Abstract
Purpose
Left bundle branch area pacing (LBBAP) has emerged as a physiological and stable form of pacing. We aim to compare the mechanical ventricular synchrony of LBBP, LBFP, and LVSP.
Methods
Proximal Left bundle branch pacing (LBBP), left bundle fascicular pacing (LBFP) and left ventricular septal pacing (LVSP) were identified in patients with bradycardia who successfully underwent LBBAP. Patients with left ventricular ejection fraction (LVEF) < 50% or QRS duration (QRSd) ≥ 120 ms were excluded. By using electrocardiograms, the left ventricular activation time (LVAT) and QRS duration (QRSd) were measured to examine electrophysiological synchrony. As indications of mechanical synchrony, global longitudinal strain (GLS), global circumferential strain (GCS), global radial strain (GRS), and peak strain dispersion (PSD) were evaluated by using 2-dimensional speckle tracking echocardiography (2D-STE).
Results
In 56 patients, data were collected during LBBP (n = 18), LBFP (n = 16), and LVSP (n = 22). LVSP resulted in a longer LVAT (91.3 ± 14.9 ms) than LBBP (77.1 ± 10.8 ms, P < 0.05) and LBFP (72.1 ± 9.6 ms, P < 0.05), but all three groups had similar QRSd. There were no differences in GLS, GCS, GRS, or PSD between LBBP, LBFP, and LVSP.
Conclusions
In patients with normal cardiac function and narrow QRS, though LBBAP with LBB capture resulted in better electrophysiological synchrony than without, the mechanical synchrony of the three groups was comparable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
His bundle pacing (HBP) has been considered the most physiological form of pacing alternative to right ventricle pacing (RVP). Despite its benefits, HBP's practical use has been limited due to higher capture thresholds, lower success rates in maintaining stable capture, and the potential need for lead revisions. This has led to the increased popularity of left bundle branch area pacing (LBBAP) as a viable alternative. Consequently, extensive research has been conducted to verify the feasibility and safety of LBBAP as well as to identify determinants of optimal clinical outcomes of LBBAP [1, 2]. It has been established that pacing-induced contraction dyssynchrony can significantly impact the left ventricular (LV) mechano-energetic function, leading to reduced cardiac efficiency and increased risk of heart failure. This highlights the importance of both electrophysiological and mechanical synchrony in pacing strategies.
The subtypes of LBBAP, including proximal left bundle branch pacing (LBBP), left bundle fascicular pacing (LBFP), and left ventricular septal pacing (LVSP), have distinct characteristics such as left bundle branch (LBB) capture, capture selectivity and lead tip location, which can impact electrophysiological synchrony [3]. Mechanical synchrony plays a critical role in achieving optimal pacing left ventricular function. Previous studies has shown ventricular superior mechanical synchrony of LBBP compared to LVSP in patients with heart failure [4, 5], yet, there is a limited body of research comparing this aspect between the different subtypes in patients without heart failure or conduction disorder.
In this study, we aim to further evaluate the mechanical synchrony of proximal LBBP, LBFP, and LVSP using 2D-STE in patients with normal cardiac function. This will provide valuable insights into the impact of different LBBP subtypes on LV mechanical synchrony and inform clinical decision making for optimal pacing strategies because of the critical role of mechanical synchrony in achieving optimal left ventricular function.
2 Methods
2.1 Study design and population
We conducted a single-center, retrospective observational study. We enrolled 56 patients who successfully underwent left bundle branch area pacing between August 2018 and April 2022 in Chaoyang Hospital. Pacing indications included sick sinus syndrome, atrioventricular block, and atrial fibrillation with symptomatic bradycardia. Patients with heart failure, left ventricular ejection fraction(LVEF) below 50%, severe valvular disease, previous history of valvular implantation or replacement, recent (< 3 monthes) myocardial infarction or myocardiopathy such as hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) were excluded from our study. Furthermore, the exclusion criteria for the study also encompassed patients with prolonged QRS duration (≥ 120 ms), such as bundle branch block and non-specific intraventricular conduction delay. Patients were divided into proximal LBBP group, LBFP group and LVSP group according to specific criteria (Figs. 1 and 2). For clear definition, the patients in the LBBP group mentioned in this article are all pacing in the proximal left bundle branch.
