Abstract
Background
Pulmonary vein isolation (PVI) is the most effective therapy to achieve rhythm control in atrial fibrillation (AF). Peri-procedural imaging is used in many but not all centers. However, the impact of imaging on safety and efficacy of PVI is not clear. The Israeli Catheter Ablation Registry (ICAR) is a great opportunity to explore this issue in real-world practice.
Aim
To describe the real-world utilization of peri-procedural imaging technologies in a large cohort of patients undergoing ablation for AF.
Methods
A prospective-multicenter cohort of AF patients who underwent PVI during the years 2019–2021. Peri-procedural imaging (CT, ICE, TEE) was utilized based on the center and operator discretion. The study endpoints were peri-procedural complications and AF recurrence at 12 months follow-up among patients with and without peri-procedural imaging.
Results
Between January 2019 and December 2021, a total of 921 patients underwent PVI. Peri-procedural imaging (at least 1 modality of CT, TEE, and or ICE) was utilized in 753 (81.8%) and no imaging among 168 (18.2%) patients. Cryoablation was the dominant energy used for PVI in both groups (92.3% of the non-imaging group, and 95.3% among imaging group), while RF was used in the rest of the patients. Fluoroscopy time was not different between the 2 groups; however, procedure duration was longer among the imaging group (90 min) compared to the non-imaging group (74.5 min, p = 0.006). By 12 months, the incidence of AF recurrence and repeated ablation were not different between the groups. Complications and re-hospitalization for cardiocerebrovascular reasons were not different among the 2 groups. Cox regression model demonstrated no association between preprocedural imaging and the risk of AF recurrence after ablation.
Conclusion
This real-world multicenter prospective registry study demonstrated that the rate of complications and the rate of recurrence of AF during 1 year follow-up were not different among patients who had PVI either with or without peri-procedural imaging.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The prevalence of atrial fibrillation (AF) is continuously rising worldwide in the context of aging population and increasing prevalence of the metabolic syndrome [1, 2]. According to a recent study, early rhythm control whether by anti-arrhythmic drugs (AADs) or catheter ablation, is associated with a lower risk of adverse cardiovascular outcomes [3]. Furthermore, catheter ablation is superior to anti-arrhythmic therapy in preventing AF recurrence [4].
Several technologies are available for catheter-based ablation including radiofrequency ablation, cryoballoon ablation, and recently pulse field ablation [5, 6]. Whatever the technology used, cardiac imaging plays a key role in patient selection and prediction of safety and efficacy for left atrium (LA) ablation procedures [7]. Computed tomography (CT) and/or magnetic resonance imaging (MRI) may be used to define anatomy prior to the ablation, transesophageal echocardiography (TEE) is often used to exclude the presence of left atrial appendage (LAA) thrombus; and intracardiac echocardiography (ICE) can be used to exclude LAA thrombus, guide trans-septal puncture and can be used for spatial guidance during the procedure. However, there is little evidence to demonstrate improved outcomes with any specific peri-procedural imaging strategy [8,9,10]. Furthermore, the available data has poorly described the real-world practice in recent years, as many centers particularly the small ones, do not have all the aforementioned facilities in every procedure and due to the introduction of several one shot ablation technologies, mainly the cryoballoon ablation. The aim of this study was to describe the real-world utilization of peri-procedural imaging modalities in a broad cohort of patients undergoing ablation for AF. The Israeli Catheter Ablation Registry (ICAR) provides an excellent opportunity to explore the impact of peri-procedural imaging on the outcomes of catheter ablation in AF patients in the real world.
2 Methods
2.1 Design and participants
The ICAR is a prospective multicenter registry of all patients undergoing AF ablation in 14 electrophysiology centers in the country during 2019–2021. The registry is a collaborative effort between the community of cardiac electrophysiologists and is managed by the Israeli Center for Cardiovascular Research (ICCR). The registry was approved by the ethics committee of each participating institution, and all patients provided written informed consent. For the purpose of the present analysis, we included only patients who underwent PVI during the years of enrollment. Patients who could not sign an informed consent were excluded.
2.2 Catheter ablation procedure
PVI was performed as previously described using cryoballoon (vast majority) or point-by-point radiofrequency (RF) ablation guided by 3D electroanatomical mapping on the discretion of centers and operators [5].
