Abstract
Background
The use of a multi-electrode Optrell mapping catheter during ventricular tachycardia (VT) or premature ventricular complex (PVC) ablation procedures has not been widely reported.
Objectives
We aim to describe the feasibility and safety of using the Optrell multipolar mapping catheter (MPMC) to guide catheter ablation of VT and PVCs.
Methods
We conducted a single-center, retrospective evaluation of patients who underwent VT or PVC ablation between June and November 2022 utilizing the MPMC.
Results
A total of 20 patients met the inclusion criteria (13 VT and 7 PVC ablations, 80% male, 61 ± 15 years). High-density mapping was performed in the VT procedures with median 2753 points [IQR 1471–17,024] collected in the endocardium and 12,830 points [IQR 2319–30,010] in the epicardium. Operators noted challenges in manipulation of the MPMC in trabeculated endocardial regions or near valve apparatus. Late potentials (LPs) were detected in 11 cases, 7 of which had evidence of isochronal crowding demonstrated during late annotation mapping. Two patients who also underwent entrainment mapping had critical circuitry confirmed in regions of isochronal crowding. In the PVC group, high-density voltage and activation mapping was performed with a median 1058 points [IQR 534–3582] collected in the endocardium.
Conclusions
This novel MPMC can be used safely and effectively to create high-density maps in LV endocardium or epicardium. Limitations of the catheter include a longer wait time for matrix formation prior to starting point collection and challenges in manipulation in certain regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Challenges in ablating ventricular arrhythmias (VA), including ventricular tachycardia (VT) and premature ventricular complexes (PVCs), can be mitigated with the use of multipolar mapping catheters. VT that is non-inducible at baseline or not hemodynamically tolerated frequently requires an operative strategy that is based on an assessment of substrate abnormalities. While alternative strategies such as pace mapping are routinely employed when the VT morphology is known, identification of abnormal substrate likely to be critical to the arrhythmia has been shown to be beneficial, particularly when other approaches are not possible [1,2,3]. While abnormal substrate may be identified with standard point-by-point mapping, high-density substrate mapping with smaller electrodes offers the potential for more rapid and detailed characterization of true conduction abnormalities [4].
Simultaneous multipolar mapping offers a potential advantage over standard point-by-point mapping due to smaller electrodes and closer interelectrode spacing resulting in a larger sampling footprint and increased resolution. This may be helpful when PVCs are infrequent or when low amplitude early local signals are present [5]. There are available data from animal models suggesting that multipolar mapping catheters (MPMC) can be beneficial to overcome these challenges in both the atrium and ventricle [6, 7], although there are little data from human ventricular studies. In this series, we aimed to evaluate the safety and feasibility of utilizing the Optrell MPMC (Biosense Webster, Irvine, CA) in patients undergoing ablation for VT and PVCs.
2 Methods
2.1 Patient selection
A single-center retrospective study was performed including consecutive patients who underwent catheter ablation for VT or PVCs utilizing the Optrell MPMC between June and November 2022. All patients provided written informed consent for the ablation procedure and the use of their data was approved by the Institutional Review Board of the Hospital of the University of Pennsylvania.
2.2 Novel multipolar mapping catheter
The Optrell MPMC is a paddle-shaped, fixed array, multi-electrode catheter that includes 48 electrodes distributed over six parallel splines as shown in Fig. 1. The catheter is designed to allow simultaneous activation over a larger anatomic region based on the surface area in direct contact with myocardium. Each spline consists of eight electrodes and each electrode has a surface area of 0.9 mm2 with a center-to-center interelectrode distance of 2.4 mm across all six splines that are capable of recording and pacing. Bipolar electrograms are generated between these small electrodes along and across the splines as shown in Fig. 1. The distal end of the shaft consists of an irrigation port and a ring electrode, which serve as the indifferent electrode and are used for matrix formation. Unipolar electrograms are generated between each detection mini-electrode and the indifferent ring electrode.