Algorithm of LBB capture. The algorithm starts with the top of the hexagon, if the transition of QRS morphology from ns-LBBP to s-LBBP or ns-LBBP to LVSP is recognized, LBB capture can be comfirmed. If not, than observe the change of LVAT during threshold test is important. LBB capture can be confirmed by abrupt shortening of LVAT(> 10 ms) or constant and short LVAT < 80 ms. After excluding the physiological criteria of LBB capture(When LBB potential is present, the stim-V5RWPT should be similar to LBB potential-V5RWPT; when LBB potential is absent, stim-V5RWPT should be similar to intrinsic V5RWPT), if V6-V1 interpeak interval > 40 ms can not be fulfilled, then we can exclude LBB capture and confirm LVSP
2.2 LBBAP device implantation procedure
The implantation procedure has been described in our previous studies [6]. To summarize, the 3830 lead (Select Secure System, Medtronic, Inc., Minneapolis, MN, USA) is advanced through the right side of the interventricular septum after crossing the tricuspid annulus. And 1.0–1.5 cm below his bundle was referred to as an anatomical marker for the initial LBBAP target zone. While screwing the lead tip towards the left ventricular subendocardium, the final deployment site was determined by unipolar paced QRS morphology and intracardiac electrogram. Successful LBBAP was defined as narrow QRS with RBBB pattern in V1 and V2 at a low threshold (< 1.0 V/0.5 ms).
2.3 Definitions of LBBP, LBFP and LVSP
According to previous studies [7,8,9] and the most recent RCT [1], in our study, the criteria we used for LBB capture was listed in Supplementary material, and the criteria we used for LBB capture were listed in Fig. 1. Briefly speaking, proximal LBBP is defined as LBBAP with capture of proximal left bundle branch. LBFP is defined as LBBAP with the capture of fascicular of left bundle, including capture of left anterior fascicle, left posterior fascicle and left septal fascicular. LVSP is LBBAP capturing left ventricular myocardium without definite LBB capture. The distingushing point of LVSP and proximal LBBP/LBFP is whether the left bundle branch is captured. The differential criteria for proximal LBBP and LBFP is the interval between LBB potential and QRS wave, as illustrated in Fig. 2. Supplemental Fig. 1 shows electrocardiogram (ECG) of the three groups and Supplemental Fig. 2 shows the measurements for LBB potential to QRS onset and amplitude.
2.4 Conventional echocardiographic examination
Conventional echocardiography was obtained 1 day to 1 week before permanent pacing device implantation and follow-up at least 6 months after the procedure. Each patient was examined by the same experienced ultrasound specialist blind to grouping in order to avoid bias. All conventional analysis was based on the current guidelines of the American Society of Echocardiography [10]. Left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter(LVESD) were measured from the parasternal long-axis view; left end-diastolic volume (EDV) and left ventricular end-systolic volume (ESV) were collected from biplane view. Left ventricular ejection fraction (LVEF) was calculated from EDV and ESV, using modified Simpson’s rule.
As our previous study described, from the apical four or five-chamber view, measurement of lead tip to LV and RV endocardium were collected during both the end-diastolic and the end-systolic phases. To provide a more detailed description of the pacing lead location, measurements were taken from the lead tip to the tricuspid ring and the apex. As illustrated in Supplemental Fig. 3, parasternal or apical four-chamber view was selected to display the position of the lead in the interventricular septum and the attachment point of the tricuspid valve leaflet.
2.5 Evaluation of echocardiographic ventricular synchrony
All patients with dual chamber devices were initially programmed in DDD mode. Before the examination, to ensure the assessment is performed during ventricular pacing, we temporarily reset the programming according to specific circumstances. The programming remains unchanged: (1) in AF patients undergone AV node ablation or patients with high degree AVB; (2) in patients with ventricular pacing percentage of almost 100%; The programming was adjusted to VVI mode and set at least 10 bpm more than the native rate to ensure ventricular capture and avoid intrinsic conduction or fusion beat. Patients with AF who failed to capture equal R-R interval for at least five cardiac cycles during the echocardiographic evaluation were excluded.
Two-dimensional speckle tracking echocardiography (2D-STE) was conducted by an experienced echocardiologist (LLS) who was blinded to the grouping of the participants. Echocardiographic images were taken using GE Vivid E9 (GE Health- care, Horten, Norway), with an M5S probe, and stored electronically for later offline analysis with EchoPAC 201 (GE Healthcare, Horten, Norway). Images from the parasternal long-axis and short-axis, apical four-chamber, and two-chamber views were captured during breath-hold at the end of expiration. Five cardiac cycles were obtained for each acquisition. The region of interest was analyzed semi-automatically and adjusted manually to encompass the whole myocardium [11].