The use of preprocedural imaging modalities (CT, TEE), and intraprocedural imaging modalities including ICE or TEE was based upon physician preference. Ablation was performed under conscious sedation or general anesthesia, per local practice. In both techniques, PV isolation was confirmed using entrance and exit blocks by pacing maneuvers. Cavotricuspid isthmus ablation in the event of documented right atrial flutter was performed at the operator discretion.
2.3 Data collection
Data were collected prospectively at the index hospital admission at the time of ablation by the local electrophysiologists and entered into a secure (firewall and password protected) web-based electronic case report form, using the REDCap software. All the electrophysiologists in all centers who were involved in this registry were familiar with study requirements and were part of the study design.
Variables collected include demographic data, AF type, concomitant and previous anticoagulation and AADs, comorbidities, left ventricular ejection fraction (EF), LA size in echocardiography, and procedural data such as skin-to-skin procedure time, fluoroscopy time, pulmonary veins anatomy, ablation strategy, and acute complications.
Following hospital discharge, endpoints were obtained by contacting each patient and by reviewing the clinic visits and hospital course if the patient had been re-hospitalized.
Typical setup for institution follow-up was clinical ambulatory visits at 3, 6 months, and at 1 year or sooner for symptoms suggestive of arrhythmia recurrence. Every visit included evaluation of symptoms, 12 leads ECG, and 48 h Holter by senior electrophysiologist. All episodes of arrhythmias detected by ECG and or 48 h Holter or highly suspected self-report episodes were counted, Furthermore, all DC cardioversions, re-do procedures or AADs were documented. All recurrent episodes had to be confirmed by a senior electrophysiologist irrespective of the method of detection.
All acute and peri-procedural complications (cardiac tamponade, thromboembolism, stroke, transient ischemic attack, phrenic nerve paralysis, heart block, pericarditis, vascular complications requiring intervention or prolonged hospital stay, atrioesophageal fistulae and death) were also registered after being confirmed by senior electrophysiologist.
After ablation, all patients were discharged on oral anticoagulation for at least 2 months. Oral anticoagulation and AADs were discontinued at the discretion of the treating physician.
For the aim of this study, patients were divided into 2 groups. The first group (the imaging group) included patients who had at least 1 peri-procedural imaging modality: CT, TEE, and/or ICE. The second group (the non-imaging group) included patients without any peri-procedural imaging modality.
2.4 Study endpoints
Demographic characteristics, comorbidity, medical therapy, echocardiographic parameters, peri-procedural data, and outcomes were compared between patients with peri-procedural imaging and patients without.
2.5 Primary endpoints
-
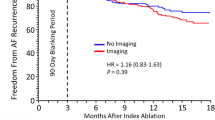
The primary effectiveness endpoint was the incidence of AF recurrence during 1 year follow-up (defined as episode lasting for at least 30 s after the 90-day blanking period) demonstrated on a clinical 12-lead ECG tracing or ambulatory monitor. In accordance with the 2017 expert consensus statement on catheter ablation, recurrences of atrial tachyarrhythmia during the first 90 days after the index ablation (the “blanking period”) were not counted in the determination of the first clinical failure for the endpoint [2].
-
The primary safety composite outcome of cardiovascular events (stroke, pericardial effusion, pericardial tamponade, heart failure, esophageal injury, PV stenosis, and death) was the occurrence of 1 or more event during the 30-day period following the ablation procedure. Safety endpoints were collected from peri-procedural complications reported by the operator and from adverse events occurring within 30 days after ablation.
2.6 Statistical analysis
Normally distributed continuous variables are presented as mean ± standard deviation. In case of non-normality, medians [interquartile ranges] are provided. Categorical variables are presented as numbers (percentages). Comparisons of proportions or means across groups were done using the chi-square test for categorical variables and unpaired t-test for continuous variables, or Mann–Whitney in case of non-normality.
Cox proportional hazard regression models were used to assess the association between peri-procedural imaging and time to efficacy endpoints. Hazard ratios (HRs) and 95% confidence intervals were calculated for the groups.
Differences were considered statistically significant at the 2-sided P value of < 0.05. Statistical analyses were performed using R version 3.6.1 (R foundation for statistical computing, Vienna, Austria).