Multi-electrode array catheter (Optrell; Biosense Webster) consists 48 electrodes distributed over 6 splines. Each spline consists of 8 electrodes and each individual electrode has a surface area of 0.9 mm2. An additional indifferent electrode is positioned at the distal shaft. The electrodes are separated with an interelectrode distance of 2.4 mm along and across all splines. These electrodes are used to evaluate and generate the propagation vectors based on the relative activation time between those electrodes
2.3 Electrophysiology study and ablation procedure
All ablation procedures were performed under conscious sedation or general anesthesia per the operator’s preference, along with direct arterial pressure and oxygen saturation monitoring. Intracardiac echocardiography (ICE; SoundStar, Biosense Webster) was used in all cases for direct visualization of cardiac anatomy, catheter positioning, and monitoring for acute complications. All cases were performed using CARTO 3 version 7.5. Heparin was administered prior to deployment of the Optrell in the right or left ventricle (RV/LV) with a target activated clotting time of 350–400 s. For LV endocardial mapping, the use of retrograde aortic and transseptal approaches were at the discretion of the operators based on the location of the arrhythmogenic substrate, severity of the aortic valve stenosis, and arterial anatomy of the patients. For retrograde aortic approach, the MPMC was placed into an 8-Fr Brite tip sheath and slowly advanced pass the aortic valve under fluoroscopic and ICE guidance. For transseptal approach, a deflectable sheath was used. The MPMC was placed in the deflectable sheath and advanced across the mitral valve into the LV. The decision for an upfront epicardial approach was based on prior history of failed endocardial ablation and the presenting morphology on a 12-lead electrocardiogram (ECG). The sheath used for epicardial access was at the discretion of the operator—most commonly either an 8.5-Fr deflectable sheath or a non-deflectable 8-Fr sheath. Heparin was held and protamine used to reverse anticoagulation prior to epicardial access.
For patients undergoing VT ablation, programmed electrical stimulation was initially performed from the RV at a drive cycle length of 400 to 600 ms, with up to 4 extrastimuli. LV stimulation was performed when VT could not be induced from the RV. For PVC patients, RV burst pacing was attempted with and without isoproterenol infusion at the starting dose of 2 µg/min and up to the highest tolerated dose (maximum 40 µg/min) or until PVCs occurred. All relevant PVCs were automatically identified among the sinus beats by the annotation software using the predefined ECG morphology acceptance criteria. At the end of the ablation procedure, the induction protocol was repeated to determine the success of the ablation. The induced arrhythmias were designated as clinical VTs or PVCs when morphologies matched the presenting VTs or PVCs recorded on the 12-lead ECGs, if available, or the VT electrograms and cycle length recorded by implantable cardioverter-defibrillators (ICD).
This MPMC was used to create a high-density electroanatomical map on the endocardial or epicardial ventricular surface. In certain challenging regions, a 3.5-mm open-irrigated ablation catheter was used to collect additional points in areas less accessible by the MPMC. Electrogram annotation was performed automatically by the mapping system during sinus rhythm, paced rhythm, or arrhythmia with subsequent manual review. Conventional bipolar voltage cut-offs were used to define normal myocardium (> 1.5 mV), border zone or intermediate-voltage myocardium (0.5–1.5 mV), and scar (< 0.5 mV) [8]. A unipolar low-voltage thresholds of < 5.5 mV was used for the right ventricle and < 8.3 mV for the left ventricle [9, 10].
For patients undergoing VT ablation, if VT was hemodynamically tolerated, a VT activation map was created using the MPMC. Entrainment mapping was performed when possible using the standard definitions described by Stevenson et al. [11] and pace mapping was performed in patients with non-tolerated VT. A detailed substrate mapping was performed in all patients during sinus rhythm or pacing. Areas with late potentials were annotated within the map. In addition, isochronal crowding was used to identify areas with slow or late conduction, conduction block, and wavefront collision during sinus or paced rhythm, as previously described [4, 12].
For VT procedures, ablation targeted regions of critical circuitry guided by the information obtained from the high-density electroanatomic mapping and entrainment or pace mapping, when available. For PVC procedures, ablation targeted the site of the earliest activation and regions of best pace maps performed with the MPMC or the ablation catheter. Radiofrequency energy was delivered using the 3.5-mm open-irrigated catheter (Thermocool SmartTouch or Thermocool SmartTouch SF, Biosense Webster) with power titrated between 20 and 50 W targeting an impedance drop of 10–12% for up to 3 min.
2.4 Acute endpoints and success of ablation
Acute procedural outcomes were defined as acute success (successful elimination of targeted clinical VT or PVC) or failure (persistence of targeted clinical VT or PVC). This was assessed following the induction protocol as outlined above. Operator feedback on the potential values and limitations of this MPMC in VA ablation procedure were collectively gathered and reported in our result.