2.6 Statistical analysis
Continuous variables were reported as mean ± standard deviation or median (Q1–Q3), and categorical variables were reported as absolute numbers or percentages. Shapiro–Wilk test and Kolmogorov–Smirnov test were used to test normality. For the sample size calculation, according to previous research by Hou et al., we estimated that the LVSP group would show a 36.4% increase in PSD. And we postulated that the LBFP group would show no change in PSD compared to the LBBP group. For LBBAP patients with LBB potential, the mean and standard deviation of PSD were 51.14 and 17.69, respectively. Therefore, to detect a 36.4% difference in PSD (51.14 × 0.364 = 18.61) between the LVSP groups, the statistical power analysis indicated that 16 participants would have to complete the trial in each group to achieve 80% power (two-sided test, a = 0.05), assuming a within-participants standard deviation of 17.69. To compare the three groups, ordinary one-way ANOVA was used, and the Holm-Sidak test for multiple comparisons. For parameters of which SDs were significantly different among three groups according to Bartlett’s test, Brown-Forsythe and Welch tests were used. Kruskal–Wallis tests were used instead for data with skewed distribution. P < 0.05 was considered to represent statistical significance. Data are shown as Tukey box plots, with the mean value indicated by a line, unless otherwise stated. Microsoft Excel (Microsoft 365 MSO version 1904) was used to collect data. GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis. The sample size calculation was estimated using PASS V.15 (NCSS, Kaysville, Utah, USA).
3 Results
3.1 Baseline characteristics
A total of 56 patients were enrolled in our study. According to previously defined criteria, there were 18 (32.1%) patients in the LBBP group, 16 (28.6%) patients in the LBFP group, and 22 (39.2%) patients in the LVSP group. Baseline characteristics including pacing indications and comorbidities are presented in Table 1. The median follow-up time was 22.1 months.
3.2 Electrophysiological characteristics and synchrony
The electrophysiological characteristics and comparison of electrophysiological synchrony are presented in Table 2. The time from LBB potential to QRS onset in was significantly longer in proximal than distal left bundle paing (28.4 ± 3.1 ms in LBBP vs. 18.3 ± 3.2 ms in LBFP, P < 0.05). LBB potential amplitudes were similar in proximal and distal LBBP, 0.47 ± 0.56mv and 0.61 ± 0.53mv, respectively. The baseline and paced QRS durations, as well as the change in QRS axis, were comparable across all three groups. Concerning characteristics of lead V1, there was no significant difference in paced QRS duration, r wave amplitude, r wave duration, or amplitude of negative wave in lead V1. The average V6-V1 interpeak interval was 31.8 ± 24.0 ms, 36.5 ± 15.3 ms, and 25.7 ± 18.1 ms in LBBP, LBFP, and LVSP groups, respectively. But no differences were identified by the ANOVA analysis of the V6-V1 interpeak interval among the three groups. The difference in RWPT in V5 and V6 among the three groups was statistically significant. V6RWPT was much shorter in LBBP and LBFP compared to LVSP (77.1 ± 10.8 ms, 72.1 ± 9.63 vs. 91.3 ± 14.9 ms). However, there was no difference between LBBP and LBFP in V6RWPT. The results of V5RWPT were similar to V6RWPT.
3.3 Location of the pacing lead
Echocardiographic description of the lead tip location is demonstrated in the Table 3. The average depth of lead tip into the RV septum in diastolic phase was 10 mm. The average distance from the lead tip to the tricuspid annular was 21.2 ± 8.2 mm. And the LBFP group had shorter distance from lead tip to apex than LBBP (38.9 ± 8.5 vs. 46.8 ± 4.7 mm).
3.4 Echocardiographic characteristics and mechanical synchrony
Table 4 shows the data of conventional echocardiography of LBBAP patients during baseline and follow up. There was no statistically significant change in heart structure or function during follow up. The results of the conventional echocardiographic evaluation were comparable among three groups.
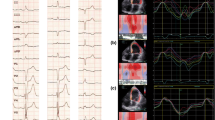
The comparison of myocardial strain in three groups by 2D-STE was illustrated in Fig. 3. As shown in the figures, there was no significant difference in GLS, GCS, GRS, or PSD among the three groups.
4 Discussion
4.1 Main findings
The results of our study demonstrated that there was no statistical difference in mechanical synchrony between proximal LBBP, LBFP, and LVSP patients.