3 Results
Between January 2019 and December 2021, a total of 921 patients underwent pulmonary vein isolation (PVI). Peri-procedural imaging (at least 1 modality of CT, TEE, and or ICE) was utilized in 753 (81.8%) and no imaging among 168 (18.2%) patients. Baseline characteristics are listed in Table 1. Age, hypertension, diabetes mellitus, stroke/transient ischemic attack (TIA), NYHA classification, CHA2DS2-VASc score, left ventricular (LV) hypertrophy, LA size, significant (at least moderate) mitral regurgitation, AF duration and classification were not significantly different between the 2 groups (Table 1). Males made up 64.9% of the imaging group and 53.6% of the non-imaging group (p = 0.008). Anticoagulants were used more frequently among non-imaging group (92.9%) compared to imaging group (86.4%, p = 0.031). Baseline AAD use was not different between the two groups (non imaging 67.7%, versus imaging 71%, p = 0.445).
The technology used for ablation is shown in Table 2. Cryoablation was the dominant energy used for PVI in both groups (92.3% of the non-imaging group, and 95.4% among imaging group), while RF was used only in 7.7% in non-imaging group and 4.6% among the imaging group. Among the imaging group, most patients (72.6%) had only 1 peri-procedural imaging modality, and 25.9% of patients had 2 imaging modalities (Table 2).
PV anatomy was not different between the 2 groups (Table 3). All PVs were acutely isolated in the two groups. Fluoroscopy time was not different between the groups (23 min among non-imaging group, vs 21 min among imaging group, p = 0.184); however, procedure duration was longer among the imaging group (90 min) compared to the non-imaging group (74.5 min, p = 0.006).
3.1 Primary effectiveness outcomes
During 12 months follow-up, 0.6% of the non-imaging group and 1.1% of the imaging group died (not related to AF). Finally, 92.3% of the non-imaging group (n = 155) and 93.9% of the imaging group (n = 707) completed the 12 months follow-up (Table 4).
3.2 AF recurrence
Utilization of AADs post ablation was not significantly different between the groups (non-imaging 18.45%, vs 21.1% imaging, p = 0.16). By 12 months after the blanking period, the incidence of AF recurrence was 13.5% among non-imaging compared to 17.5% among imaging group p = 0.278) (Table 4). Repeated ablation at 12 months was 6.5% in the non-imaging group compared to 8.3% among the imaging group (p = 0.533). Rehospitalization was more common among the imaging group (24.3%) compared to non-imaging group (15.9%, p = 0.033). However, re hospitalization for cardiovascular reasons was not different among the 2 groups (Table 4).
Of note, we also compared patients with 2 imaging modalities to patients with 1 imaging modality and did not find any difference in AF recurrence (16% vs 18.8%, respectively. p = 0.6).
Cox regression model demonstrated no association between preprocedural imaging and the risk of AF recurrence after blanking period with adjusted HR of 1.4 (95% confidence interval 0.88–2.22; p = 0.16). Persistent AF was found to be a predictor of AF recurrence after blanking period with adjusted HR 1.73 (95% confidence interval 1.14–2.63, p = 0.01).
3.3 Adverse events
Adverse events were infrequent and did not vary significantly between imaging and non-imaging groups (Table 5). The most common complication was vascular complications (1.3% among imaging group and 1.7% among non-imaging group). Neurologic event occurred in 0.7% of imaging group and 0.6% of non-imaging group. One patient from the imaging group (0.1%) had tamponade.
4 Discussion
The main findings of this real-world multicenter prospective registry are as follows: (I) wide range of variability in using peri-procedural imaging modalities in AF ablation. (II) The rate of peri-procedural complications and the rate of AF recurrence during 1 year follow-up were not different among patients who underwent PVI with or without peri-procedural imaging. (III) The use of peri-procedural imaging was not found to be a predictor of AF recurrence during 1 year follow-up.