2.5 Statistical analysis
Continuous variables are presented as mean ± standard deviation or median and interquartile range (IQR). Categorical variables are presented as numbers and percentages. Analyses were performed using Stata, version 14.1 (StataCorp, College Station, TX).
3 Results
3.1 Patient population
Our cohort consisted of 20 consecutive patients (80% male, age 61 ± 15 years, mean left ventricular ejection fraction of 42 ± 12%). A total of 42 electroanatomic maps were created, during 13 VT ablations and 7 PVC ablations. Baseline demographic and patient characteristics of the 20 patients are summarized in Table 1. Of the 20 patients, 17 (85%) had left ventricular dysfunction. Of these 17 patients, 8 patients (40%) had ischemic cardiomyopathy, and 9 patients (45%) had non-ischemic cardiomyopathy. Eleven patients (55%) had prior ablation procedures and 1 patient (5%) had previously undergone epicardial ablation. Sixteen patients were previously treated unsuccessfully with antiarrhythmic drugs.
3.2 Procedural characteristics for VT
Procedural data recorded for each patient are summarized in Table 2. Of the 13 patients who underwent VT ablation, 8 were placed under general anesthesia including all 4 epicardial mapping cases. Eight patients had LV endocardial maps and one patient had both RV and LV endocardial maps created using the MPMC.
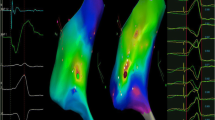
In total, 28 high-density maps (19 endocardial and 9 epicardial maps) were generated using the MPMC. Of those, 21 were voltage/isochronal late activation mapping (ILAM) maps (6 during sinus rhythm, 3 during atrial pacing, 9 during RV pacing, and 3 during LV pacing) and 7 were activation maps in VT. Detailed substrate mapping was performed in all 13 cases. The median number of endocardial and epicardial mapping points collected using the MPMC were 2753 [1471–17,024] and 12,830 [2319–30,010], respectively. The median durations of endocardial and epicardial mapping were 22.3 [15.2–33.3] min and 60.5 [31.8–96.4] min, respectively. All patients had pace mapping performed from the scar to identify potential exit sites. Late potentials (LPs) were recorded in 11 (85%) cases using the MPMC, 7 of these were during endocardial mapping and 4 were during epicardial mapping. Seven of the 11 patients with LPs had isochronal crowding demonstrated in ILAM during native conduction or left or right ventricular pacing, defined as ≥ 3 isochrones within a 1-cm radius [12]. Activation maps for induced or spontaneous VTs were performed in 7 (54%) patients; 5 were endocardial maps and 2 were epicardial maps. Entrainment mapping was performed in 2 patients and critical arrhythmia circuitry (entrance, exit, or isthmus sites) corresponded to areas of isochronal crowding in these patients as shown in Fig. 2.
A Late annotation map obtained during right ventricular pacing with isochronal crowding in regions of entrainment confirmed VT entrance, isthmus, and exit. B Late potentials seen during right ventricular pacing at the site of VT isthmus, where ablation terminated VT. C Ablation at this site of entrainment confirmed isthmus terminated the VT. VT ventricular tachycardia
3.3 Procedural characteristics and outcomes for VT
The results of VA ablation are summarized in Table 3. In the VT group, mean procedure time was 300 ± 80 min, mean fluoroscopic time was 27 ± 16 min, and mean radiofrequency time was 48 ± 22 min. At baseline, among the 13 patients who underwent VT ablation, at least one VT was inducible in 11 patients (9 out of 11 inducible for clinical VT) and 2 patients were not inducible for any VT. The mean number of clinical VTs induced was 0.7 ± 0.5 with a mean cycle length of 467 ± 74 ms. The mean number of non-clinical VTs induced was 1.9 ± 2.1 with a mean cycle length of 344 ± 59 ms. Patients required a median of 103 [24–216] points in the endocardium and 77 [59–82] points in the epicardium to be sampled point-by-point using the ablation catheter after completion of the MPMC map (Table 4).
Among patients where clinical VTs were inducible at baseline, acute success was achieved in 8 out of 9 patients. Success was not achieved in 1 patient as the critical circuitry was felt to be in epicardial in a patient with prior coronary artery bypass grafting. Post-ablation, 5 patients underwent non-invasive programmed stimulation and remained non-inducible for any VT. One patient with end-stage heart failure and VT storm died the day following the ablation due to progression of end-stage heart failure. No other acute adverse events (no pericardial effusion and no change in valvular regurgitation) related to the use of MPMC were noted.
