Abstract
Background
Isoproterenol, a non-specific beta agonist, is commonly used during electrophysiology studies (EPS). However, with the significant increase in the price of isoproterenol in 2015 and the increasing number of catheter ablations performed, the cost implications cannot be ignored. Dobutamine is a less expensive synthetic compound developed from isoproterenol with a similar mechanism to enhance cardiac conduction and shorten refractoriness, thus making it a feasible substitute with a lower cost. However, the use of dobutamine for EPS has not been well-reported in the literature.
Objective
To determine the site-specific effects of various doses of dobutamine on cardiac conduction and refractoriness and assess its safety during EPS.
Methods
From February 2020 to October 2020, 40 non-consecutive patients scheduled for elective EPS, supraventricular tachycardia, atrial fibrillation, and premature ventricular contraction ablations at a single center were consented and prospectively enrolled to assess the effect of dobutamine on the cardiac conduction system. At the end of each ablation procedure, measures of cardiac conduction and refractoriness were recorded at baseline and with incremental doses of dobutamine at 5, 10, 15, and 20 mcg/kg/min. For the primary analysis, the change per dose of dobutamine from baseline to each dosing level of dobutamine received by the patients, comparing atrioventricular node block cycle length (AVNBCL), ventricular atrial block cycle length (VABCL) and sinus cycle length (SCL), was tested using mixed-effect regression. For the secondary analysis, dobutamine dose level was tested for association with relative changes from baseline of each electrophysiologic parameter (SCL, AVNBCL, VABCL, atrioventricular node effective refractory period (AVNERP), AH, QRS, QT, QTc, atrial effective refractory period (AERP), ventricular effective refractory period (VERP), using mixed-effect regression. Changes in systolic and diastolic blood pressures were also assessed. The Holm-Bonferroni method was used to adjust for multiple testing.
Results
For the primary analysis there was no statistically significant change of AVNBCL and VABCL relative to SCL from baseline to each dose level of dobutamine. The SCL, AVNBCL, VABCL, AVNERP, AERP, VERP and the AH, and QT intervals all demonstrated a statistically significant decrease from baseline to at least one dose level with incremental dobutamine dosing. Two patients (5%) developed hypotension during the study and one patient (2.5%) received a vasopressor. Two patients (5%) had induced arrhythmias but otherwise no major adverse events were noted.
Conclusion
In this study, there was no statistically significant change of AVNBCL and VABCL relative to SCL from baseline to any dose level of dobutamine. As expected, the AH and QT intervals, and the VABCL, VERP, AERP and AVNERP all significantly decreased from baseline to at least one dose level with an escalation in dobutamine dose. Dobutamine was well-tolerated and safe to use during EPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Isoproterenol is a non-specific beta agonist commonly used during electrophysiology studies (EPS). Its β1-stimulation promotes tachyarrhythmia induction by improving cardiac conduction and shortening AV nodal refractoriness. However, the financial burdens of using isoproterenol are significant given the high cost of the medication and the increasing number of catheter ablations [1,2,3,4].
Dobutamine is a less expensive synthetic compound developed from isoproterenol that is predominantly a β1-agonist with mild β2 and α1-activities [5]. It has been well-studied and used in cardiac stress imaging as well as treatment for cardiogenic shock [5]. Therefore, dobutamine is a feasible, potentially less expensive alternative to isoproterenol. However, its use for EPS has not been extensively studied.
The purpose of this study was to determine the site-specific effects of various doses of dobutamine on cardiac conduction and refractoriness and assess its safety during EPS.
2 Methods
This study was approved by the Institutional Review Board (IRB #: 19–0934) of Northwell Health and exempted from the investigational new drug (IND) based upon a Food and Drug Administration (FDA) review.
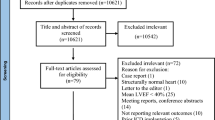
From February 2020 to October 2020, 40 non-consecutive patients scheduled for elective EPS, supraventricular tachycardia (SVT), atrial fibrillation (AF), and premature ventricular contraction (PVC) ablations at a single center were consented and prospectively enrolled for the use of dobutamine. The inclusion criteria were patients between the ages of 18 and 80 and those undergoing EPS. Patients were excluded from the study for the following conditions: (1) hypertrophic obstructive cardiomyopathy or other forms of left ventricular outflow tract obstruction, (2) severe aortic stenosis, (3) prior sustained ventricular tachycardia or ventricular fibrillation, (4) prior allergic reaction to dobutamine or sulfates, (5) patients with stable and unstable angina and (6) pregnancy.