4.2 Ventricular synchrony with vs. without LBB capture
The direct evidence to confirm LBB capture is retrograde his bundle potential or anterograde distal left conduction system activation [7]. However, this invasive method using his lead or multielectrode linear catheter is not routine in clinical practice. Therefore, various indirect evidence was combined to indicate LBB capture. LBB potential, a high-frequency signal from LBB lead, is a good indication of LBB capture suggests that the electrode is located either within or very close to the left bundle branch. However, the capture of the left bundle branch system also depends on the pacing output. LBB potential alone is not sufficient to conclusively confirm LBB capture or to rule out LVSP [7]. In contrast to previous studies, we used the criteria published by Wu et al. [9] and the most recent MELOS study [1] definition to diagnose LBB capture and distinguish between its subtypes in our cohort.
In patients with heart failure or intraventricular conduction delay, LBB capture plays a significant role in both electrophysiological and mechanical synchrony. Hou et al. [4] demonstrated that LBBAP patients with LBB potential had a shorter left ventricular activation time (LVAT), indicating a relatively delayed activation of the left ventricular free wall when pacing the septum without engaging the His-Purkinje system. Consistent with improved electrical synchrony, they also observed significantly better left ventricular mechanical synchrony in LBBAP patients with LBB potential compared to those without, as measured by phase analyses of SPECT MPI. Similarly in a more recent study based on tissue synchronization imaging (TSI) of 12 LV segments [5], LBBP resulted in better both electrophysiological and mechanical synchrony compared to LVSP in heart failure patients with cardiac resynchronization therapy indication.
However, this finding cannot be replicated in patients with narrow QRS and normal cardiac function. In our study, we found better electrical intraventricular synchrony in LBBP compared to LVSP, while no difference was detected in mechanical synchrony between LBBAP with LBB capture and without. These results are in line with those reported by Wang et al. and Huang et al. [12, 13]. They observed similar LV mechanical synchrony regardless of LBB potential appearance.
Although the exact mechanism behind the inconsistent results in patients with and without heart failure is not clear, there might be several possible explanations. First, in the context of normal cardiac function, no significant differences in QRS duration were observed regardless of left bundle branch (LBB) capture. Although endocardial activation mapping has yet to be performed, it is postulated that the ventricular activation sequence may be similar in cases of LBB activation potential with capture as well as without. Additionally, significant but slightly shortened LVAT does not always translate into increased myocardial strain measured by 2D-STE. In contrast, in patients with intrinsic dyssynchrony like HF or conduction disturbance, the improvement of electrophysiological synchrony by recruiting His-Purkinje system might be sufficient to result in improvement of mechanical synchrony. Second, we speculated that capturing the conduction system might reverse the electrical remolding of failing hearts [14]. This remodeling involves changes to gap junctions, ion channels, and molecular processes, all of which can impact the mechanical synchronization of the heart.
In summary, we suggested that, in patients without LV dysfunction, the long-term outcome of LBBAP without LBB capture may be noninferior to LBBAP with LBB capture.
4.3 Proximal LBBP vs. LBFP
A formal definition to distinguish between proximal LBBP and LBFP, which are also referred to as the proximal and distal left conduction system, has not been established yet. However, Jastrzebski et al. proposed that a time interval of 25 ms between the left bundle branch potential and the onset of the QRS complex can serve as a cutoff point for identifying LBFP, based on the anatomy of the conduction system [1]. As Huang et al. reported, 1.0–1.5 cm below the his bundle in RAO 30o fluoroscopic view was referred to as an anatomical marker for the initial LBBAP target zone. In our study, the position of the lead tip in LBFP was achieved 7.94 mm more apical than that of proximal LBB pacing. The pacing of LBB fascicles was achieved in 28.6% of enrolled patients. It can be postulated that the proportion of LBFP might be higher if the lead tips were not strictly positioned 1.0–1.5 cm from his bundle.
Controversies exist regarding the results of studies comparing electrophysiological ventricular synchrony between proximal LBBP and LBFP. Lin et al. [15] reported comparable results of LVAT and QRSd in LBFP and LBBP patients, whereas Liu et al. [16] observed slightly prolonged QRSd and larger paced QRS area in the LBFP group. In a more recent research [3], Weiping et al. found that, in the same patient with selective pacing of LCS, shorter LVAT and longer QRS were observed when pacing the LBB distally than proximally, indicating shorter Purkinje-to-myocardium interval and longer time to activate RBB. Our study showed similar results of LVAT and QRSd in the two groups, and for the first time demonstrated that mechanical synchrony was comparable in proximal and distal pacing patients. To explain these findings, we hypothesized that the comparable electrophysiological and mechanical synchrony between proximal LBBP and LBFP may be due to proximity of proximal LBB and it fasciculus and rapid conduction in his-Purkinje system. The conduction speed of activation in his-Purkinje system is 4-6 m/s. The proximal portion of LBB originates beneath the endocardium of the subaortic septal region and runs for 10-15 mm before ramifying in trifasicular fashion [17]. Therefore, the estimated LV activation delay of only 1.7–3.8 ms may not be significant enough to improve ventricular synchrony both electrophysiologically and mechanically. In addition, the delayed RBB activation in distal LBB pacing because of prolonged retrograde LBB to his conduction may be compensated by myocardial capture of the septum.