3.4 Procedural characteristics for PVC
Seven patients underwent PVC ablation: 6 under conscious sedation and 1 under general anesthesia as shown in Table 2. Four patients had LV endocardial mapped, 2 patients had RV endocardial mapped, and 1 patient had both endocardial mapped obtained using the MPMC. A total of 14 maps were acquired with a median of 1058 [534–3582] points per map obtained over a median time of 23.1 [10.1–30.1] min. Pace mapping was performed in all patients.
3.5 Procedural characteristics and outcomes for PVC
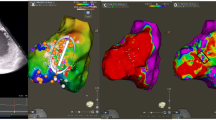
In the PVC group, mean procedure time was 176 ± 52 min, mean fluoroscopic time was 23 ± 13 min, and mean radiofrequency time was 19 ± 11 min. Of the 7 patients, 6 patients had a clinical PVC targeted with ablation. One patient had only RV voltage map acquired and no ablation was performed as no PVC was observed during the procedure. Patients required a median of 44 [7–55] points to be sampled point-by-point using the ablation catheter after completion of the MPMC map (Table 4). Among those 6 patients with PVCs observed, acute success was achieved in 5 patients (right ventricular outflow tract, left ventricular outflow tract (Fig. 3), inferior mitral annulus, right parahisian, and anterolateral papillary muscle PVC sites of origin). Success was not achieved in 1 patient as the PVC, which was felt to be epicardial in origin and in close proximity to a ramus intermedius coronary artery, limiting ablation from the coronary venous system. No acute adverse events (no pericardial effusion and no change in valvular regurgitation) occurred in this group post-PVC ablation.
A High-density endocardial left ventricular activation map with the Optrell MPMC showing the earliest activation site (indicated by a white star) of the clinical premature ventricular complex adjacent to the lateral left coronary cusp. B Simultaneous activation recorded by MPMC beneath left coronary cusp revealing local electrogram preceding the PVC QRS by 28 ms. PVCs were eliminated with ablation at this site. MPMC multipolar mapping catheter, PVC premature ventricular complex
3.6 Potential values and pitfalls of Optrell MPMC
After the procedures, all operators were assed to report advantages and disadvantages of the MPMC as well as strategies they had employed to overcome any challenges. Feedback on the MPMC use is summarized in Table 5. There was less ectopy noted during MPMC manipulation in the ventricles and throughout the phase of mapping than had been observed with other MPMC, although some degree of ectopy was still present. The use of Optrell MPMC was able to identify low amplitude signals that were not seen on ablation catheter and collect more points over a given anatomic location in a shorter duration. Mapping with the MPMC appeared to be most beneficial in regions of large substrate, especially in apical aneurysm or a lateral region. Notably, operators did experience a longer wait time for matrix formation prior to starting point collection in the ventricular chambers. Operators encountered difficulty in manipulating the MPMC in the epicardial surface and generating epicardial matrix during mapping. This finding was substantiated by a longer median mapping time in the epicardium as compared to endocardium (60.5 [31.8–96.4] min versus 22.3 [15.2–33.3] min) in our case series. All operators noted that adding approximately 50 cm3 of fluid to the epicardial space improved manipulation in all our epicardial cases in this series. Likely due to the relatively large and stiff profile of the paddle of the mapping catheter, it was more challenging to map highly trabeculated surfaces, such as non-outflow tract regions of the right ventricle, the basal septum of the left ventricle, and regions near papillary muscles or valve apparatus. Due to these limitations, it was especially challenging to map PVCs, which commonly arise from these structures and therefore point-by-point mapping was often required to adequately complete activation maps. Similarly, manipulation of the MPMC around intracavitary structures especially in smaller/structurally normal hearts was challenging relatively to dilated ventricles. Consistent with this, the most common areas where re-sampling with a point-by-point technique using the ablation catheter was most commonly required near valve apparatus (especially the mitral annulus and near the aortic valve) and on the papillary muscles (Table 6). In addition, manual reviews of the points collected were necessary in our case series to create accurate substrate maps with frequent automatic annotation of far-field signals. Improved automatic processing would improve the feasibility of use on daily basis as a real-time tool to guide ablation strategy, particularly in regions where poor contact often led to annotation of far-field signals. During PVC ablation, when the MPMC could be positioned in contact with the anticipated site of origin, operators could rapidly identify a small region of accurate pace maps based on rapidly pacing from multiple poles on the MPMC.