All procedures were performed in the EP laboratory under general anesthesia or conscious sedation monitored by an anesthesiologist. No effort was made in this study to influence the manner in which patients were sedated by the anesthesiologists relative to the study. However, the anesthesiologists were asked to avoid administering medications such as glycopyrrolate and catecholamines during the study period unless necessary. Multi-electrode catheters were inserted via the femoral vein and positioned fluoroscopically at the His-bundle position, coronary sinus, and right ventricular apex. Stimulation was performed with a programmable stimulator EP-4™ (St. Jude Medical, Little Canada, MN, USA). The procedures were performed by three experienced electrophysiologists and the EPS protocol was performed as previously reported [5].
At the conclusion of each ablation, the baseline blood pressure and the following parameters were recorded: (1) sinus cycle length (SCL), (2) AH interval, (3) HV interval, (4) QRS duration, (5) QT interval, (6) AV node block cycle length (AVNBCL), (7) AV node effective refractory period (AVNERP), and (8) VA block cycle length (VABCL), (9) atrial effective refractory period (AERP) and (10) ventricular effective refractory periods (VERP). Dobutamine was then incrementally infused at 5, 10, 15, and 20 mcg/kg/min with a waiting period of five minutes between each dose escalation before the blood pressure and the same parameters noted from baseline were recorded. Blood pressures were recorded from an arterial line or manual cuff at five-minute interval. Electrogram intervals were measured using CardioLab™ (GE Healthcare, Chicago, IL, USA). The study endpoint was at protocol completion with measurements at baseline and at each incremental dose of dobutamine. If any sustained arrhythmia was induced, the arrhythmia was ablated and the study was stopped.
Stimulation was performed by pacing at the coronary sinus and the right ventricular apex at cycle lengths just shorter than the prevailing sinus cycle length, and then at progressively shorter cycle lengths to the point of AV or VA block. Programmed stimulation was then performed at each site beginning with an 8-beat drive train at 600 ms in the atrium and the ventricle with single extrastimuli beginning in late diastole, and then progressively earlier in 10-ms decrements (with increasing doses of dobutamine, the drive train cycle was decreased to avoid competition during sinus tachycardia). The SCL, and AH and HV intervals were measured from an average of at least ten consecutive intervals recorded from the His-bundle catheter. The AVNBCL and VABCL were determined as the longest pacing cycle length from the coronary sinus and right ventricular apex, respectively, which resulted in AV nodal block during gradually increasing pacing rates. The anterograde AVNERP was measured as the longest A1-A2 interval (measured in the His bundle recording), at a drive cycle length of 600 ms, in which the A2 failed to propagate through the AV node. Similarly, the retrograde AVNERP was the longest V1-V2 coupling interval, at a drive cycle length of 600 ms, at which the premature stimulus failed to propagate to the atrium. Pacing cycle lengths of 600, 500, 450 and 400 ms were used to measure the refractory periods, in view of the shortening of the sinus cycle length in response to dobutamine. We reported a single mean refractory period for each dose of dobutamine, even with varying drive cycle lengths required during dose escalation of dobutamine.
If despite ablation, an arrhythmia was induced, the protocol was stopped and the arrhythmia ablated. No further attempts to continue or repeat the study were made.
2.1 Statistical methods
Retrospective data of the use of dobutamine in our EP lab since March 2015 was used for the sample size calculation, which showed that the mean and standard deviation for the difference between the change as a percent from baseline in the SCL and the change from baseline in the AVNBCL is 7.7% ± 16.0% at 15 mcg/kg/min of dobutamine (Table 1 Supplement). We enrolled 40 patients based on the finding that a study of 37 patients yields 80% power with respect to the primary endpoint with an alpha of 0.05 using a paired 2-tail t-test.
The change per dose of dobutamine from baseline to each dosing level of dobutamine received by the patients, comparing AVNBCL, VABCL and SCL, was tested using mixed-effect regression.
