspectives on old data. Trends Ecol Evol 24:625–633
Tolman T, Lewington R (1997) Butterflies of Britain and Europe. HarperCollins, London
Van Swaay C, Cuttlelod A, Collins S et al (2010) European red list of butterflies. Publications Office of the European Union, Luxembourg
Weiss SB, Murphy DD, White RR (1988) Sun, slope and butterflies: topographic determinants of habitat quality for Euphydryas editha. Ecology 69:1486–1496
Wilson RJ, Gutiérrez D (2012) Effects of climate change on the elevational limits of species ranges. In: Beever EA, Belant JL (eds) Ecological consequences of climate change: mechanisms, conservation, and management. Taylor and Francis, Boca Raton, pp 107–131
Wilson RJ, Gutiérrez D, Gutiérrez J, Martínez D, Agudo R, Monserrat VJ (2005) Changes to the elevational limits and extent of species ranges associated with climate change. Ecol Lett 8:1138–1146
Acknowledgments
V. J. Monserrat provided access to historical data. J. Gutiérrez Illán, J. Harcourt, D. M. Gray, S. Ashton, and S. B. Díez assisted with fieldwork and data processing. Research was funded by Universidad Rey Juan Carlos/Comunidad de Madrid (URJC-CM-2006-CET-0592), the Spanish Ministry of Education and Science with an F.P.U. Scholarship and Research Projects (REN2002-12853-E/GLO, CGL2005-06820/BOS, CGL2008-04950/BOS and CGL2011-30259), and the Royal Society of London (International Joint Project “Climate change and metapopulation dynamics at a contracting range margin”). Access and research permits were provided by Comunidad de Madrid, Parque Natural de Peñalara, Parque Regional de la Cuenca Alta del Manzanares, Parque Regional del Curso Medio del Río Guadarrama, Patrimonio Nacional and Ayuntamiento de Cercedilla.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilson, R.J., Bennie, J., Lawson, C.R. et al. Population turnover, habitat use and microclimate at the contracting range margin of a butterfly. J Insect Conserv 19, 205–216 (2015). https://doi.org/10.1007/s10841-014-9710-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9710-0