4 Discussion
To our knowledge, this is the first study to report the clinical utility and safety of the Optrell MPMC for mapping VAs in humans, which was both feasible and safe in this cohort of 20 consecutive patients (13 patients with VTs and 7 patients with PVCs). This novel MPMC facilitates high-density substrate mapping in VA ablation procedures with a median of > 2500 and > 10,000 points collected during endocardial and epicardial mapping, respectively. As demonstrated by Viswanathan et al. and Acosta et al., high-density mapping with MPMC resulted in better mapping resolution and mapping with MPMC took shorter time to generate a substrate map [13, 14]. Interestingly, procedural and fluoroscopy times were higher in our experience when compared to previous studies [13, 15,16,17]. These longer procedural and fluoroscopy times are likely explained by the presence of learning curve when using the MPMC for substrate mapping and the need for point-by-point mapping at areas harder to reach by the MPMC.
In our cohort, the mapping catheter acquired high-quality local electrograms when the catheter was in contact with the myocardium. LPs were identified in 11/13 (85%) of VT procedures with automatic annotation during substrate mapping. Of the 7 VT cases with isochronal crowding demonstrated in ILAM during substrate mapping, a focused target for ablation was suggested within a larger substrate. Two of those patients had entrainment mapping performed and had critical circuitry identified in areas with isochronal crowding, consistent with other data suggesting the importance of these regions as shown in Fig. 2. This can provide a valuable opportunity to abbreviate an otherwise excessively lengthy procedure or to avoid the need for ablation in high-risk regions (such as near coronary arteries). Our study showed that radiofrequency catheter ablation performed at these sites resulted in acute success in those 7 patients (non-inducible for clinical VTs post-ablation).
Aziz et al. have previously reported the use of multi-electrode catheters for ILAM. The authors reported that 95% of cases with successful termination of VT during radiofrequency delivery had VT co-localized to areas with isochronal crowding (conduction deceleration). By targeting ablation to a region with isochronal crowding, the VT-free survival was reportedly 70% at 12 ± 10 months follow-up [4]. Thus, ablation strategies targeting LPs and isochronal crowding are potentially valuable, especially in the event of unmappable VTs due to hemodynamic instability or non-inducibility. Our data suggest that these regions are identified with MPMC and the associated ILAM software is likely to be similarly valuable.
In our series of 7 PVC ablations, maps obtained using the MPMC had a median of 1000 points, far more than we would typically obtain in a point-by-point PVC activation map. The use of MPMC can be particularly important in the event of infrequent PVCs at the time of procedure, allowing an entire region of activation to be annotated from a single complex. The close interelectrode spacing on the mapping catheter also allows precise endomyocardial capture at a lower output during pace mapping and, although not prospectively compared, we did identify small regions of accurate pace maps relatively rapidly compared to what may be achieved with point by point pace maps from an ablation catheter. In addition, PVC ablation guided by the use of high-density system has been shown to have encouraging acute and intermediate clinical success [13, 18, 19].
4.1 Limitations
This study includes the typical limitations of a retrospective series with a small number of patients at a single center. These results are therefore hypothesis-generating, and prospective studies are required to further investigate the use of this novel mapping catheter in VA procedures and to compare its use to other catheters including conventional point-by-point mapping and other MPMC. The wait time for matrix formation has been addressed in the most recent release of CARTO 3 version 7.5 software although our analysis was performed on cases prior to this update.
5 Conclusions
This study demonstrates that the use of Optrell MPMC in VA ablation procedures appears to be feasible and safe for high-density mapping of the left and right ventricular endocardium and epicardium. This novel MPMC has the potential to facilitate substrate mapping, define VT ablation targets rapidly, and provide detailed PVC activation maps.
Abbreviations
- AAD:
-
Antiarrhythmic drug
- ECG:
-
Electrocardiography
- ICD:
-
Implantable cardioverter-defibrillator
- ILAM:
-
Isochronal late annotation mapping
- LPs:
-
Late potentials
- PVC:
-
Premature ventricular complex
- VA:
-
Ventricular arrhythmia
- VT:
-
Ventricular tachycardia
References
Tzou WS, Frankel DS, Hegeman T, et al. Core isolation of critical arrhythmia elements for treatment of multiple scar-based ventricular tachycardias. Circ Arrhythm Electrophysiol. 2015;8:353–61.
de Chillou C, Groben L, Magnin-Poull I, et al. Localizing the critical isthmus of postinfarct ventricular tachycardia: the value of pace-mapping during sinus rhythm. Heart Rhythm. 2014;11:175–81.