Dobutamine dose level was tested for association with relative changes from baseline of each electrophysiologic parameter (SCL, AVNBCL, VABCL, AVNERP, AH, QT and QTc intervals) using mixed-effects regression. The Holm-Bonferroni method was used to adjust for multiple testing. Estimates of the change from baseline in each electrophysiologic parameter at each dose of dobutamine were provided along with 99% confidence intervals and plots.
3 Results
Between February 2020 to October 2020, 40 non-consecutive patients median age 63 years (IQR 55–69), 11 (28%) females, scheduled for elective EP procedures at a single center consented and received dobutamine at the end of the procedure for EPS. The patient demographics and the diagnoses for the procedure indications are listed in Table 1.
There was no significant difference in the change in AVNBCL relative to the SCL and the change in the VABCL relative to the SCL at each incremental dose of dobutamine (Table 2, 3 and 4, Fig. 1 and 2).
The SCL shortened with incremental doses of dobutamine (Table 2, 3, 4, Fig. 1). The change only became statistically significant at 10 mcg/kg/min and greater doses. The largest percentage decrease in the SCL from one consecutive dose escalation to the next was noted between 5 and 10 mcg/kg/min. Similarly, antegrade and retrograde AV nodal conduction shortened with each dose of dobutamine and the largest decrease in AVNBCL and VABCL between consecutive dose escalations was also noted between 5 and 10 mcg/kg/min (Table 2, 3, 4, Fig. 1 and 2). The AH interval shortened at 15 mcg/kg/min and greater doses (Table 2, 3, 4, Fig. 3) but the HV interval did not show evidence of change (Table 2, 3, 4, Fig. 4). The QRS duration did not change significantly from baseline to each incremental dose of dobutamine (Table 2, 3, 4, Fig. 5). The QT interval decreased with escalation in dobutamine dose starting at 15 mcg/kg/min (Table 2, 3, 4, Fig. 5). The QTc increased from baseline to each dose of dobutamine (Table 2, 3, 4, Fig. 5) but the association between dose of dobutamine and QTc was not statistically significant (P = 0.3873).
The decrease in AVNERP reached statistical significance for all doses of dobutamine (Table 2, 3, 4, Fig. 6). Although there was no significant decrease in the AERP from baseline up to 15 mcg/kg/min of dobutamine, there was a significant decrease in the AERP from baseline to 20 mcg/kg/min (Table 2, 3, 4, Fig. 6). Although there was no significant decrease in the VERP from baseline to 5 mcg/kg/min of dobutamine, there was a significant decrease in the VERP from baseline to 10 mcg/kg/min, to 15 mcg/kg/min and to 20 mcg/kg/min dobutamine (Table 2, 3, 4, Fig. 6).
Changes in diastolic and systolic blood pressure with escalating doses of dobutamine are shown in Table 2, 3, 4 and Fig. 7. The systolic blood pressure increased significantly by 11%to a maximum at 15 mcg/kg/min and then decreased slightly at 20 mcg/kg/min. The diastolic blood pressure decreased significantly by 8% to a minimum at 20 mcg/kg/min.
Three patients had no retrograde conduction at baseline. One patient developed retrograde conduction at 5 mcg/kg/min of dobutamine, one patient at 20 mcg/kg/min, and one patient had no retrograde conduction during the study.
Four patients (10%) were hypotensive, defined as systolic blood pressure < 90 mmHg, at baseline (87, 83, 86, and 84), secondary to the effects of sedation. All four patients were able to tolerate dobutamine with no limitations. Hypotensive episodes were recorded in two additional patients (5%), a total of six patients (15%), during the study but none required a vasopressor nor subsequently developed end organ damage as a result. Another patient (2.5%) received a vasopressor for systolic blood pressure in the 90 s based on the discretion of the anesthesiologist during 15 mcg/kg/min of dobutamine infusion and the dobutamine dose was not increased to 20 mcg/kg/min; therefore, data could not be collected for 20 mcg/kg/min. One patient (2.5%) developed junctional rhythm at 20 mcg/kg/min of dobutamine but remained normotensive.
One patient (2.5%) developed atrial fibrillation at 10 mcg/kg/min and another patient (2.5%) developed AVNRT at 15 mcg/kg/min. For both patients, dobutamine was held and subsequently tolerated ablation with no further adverse events. These two patients were included in the analysis, but data could only be collected for the dosages they received.