Despite the different results of previous studies in terms of LVAT and QRSd comparison, the reduction of electrophysiological synchrony did not necessarily lead to a difference in mechanical synchrony. Therefore, as indicated in the results of similar synchrony in proximal and distal pacing, the clinical outcome might also be comparable in patients without heart failure and wide QRS.
4.4 Clinical implications
Selecting the appropriate location of the lead tip appears to be essential to optimize long-term outcomes and simplify procedure techniques. In the aspect of anatomy, the LBB trunk is short and quickly fans out as fasciculi at the level of the septal leaflet of the tricuspid valve, and then the LBB fibers radiate as a subendocardial network. It is easier to reach the wider target area including both trunk and ramifications of LBB through the transseptal technique, rather than strictly positioning the lead tip at the short, narrow, and insulated area of the LBB trunk. In addition, as has been shown in the previous study of our group, lead tip with LBB capture is closer to the LV subendocardium and longer procedure time, which may be associated with higher complication risk and lower success rate of the procedure. Consequently, the noninferiority of LBFP and LVSP to LBBP may reduce attempts of lead deployments to achieve capture of the LBB trunk, thus decreasing the risk of septum perforation, damage to RBB or septal artery, etc.
4.5 Study limitations
First, it is important to note that the sample size of our cohort was relatively small. This may limit the generalizability of the findings and reduce the statistical power of the analysis. Second, since patients with heart failure, CRT indication, or conduction system block were excluded from our study, the results cannot be extended to the general population. Third, in some patients, the evaluation of myocardial strain was performed during VVI mode to ensure ventricular pacing. Dyssynchrony of the atrium and ventricle cannot be avoided, which may influence the interpretation of the results, especially in comparison to mechanical interventricular synchrony. Fourth, one of the total enrolled 56 patients was diagnosed with LBB capture without LBB potential according to abrupt shortened LVAT and V5RWPT < 80 ms. It was difficult to differentiate between proximal LBBP and LBFP according to QRS axis. Fifth, Previous research has indicated that there may be variation in heart function between chronic and acute pacing patients. In our current cohort, however, only 4 (%) patients (one from the LBBP group, two from the LBFP group, and one from the LVSP group) were temporarily reprogrammed to VVI mode for the echo measurement, which may unlikely impact the final results Sixth, Confounding factors, such as capture selectivity, and intrinsic conduction block, may influence overall ventricular synchrony. Right ventricular recruitment is achieved due to both anterograde RBB conduction and the left-to-right transseptal activation through intercellular conduction/inter-connection fibers between the left and right branches. Therefore, interventricular dyssynchrony can be influenced in the following circumstances: (1) fusion of LBB pacing and intrinsic activation, which can be improved by optimization of AV interval; (2) earlier recruitment of RV by capture of myocardium, especially in patients with RBBB and bidirectional LBBB. A strict definition of pacing selectivity and optimization of the AV interval should be implemented to avoid these biases.Finally, in our study, parameters such as LVAT, QRSd, and myocardial strain were used as indications of ventricular synchrony. There may be individual variability and poor correlation between these synchrony parameters and clinical outcomes like mortality and heart failure hospitalization.
5 Conclusion
In patient without heart failure, LBBAP with LBB capture demonstrated better electrophysiology than without. However, LVSP can achieve comparable mechanical ventricular synchrony compared to LBBP. In addition, there was no difference in both electrophysiological and mechanical synchrony between proximal and distal pacing of LCS.
Data Availability
Data are available from the corresponding author upon reasonable request.
References
Jastrzębski M, Kiełbasa G, Cano O, Curila K, Heckman L, De Pooter J, et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022;ehac445.
Ravi V, Hanifin JL, Larsen T, Huang HD, Trohman RG, Sharma PS. Pros and cons of left bundle branch pacing: a single-center experience. Circ Arrhythm Electrophysiol. 2020;13(12):e008874. https://doi.org/10.1161/CIRCEP.120.008874.