The goal of peri-procedural imaging is to obtain a detailed anatomical description of the pulmonary veins, to exclude presence of thrombus in the LA/LAA, to define the prognostic factors, and to guide trans-septal puncture [11]. Multidetector CT angiography effectively and simply meets nearly all of these needs. The CT may also help to detect a LAA that had not been previously assessed by TEE [12]. It also allows the operator to quickly create the correct electroanatomical map (EAM) and proceed with ablation with or without any attempt at image integration when 3D system is used. About 55% of the patients in the imaging group in our cohort had CT. However, there is a wide range of variability in using peri-procedural imaging and the evidence for efficacy is controversial. Based on nationwide claims data, Steinberg et al. [13] identified associated imaging studies in addition to EAM among 11, 525 patients who underwent AF ablation (from 2007 to 2009) before and during ablation. In addition to electroanatomic mapping, 53% underwent TEE, 67% received ICE, and 50% underwent a pre-procedure CT or MRI. After adjustment, the use of pre-ablation CT or MRI was associated with a significantly lower risk of stroke or TIA (0.4% vs 0.9%, p = 0.002), and the use of ICE was associated with a lower risk of repeat ablation (5.7% vs 8.5%, p = 0.02) but higher risk of bleeding (1.1% vs 0.7%, p = 0.009). Other studies reported lower arrhythmia recurrence rate after AF ablation using EAM with image integration versus without image integration [14, 15]. However, a more recent study reported no significant improvement in clinical outcome between the image integration group vs no image integration group [16]. On the other hand, there was a significant decrease in fluoroscopy time in the image integration group vs. without image integration group.
A meta-analysis of randomized controlled trials by Mammadi et al. showed that CT image integration with EAM to guide RF ablation for AF does not improve clinical and procedural outcomes [17]. The authors explained their conclusions by the fact that the integrated map may not be completely accurate as slight shifts may occur from the pre-procedure acquired map and the intraoperative map.
The common practice during the period of our registry is different in some aspects than before. The technique of ablation has evolved since the introduction of AF ablation and it is now recognized that the challenge of catheter ablation of AF is no longer the anatomy but rather the durability of the lesions that circumferentially isolate the pulmonary veins. The durability is more often due to adequate tissue contact, power, duration of energy delivered and ablation strategy [12]. Nowadays, the cryoballoon constitutes an established alternative to radiofrequency ablation for PVI, which offers the possibility to isolate the PVs with a single application. As mentioned in the results section, the vast majority of patients in our registry had PVI using cryoballoon. In general, pre-procedural and or intra-procedural imaging beside fluoroscopy is not mandatory in AF ablation using cryoballoon. Knowing the anatomy of the pulmonary veins by pre-procedural CT could help to plan the procedure but is not crucial. One study elucidated several key anatomical features of pulmonary veins based on pre-procedural CT possibly affecting acute success, AF recurrence and complications in patients with AF using cryoballoon ablation [18]. However, previous studies have shown that the efficacy of cryoballoon ablation is independent of the PV anatomy. One study suggested that mid-term outcomes of cryoballoon ablation for paroxysmal AF ablation are similar to those of radiofrequency, regardless of PV anatomy [19], and the presence of anatomical variants of PVs should not discourage the referral of patients with paroxysmal AF for cryoballoon ablation [19].
The variability in utilizing peri-procedural imaging to exclude LAA thrombus is even more prominent mainly in the era of novel direct oral anticoagulants (DOACs). An increasing number of patients with AF are managed with DOACs, and these are generally a preferred choice over warfarin in eligible patients. Similarly, an increasing number of patients are undergoing ablation procedures for AF with peri-procedural anticoagulation with DOACs in contrast to warfarin which was the main oral anticoagulant for many years. Performing AF ablation with uninterrupted anticoagulation is a recommended strategy that has been shown in multiple studies to provide stroke protection without increasing bleeding risks [2]. For the purpose of preventing peri-procedural thromboembolism, many centers perform TEE, CT, or intraprocedural imaging of the LAA using ICE to assess for LAA thrombi in addition to uninterrupted anticoagulation strategy. There is a wide range of variability in using TEEs in patients undergoing AF ablation and in a task force survey, about 50% of expert members indicated that they perform TEE in all patients undergoing AF ablation regardless of individual stroke risk [20]. On the other hand, one study illustrates that performing AF ablation with ICE guidance on uninterrupted rivaroxaban for at least 4 weeks even without TEE is feasible and safe [21]. Diab et al. reported based on a prospectively maintained data registry that in DOAC compliant patients who present for ablation in AF/atrial flutter, the procedures could be performed without TEE screening or ICE imaging of the appendage, with low risk of complications [22]. In our study, the thromboembolic event rate was low and not statistically different between the 2 groups. Accordingly, it may be feasible to perform AF ablation using uninterrupted anticoagulation strategy without peri-procedural imaging of the LAA. However, this important issue should be clarified by randomized studies.