We did not perform ventricular pacing for VABCL or VERP in 13 patients who underwent AF ablations given the length of the procedure and the effect of ventricular pacing on the blood pressure.
The prices of isoproterenol and dobutamine are shown in Table 5 for 2015 and 2023. Whereas the price of isoproterenol decreased by a factor of 7.4, the price of dobutamine increased by a factor of 2.6.
4 Discussion
Isoproterenol, a non-selective beta agonist, is commonly used during EPS for its effects on enhancing conduction and shortening refractoriness of the AV node, particularly in sedated patients [6, 7]. However, the cost of isoproterenol increased exponentially following ownership changes in 2015 such that the wholesale acquisition cost (WAC) per milligram increased from $26.20 in 2012 to $1,790.11 in 2015 [3, 4]. The cost implications were significant given the increasing number of catheter ablations. An estimated 10,000 atrial fibrillation ablation procedures were performed in the United States in 1992. The number increased to approximately 50,000 in 2013 and is continuing to rise [1, 2]. Healthcare systems and electrophysiologists coped with the financial burden by rationing the use of isoproterenol. A study reported 40% reduction in the number of hospitalized patients treated with isoproterenol from 2012 to 2015 [3].
Another response to the cost increase was substituting isoproterenol with dobutamine. The cost of dobutamine has remained steady with the WAC per milligram of $17.78 in 2012 and $16.50 in 2015, and the number of patients treated with dobutamine increased during this time [3]. Isoproterenol is a potent β1-adrenergic agent associated with chronotropic and proarrhythmic responses. Dobutamine was synthesized in hopes of mitigating the side effects of isoproterenol. Removing the hydroxyl group from isoproterenol led to the discovery of dobutamine, which has two isomers [8]. The S(-)-enantiomer dobutamine is a potent α1-adrenoceptor agonist with minor β1 and β2-adrenoceptor agonist activities. In contrast, the R( +)-enantiomer dobutamine possesses minimal α1-agonist effects with predominantly β1 and β2-adrenoceptor agonist activities. The net effect of dobutamine is mostly β1-activity with mild β2 and α1-agonist effects. In addition, α1-activity helps offset β2-activity thus mitigating vasodilation-mediated hypotension, which is reported with high-doses of isoproterenol [9].
Dobutamine is commonly used for cardiac stress imaging studies to assess the severity of coronary artery disease and its utilization has been well-studied [10,11,12,13]. Dobutamine stress echocardiography (DSE) was introduced as an alternative method for patients who cannot tolerate exercise, to provoke myocardial ischemia, leading to ST-segment changes on the ECG and regional wall motion abnormalities on two-dimensional echocardiography [11]. While dobutamine has a half-life of 2 min and may take up to 10 min to achieve a steady state, DSE is routinely performed with 3 min intervals of dose increase, derived from the standard exercise Bruce protocol. There is no evidence to support this protocol, but the dose-increase before reaching steady state has been largely adopted for safety concerns [11]. We used a hybrid approach in our study and started with 5 mcg/kg/min at increments of 5 mcg/kg/min up to 20 mcg/kg/min for five minutes each. Buxton similarly used a dose increment at 5 min each [7]. The half-life of isoproterenol is 2.5 to 5.0 min, longer than that of dobutamine.
Buxton et al. studied the site-specific effects of isoproterenol at varying doses in 38 patients [7], and we conducted our study in a similar manner with dobutamine. Isoproterenol decreased the sinus cycle length at each incremental dose with the largest drop from 0.007 to 0.014 mcg/kg/min [7]. Dobutamine also decreased the sinus cycle length significantly by 10 mcg/kg/min with the largest decrease from 5 to 10 mcg/kg/min. Interestingly, the largest decrease in the AVNBCL and the VABCL also occurred between 5 and 10 mcg/kg/min. Similar to isoproterenol, dobutamine decreased the AH interval significantly by 15 mcg/kg/min, but there was no significant change in the HV interval. The lack of significant effect on the His-Purkinje system was consistent with previously reported studies in both animals and humans [7]. In contrast to the effects of isoproterenol more notable in the AV node compared to the sinus node, our study showed no significant difference in changes in the SCL relative to the changes in the AVNBCL or the VABCL over time with dose escalation. The AVNERP was often less than or equal to the AERP and therefore unable to be measured. Three patients (7.5%) had no retrograde conduction at baseline but two demonstrated retrograde conduction with 5 and 20 mcg/kg/min, which suggests bidirectional conduction enhancement.