Parreira L, Marinheiro R, Carmo P, et al. Isolated diastolic potentials as predictors of success in ablation of right ventricular outflow tract idiopathic premature ventricular contractions. PLoS One. 2019;14: e0211232.
Aziz Z, Shatz D, Raiman M, et al. Targeted ablation of ventricular tachycardia guided by wavefront discontinuities during sinus rhythm: a new functional substrate mapping strategy. Circulation. 2019;140:1383–97.
Matto F, Venugopal D, Bhave PD, Rhodes TE, Mazur A. Utility of high resolution mapping to guide ablation of ventricular arrhythmias from the aortic sinuses of Valsalva. J Interv Card Electrophysiol. 2023;66:51–9.
Yavin HD, Bubar ZP, Higuchi K, Sroubek J, Yarnitsky J, Anter E. Propagation vectors facilitate differentiation between conduction block, slow conduction, and wavefront collision. Circ Arrhythm Electrophysiol. 2021;14: e010081.
Younis A, Yarnitsky J, Rodriguez H, Bubar ZP, Zilberman I, Anter E. Multipolar electrograms: a novel algorithm for accurate annotation of near-field potentials in scar. Heart Rhythm. 2022;19:S511.
Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–96.
Hutchinson MD, Gerstenfeld EP, Desjardins B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:49–55.
Polin GM, Haqqani H, Tzou W, et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83.
Stevenson WG, Friedman PL, Sager PT, et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997;29:1180–9.
Irie T, Yu R, Bradfield JS, et al. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia: systematic analysis of isochronal late activation mapping. Circ Arrhythm Electrophysiol. 2015;8:390–9.
Viswanathan K, Mantziari L, Butcher C, et al. Evaluation of a novel high-resolution mapping system for catheter ablation of ventricular arrhythmias. Heart Rhythm. 2017;14:176–83.
Acosta J, Penela D, Andreu D, et al. Multielectrode vs. point-by-point mapping for ventricular tachycardia substrate ablation: a randomized study. Europace. 2018;20:512–9.
Di Biase L, Burkhardt JD, Lakkireddy D, et al. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol. 2015;66:2872–82.
Fernandez-Armenta J, Penela D, Acosta J, et al. Substrate modification or ventricular tachycardia induction, mapping, and ablation as the first step? A randomized study. Heart Rhythm. 2016;13:1589–95.
Proietti R, Dowd R, Gee LV, et al. Impact of a high-density grid catheter on long-term outcomes for structural heart disease ventricular tachycardia ablation. J Interv Card Electrophysiol. 2021;62:519–29.
Sousa PA, Antonio N, Barra S, Elvas L, Goncalves L. Pattern matching filter and multielectrode mapping catheter - a new approach for complex premature ventricular contraction ablation. Rev Port Cardiol (Engl Ed). 2021;40:423–31.
Martins RP, Benali K, Galand V, et al. Ablation of multifocal premature ventricular contractions using automated pace-mapping software. Rev Port Cardiol. 2022;41:653–62.
Funding
This work was supported by the Pennsylvania Steel Company EP Research Fund and the Winkelman Family Fund in Cardiovascular Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Retrospective use of data was approved by the IRB of the University of Pennsylvania.
Conflict of interest
DSF: lecture honoraria from and investigator for Biosense Webster. SN: consultant for CardioSolv, ImriCor, Dyne Pharmaceuticals, and Circle CVI; and principal investigator for research funding from Biosense Webster, ImriCor, and ADAS software. FM: consultant for Biosense Webster, Abbot Medical, and Medtronic; and has served as an investigator with research funding from Biosense Webster. TM: consultant for Biosense Webster. The remainder of the authors report nothing relevant to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, J.L., Guandalini, G.S., Hyman, M.C. et al. Substrate and arrhythmia characterization using the multi-electrode Optrell mapping catheter for ventricular arrhythmia ablation—a single-center experience. J Interv Card Electrophysiol 67, 559–569 (2024). https://doi.org/10.1007/s10840-023-01618-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-023-01618-5