Fluoroscopy time was not different between the 2 groups, but procedure duration was longer among the imaging group, compared to the non-imaging group. However, this data should not underestimate the potential role of peri-procedural imaging in reducing fluoroscopy time. Most of the patients in this registry (about 95%) had cryoballoon ablation and the positioning of cryoballoon and confirming venous occlusion rely mainly on fluoroscopy in our common practice. Efforts have been made to reduce fluoroscopic time to “as low as reasonably achievable,” as recommended by the American College of Cardiology with the ALARA statement [23]. Consequently, fluoroless or near-fluoroless RF catheter ablation for AF has been enabled using a three-dimensional (3D) EAM system, TEE, or ICE [24, 25]. Recent studies have reported a reduction in radiation with TEE, ICE, or pressure-guided cryoablation [26, 27]. Moreover, one study showed that ICE–guided fluoroless cryoballoon ablation for paroxysmal AF was a feasible strategy without compromising acute and long-term success or complication rates compared to conventional fluoroscopy-guided cryoballoon ablation [28].
4.1 Limitations
Our study has several limitations. (I) This is a non-randomized study. (II) The type of peri-procedural imaging used during the procedure depends on the availability of these modalities and on the preference and the experience of the operators in every center. Thus, there is a high variability in utilizing peri-procedural modalities among the different centers and operators. (III) Our results cannot be generalized to other technologies since the vast majority of patients had cryoballoon ablation. Of note, similar results were found when the analysis included only patients who had cryoablation. (IV) We did not use implantable loop recorder for the detection of asymptomatic recurrence of AF. Thus, asymptomatic episodes of AF could be missed. We think that asymptomatic episodes would not significantly affect the results, as the missing data might be balanced between the two groups.
5 Conclusions
This real-world multicenter prospective registry study demonstrated that the rate of peri-procedural complications and the recurrence of AF during 1 year follow-up were not different among patients who underwent PVI either with or without peri-procedural imaging. The use of peri-procedural imaging was not found to be a predictor of AF recurrence during 1 year follow-up. Randomized studies are needed to evaluate the impact of each modality on the efficacy and safety of each technology used for PVI.
References
Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–98. https://doi.org/10.1378/CHEST.11-2888.
Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. EP Europace. 2018;20:e1–160. https://doi.org/10.1093/EUROPACE/EUX274.
Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–16.
Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–74. https://doi.org/10.1001/JAMA.2019.0693.
Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–45.
Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, Mountantonakis SE, Gibson DN, Harding JD, Ellis CR, Ellenbogen KA, DeLurgio DB, Osorio J, Achyutha AB, Schneider CW, Mugglin AS, Albrecht EM, Stein KM, Lehmann JW, Mansour M, ADVENT Investigators. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med 2023;389:1660–1671. https://doi.org/10.1056/NEJMoa2307291.
Bhagirath, et al. Multimodality imaging for patient evaluation and guidance of catheter ablation for atrial fibrillation - current status and future perspective. Int J Cardiol. 2014;175:400–8.
Bunch TJ, Day JD. Examining the risks and benefits of transesophageal echocardiogram imaging during catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:621–3.
Ferguson JD, Helms A, Mangrum JM, et al. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol. 2009;2:611–9.
Hamdan A, Charalampos K, Roettgen R, et al. Magnetic resonance imaging versus computed tomography for characterization of pulmonary vein morphology before radiofrequency catheter ablation of atrial fibrillation. Am J Cardiol. 2009;104:1540–6.
Ohana M, Bakouboula B, Labani A, Jeung M-Y, El Ghannudi S, Jesel-Morel L, Roy C. Imaging before and after catheter ablation of atrial fibrillation. Diagn Interv Imaging. 2015;96:1113–23.
Teo WS. Is preprocedural imaging before radiofrequency catheter ablation of atrial fibrillation and image integration useless? J Arrhythmia. 2021;37:556–7.
Steinberg BA, Hammill BG, Daubert JP, Bahnson TD, Douglas PS, Qualls LG, Pokorney SD, Calkins H, Curtis LH, Piccini JP. Periprocedural imaging and outcomes after catheter ablation of atrial fibrillation. Heart. 2014;100:1871–7. https://doi.org/10.1136/heartjnl-2014-306067.
Della BP, Fassini G, Cireddu M, et al. Image integration-guided catheter ablation of atrial fibrillation: a prospective randomized study. J Cardiovasc Electrophysiol. 2009;20:258–65.