At 5 mcg/kg/min of dobutamine, the VABCL decreased by a significant degree, indicating that even at low doses, there can be a significant effect on retrograde conduction, thus theoretically facilitating induction of specific arrhythmias like AVNRT at relatively low doses. Isoproterenol had been shown to improve retrograde conduction through the AV conducting system, thus facilitating induction of AVNRT [7].
Masoni studied the effects of dobutamine on the electrophysiological properties of the conduction system in 19 patients without heart block, 5 with second degree and 5 with third degree AV block using 5, 10 and 15 mcg/kg/min of dobutamine [14]. Dobutamine decreased the sinus cycle length, the atrial effective refractory period, and the AH interval. They also demonstrated an improvement AV nodal conduction proximal to the His bundle and a minimal increase in heart rate in patients with infra-Hisian block. There was no change in the HV interval. These findings are consistent with the results of our study. Bianchi demonstrated moderate heart rate increases with dobutamine, a very significant facilitation of A-H conduction and no significant effect on the HV interval with 10 and 15 mcg/kg/minute of dobutamine [15].
The safety of the use of dobutamine was an important observation of our study. Mazeika et al. utilized the same increments of dobutamine at 5, 10, 15, and 20 mcg/kg/min for eight minutes each during DSE and reported that all adverse reactions were minimal and resolved within two minutes of discontinuing the dobutamine infusion. Notably, seven patients (14%) had dobutamine-induced symptomatic hypotension of which three had transient junctional rhythms [12]. In our study, four patients (10%) were hypotensive at baseline (SBP 87, 83, 86, and 84) and a total of six patients (15%) developed hypotension during the study. One patient (2.5%), who did not meet our definition of hypotension (SBP < 90 mmHg), was preemptively given a vasopressor by the anesthesiologist during the dobutamine infusion of 15 mcg/kg/min and did not experience any adverse events. Another patient (2.5%) developed junctional rhythm during 20 mcg/kg/min of dobutamine infusion but remained normotensive and spontaneously recovered normal sinus rhythm.
Multiple studies have shown that even higher doses of dobutamine for prolonged duration causes low incidence of serious side effects [11]. Gianni et al. reported that two patients (4.2%) with significant history of myocardial ischemia experienced paradoxical hypotension during the high-dose dobutamine infusion and two other patients (4.2%) had hypertensive responses while on norepinephrine for anesthesia-induced hypotension. The incidence of atrial arrhythmias and outflow tract premature ventricular contractions with high-dose dobutamine was comparable to high-dose isoproterenol [9]. In our study, AF was induced in one patient (2.5%) at 10 mcg/kg/min and AVNRT was induced in one patient (2.5%) at 15 mcg/kg/min of dobutamine at which point the study protocol was terminated.
Although the result from the mixed-effects model showed the association between dose of dobutamine and QTc was not statistically significant (P = 0.3873), the QTc significantly increased from baseline at all doses (each p < 0.01). One would have expected the QTc to have remained constant as the dose of dobutamine increased and the SCL decreased with dose escalation, since the purpose of the corrected QT is to normalize the QT interval according to rate. The reason for the increase in QTc in this study is unclear. The QTc using Bazett’s formula is frequently used in general practice to correct QT for rate, although its applicability has been questioned in multiple studies. However, Dahlberg and colleagues concluded that Bazett’s formula was preferable in long QT1 and 2 patients [16].
Although the price of isoproterenol in 2015 was much higher than it is today, it was the impetus for us to study alternatives to isoproterenol. We found a significant cost saving substituting dobutamine for isoproterenol in 2015. However, since 2015 the difference in cost between the two medications has narrowed (Table 5). Whereas the price of isoproterenol decreased by a factor of 7.4, the price of dobutamine increased by a factor of 2.6. Another consideration is that the dosing of isoproterenol and dobutamine is quite different. One would use a 0.2 mg/cc vial of isoproterenol ($48.47) and a 250 mg/20 cc vial of dobutamine ($10.75) [17].