Sun W, Upadhyay GA, Tung R. Influence of capture selectivity and left intrahisian block on QRS characteristics during left bundle branch pacing. JACC: Clin Electrophysiol. 2022;8(5):635–47.
Hou X, Qian Z, Wang Y, Qiu Y, Chen X, Jiang H, et al. Feasibility and cardiac synchrony of permanent left bundle branch pacing through the interventricular septum. EP Europace. 2019;21(11):1694–702.
Zhang W, Chen L, Zhou X, et al. Resynchronization effects and clinical outcomes during left bundle branch area pacing with and without conduction system capture. Clin Cardiol. 2023;46(3):287–95. https://doi.org/10.1002/clc.23969.
Gao M, Tian Y, Shi L, Wang Y, Xie B, Qi J, et al. Electrocardiographic morphology during left bundle branch area pacing: Characteristics, underlying mechanisms, and clinical implications. Pacing Clin Electrophysiol. 2020;43(3):297–307.
Wu S, Chen X, Wang S, et al. Evaluation of the Criteria to Distinguish Left Bundle Branch Pacing From Left Ventricular Septal Pacing. JACC Clin Electrophysiol. 2021;7(9):1166–77. https://doi.org/10.1016/j.jacep.2021.02.018.
Jastrzębski M, Burri H, Kiełbasa G, Curila K, Moskal P, Bednarek A, et al. The V6–V1 interpeak interval: a novel criterion for the diagnosis of left bundle branch capture. EP Europace. 2022;24(1):40–7.
Jastrzębski M, Kiełbasa G, Curila K, Moskal P, Bednarek A, Rajzer M, et al. Physiology-based electrocardiographic criteria for left bundle branch capture. Heart Rhythm. 2021;18(6):935–43.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.
El Missiri AM. Echocardiographic assessment of left ventricular mechanical dyssynchrony – A practical approach. Egypt Heart J. 2014;66(3):217–25.
Huang J, Guo L, Zhang W, Li R, He B. Left bundle branch potential predicts better electrical synchrony in bradycardia patients receiving left bundle branch pacing. Bmc Cardiovasc Disor. 2022;22(1):376.
Wang S, Lan R, Zhang N, Zheng J, Gao Y, Bai J, et al. LBBAP in patients with normal intrinsic QRS duration: Electrical and mechanical characteristics. Pacing Clin Electrophysiol. 2021;44(1):82–92.
Cvijić M, Žižek D, Antolič B, Zupan I. Time Course of Electrical Remodeling of Native Conduction After Cardiac Resynchronization Therapy and Its Impact on Clinical Outcome. J Cardiac Fail. 2017;23(3):257–61.
Lin J, Hu Q, Chen K, Dai Y, Chen R, Sun Q, et al. Relationship of paced left bundle branch pacing morphology with anatomic location and physiological outcomes. Heart Rhythm. 2021;18(6):946–53.
Liu X, Gu M, Niu HX, Chen X, Cai C, Zhao J, et al. A Comparison of the Electrophysiological and Anatomic Characteristics of Pacing Different Branches of the Left Bundle Conduction System. Front Cardiovasc Med. 2022;5(8):781845.
Elizari M. The normal variants in the left bundle branch system. J Electrocardiol. 2017;50(4):389–99.
Funding
This study was supported by grants from Beijing Natural Science Foundation (Grant No. 7202050).
Author information
Authors and Affiliations
Contributions
Concept/design: Xing-Peng Liu. Data collection: Chaodi Cheng, Lan-Lan Sun, Xinyi Peng, Tong Zhao, Ziyu Wei. Procedure performing: Xing-Peng Liu, Ying Tian, Liang Shi, Yan-Jiang Wang. Data analysis/ interpretation: Chao-Di Cheng. Drafting article: Chaodi Cheng. Critical revision of the article: Xing-Peng Liu. Funding secured by: Xing-Peng Liu.
Corresponding author
Ethics declarations
Ethical approval
This study protocol was approved by the Ethics Committee of Beijing Chaoyang Hospital. And informed consent was obtained from all included patients.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, C., Sun, L., Peng, X. et al. Difference of ventricular synchrony between LBBP, LBFP and LVSP: A speckle tracking echocardiographic study. J Interv Card Electrophysiol 67, 539–547 (2024). https://doi.org/10.1007/s10840-023-01620-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-023-01620-x