Bertaglia E, Bella PD, Tondo C, et al. Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMerge Italian Registry. Europace. 2009;11:1004–10.
Caponi D, Corleto A, Scaglione M, et al. Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome?: a randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace. 2010;12:1098–104.
Mammadi A, Demirtola AI, Diker E. Impact of image integration on clinical and procedural outcomes of radiofrequency catheter ablation of atrial fibrillation: a meta-analysis of randomized controlled trials. J Arrhythmia. 2021;37:550–5. https://doi.org/10.1002/joa3.12508.
Hayashi T, Murakami M, Saito S, Iwasaki K. Characteristics of anatomical difficulty for cryoballoon ablation: insights from CT. Open Heart. 2022;9:e001724. https://doi.org/10.1136/openhrt-2021-001724.
Khoueiry Z, Albenque JP, Providencia R, Combes S, Combes N, Jourda F, Sousa PA, Cardin C, Pasquie JL, Cung TT, Massin F, Marijon E, Boveda S. Outcomes after cryoablation vs. radiofrequency in patients with paroxysmal atrial fibrillation: impact of pulmonary veins anatomy. Europace. 2016;18:1343–51. https://doi.org/10.1093/europace/euv419.
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171–257. https://doi.org/10.1007/s10840-012-9672-7.
Tsyganov A, Shapieva A, Sandrikov V, Fedulova S, Mironovich S, Dzeranova A, Lyan E. Transesophageal vs. intracardiac echocardiographic screening in patients undergoing atrial fibrillation ablation with uninterrupted rivaroxaban. BMC Cardiovascular Disorders. 2017;17:171. https://doi.org/10.1186/s12872-017-0607-1.
Diab M, Wazni OM, Saliba WI, Tarakji KG, Ballout JA, Hutt E, Rickard J, Baranowski B, Tchou P, Bhargava M, Chung M, Varma N, Martin DO, Dresing T, Callahan T, Cantillon D, Kanj M, Hussein AA. Ablation of atrial fibrillation without left atrial appendage imaging in patients treated with direct oral anticoagulants. Circ Arrhythm Electrophysiol. 2020;13:e008301. https://doi.org/10.1161/CIRCEP.119.008301.
Limacher MC, Douglas PS, Germano G, Laskey WK, Lindsay BD, McKetty MH, et al. ACC expert consensus document. Radiation safety in the practice of cardiology. American College of Cardiology. J Am Coll Cardiol. 1998;31:892–913.
Cha MJ, Lee E, Oh S. Zero-fluoroscopy catheter ablation for atrial fibrillation: a transitional period experience. J Arrhythm. 2020;36:1061–7.
Falasconi G, Penela D, Soto-Iglesias D, Jáuregui B, Chauca A, Antonio RS, et al. A standardized stepwise zero-fluoroscopy approach with transesophageal echocardiography guidance for atrial fibrillation ablation. J Intervent Cardiac Electrophysiol. 2022;64:629–39.
Siklódy CH, Minners J, Allgeier M, Allgeier HJ, Jander N, Weber R, et al. Cryoballoon pulmonary vein isolation guided by transesophageal echocardiography: novel aspects on an emerging ablation technique. J Cardiovasc Electrophysiol. 2009;20:1197–202.
Alyesh D, Venkataraman G, Stucky A, Joyner J, Choe W, Sundaram S. Acute safety and efficacy of fluoroless cryoballoon ablation for atrial fibrillation. J Innov Card Rhythm Manag. 2021;12:4413–20.
Ahn J, Shin DG, Han SJ, Lim HE. Safety and efficacy of intracardiac echocardiography-guided zero-fluoroscopic cryoballoon ablation for atrial fibrillation: a prospective randomized controlled trial. Europace. 2023;25:euad086. https://doi.org/10.1093/europace/euad086.
Acknowledgements
Thanks to Mr. Oded Sabah, BS.c, for his contribution to the statistical analysis of the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study followed the Declaration of Helsinki. Ethical approval was obtained from the ethics committee of Tzafon Medical Center, Tiberias, Israel. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marai, I., Elias, A., Rozen, G. et al. The impact of peri-procedural imaging on safety and efficacy of atrial fibrillation ablation: insights from the Israeli AF Catheter Ablation Registry (ICAR). J Interv Card Electrophysiol (2024). https://doi.org/10.1007/s10840-024-01887-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10840-024-01887-8