5 Limitations
This was a single center study with limited number of patients. Our protocol did not go beyond 20 mcg/kg/min of dobutamine and does not qualify as a high dose dobutamine study. Anesthesia was not uniformly given with either general anesthesia or conscious sedation, which may have had variable effects on the cardiac conduction. In addition, anti-arrhythmic and/or rate control agents were not uniformly held before the procedure as the decision was up to the electrophysiologist based on the individual patient. Anti-arrhythmic, anti-hypertensive and/or rate control agents could have influenced the results of this study. Selection of patients was not consecutive because it was not always feasible to perform the study protocol due to time constraints. Moreover, we did not perform a VABCL or VERP in 13 patients who underwent AF ablations given the length of the procedure and the effect of ventricular pacing on the blood pressure. There were other limited missing variables which the authors deemed insignificant, including 3 patients who could not finish the protocol because of either arrhythmia induction in 2 patients and hypotension requiring a vasopressor in 1 patient. Exclusion of patients due to time constraints could possibly introduce bias and affect the study results, especially if we excluded patients when procedures were long. Safety is always the priority, and if patients were under anesthesia for a prolonged period, we did not want to extend the procedure and place the patient at greater risk. Therefore, the more complicated, sicker patients with more cardiac substrate and longer procedure times (those patients undergoing atrial fibrillation ablation) were either excluded or ventricular pacing was not performed. Patients included in this study underwent electrophysiologic testing for a variety of indications. The heterogeneity of indications must be considered when interpreting results.
We did not find a significant difference in the changes in SCL relative to changes in AVNBCL or VABCL with incremental increases in dose of dobutamine. However, out study included only 40 patients and we cannot rule out the possibility of a type II error. The study is not powered to detect smaller effect size that could be clinically significant. Future studies with a larger sample size should be conducted to further validate these findings.
The AVNERP data were included in the analysis; however, the results should be viewed in the context of limitations. The effects of dobutamine on the anterograde AVNERP could not be determined consistently because, in many cases, it was shorter than the AERP. The SCL shortened with each dobutamine dose escalation and required shortening of the drive cycle length when measuring effective refractory periods. Therefore, shortening of the atrial and ventricular ERP could have been due to either a shortening of the drive train cycle length and/or the effects of dobutamine. Shorter drive train cycle length also led to AV nodal block precluding measurement of the AVNERP. AVNERP was not reported when it was shorter than the AERP. The retrograde AVNERP could not be assessed consistently because retrograde conduction was limited by His-Purkinje refractoriness or inability to see a stable retrograde His deflection.
Although we did discuss the electrophysiologic differences between isoproterenol and dobutamine, we did not perform a direct comparison between the two drugs since none of the patients in this study received isoproterenol. The information regarding isoproterenol was from previous studies. Therefore, we do not have information on whether there could have been different arrhythmias induced with isoproterenol and the electrophysiological response to isoproterenol could have been different in these patients. A direct comparison between dobutamine and isoproterenol either in the same patients or as a randomized study would help clarify whether the two agents are equivalent in terms of inducibility of arrhythmia prior to ablation and providing an equivalent, satisfactory end-point post ablation (do the two drugs predict future suppression of arrhythmia equally and accurately?).
6 Conclusions
There was no difference in the magnitude of decrease of the AVNBCL and VABCL relative to the magnitude in decrease of the SCL at each incremental dose of dobutamine. However, the AVNBCL, the VABCL and the SCL decreased significantly from baseline with each incremental dose of dobutamine (except the 3% decrease in SCL from baseline to 5 mcg/kg/min dobutamine was not statistically significant). Dobutamine was also seen to enhance retrograde AV nodal conduction in two out of three patients who displayed no retrograde conduction at baseline. While hypotension and arrhythmia-inductions were noted, no significant hypertensive nor further adverse events were noted.
Data Availability
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research, supporting data is not available.
References
Zipes DP, DiMarco JP, Jackman WM, et al. ACC/AHA Task Force Report: Guidelines for Clinical Intracardiac Electrophysiological and Catheter Ablation Procedures. J Am Coll Cardiol. 1995;26(2):555–73.
D’Silva A, Wright M. Advances in imaging for atrial fibrillation ablation. Radiol Res Pract. 2011;2011:714864.
Khot UN, Vogan ED, Militello MA. Nitroprusside and Isoproterenol Use after Major Price Increases. N Engl J Med. 2017;377(6):594–5. https://doi.org/10.1056/NEJMc1700244.
Knight BP. The rising costs of isoproterenol. EP Lab Dig. 2013;17(3).
Tuttle RR, Mills J. Dobutamine: development of a new catecholamine to selectively increase cardiac contractility. Circ Res. 1975;36(1):185–96.
Vanegas DI, Perez CDJ, Montenegro JDJ, et al. Dobutamine use for arrhythmia induction during electrical programmed heart stimulation. Rev Colomb Cardiol. 2006;12(7):479–83.
Cossú SF, Rothman SA, Chmielewski IL, Hsia HH, Vogel RL, Miller JM, Buxton AE. The effects of isoproterenol on the cardiac conduction system: site-specific dose dependence. J Cardiovasc Electrophysiol. 1997;8(8):847–53. https://doi.org/10.1111/j.1540-8167.1997.tb00845.x.
Cismaru G. Arrhythmia induction in the EP lab. 1st ed. Cham, Switzerland: Springer Nature Switzerland AG;2019:71-86.
Gianni C, Sanchez JE, Mohanty S, Trivedi C, Della Rocca DG, Al-Ahmad A, Burkhardt JD, Gallinghouse GJ, Hranitzky PM, Horton RP, Di Biase L, Natale A. High-Dose Dobutamine for Inducibility of Atrial Arrhythmias During Atrial Fibrillation Ablation. JACC Clin Electrophysiol. 2020;6(13):1701–10. https://doi.org/10.1016/j.jacep.2020.07.018.
Piérard LA, Berthe C, Albert A, Carlier J, Kulbertus HE. Haemodynamic alterations during ischaemia induced by dobutamine stress testing. Eur Heart J. 1989;10(9):783–90. https://doi.org/10.1093/oxfordjournals.eurheartj.a059571.
Lu D, Greenberg MD, Little R, Malik Q, Fernicola DJ, Weissman NJ. Accelerated dobutamine stress testing: safety and feasibility in patients with known or suspected coronary artery disease. Clin Cardiol. 2001;24(2):141–5. https://doi.org/10.1002/clc.4960240208.
Mazeika PK, Nadazdin A, Oakley CM. Dobutamine stress echocardiography for detection and assessment of coronary artery disease. J Am Coll Cardiol. 1992;19(6):1203–11. https://doi.org/10.1016/0735-1097(92)90325-h.
Burger AJ, Notarianni MP, Aronson D. Safety and efficacy of an accelerated dobutamine stress echocardiography protocol in the evaluation of coronary artery disease. Am J Cardiol. 2000;86(8):825–9. https://doi.org/10.1016/s0002-9149(00)01100-0.
Masoni A, Alboni P, Malacarne C, Codeca L. Effects of dobutamine on electrophysiological properties of the specialized conduction system in man. J Electrocardiol. 1979;12(4):361–70. https://doi.org/10.1016/s0022-0736(79)80004-7.
Bianchi C, Diaz R, Gonzales C, Beregovich J. Effecto of dobutamine on atrioventricular conduction. Am Heart J. 1975;90(4):474–8.
Dahlberg P, Diamant U, Gilljam T, Rydberg A, Bergfeldt L. QT correction using Bazett’s formula remains preferable in long QT syndrome type 1 and 2. Ann Noninvasive Electrocardiol. 2021;26:12804.
Northwell Health Pharmacy Department. Contracted Price for Isoproterenol and Dobutamine in 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board (IRB #: 19–0934) of Northwell Health and exempted from the investigational new drug (IND) based upon a Food and Drug Administration (FDA) review. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
this study was obtained from all participants included in the study.
Conflict of interest
There was no outside funding for this study. The authors did not receive support from any organization for the submitted work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ismail, H., Gabriels, J.K., Chang, D. et al. Site-specific effects of dobutamine on cardiac conduction and refractoriness. J Interv Card Electrophysiol 67, 71–82 (2024). https://doi.org/10.1007/s10840-023-01573-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-023-01573-1