Abstract
Purpose
To evaluate the association between different right ventricular (RV) lead positions as assessed by cardiac computed tomography (CT) and echocardiographic and clinical outcomes in patients receiving cardiac resynchronization therapy (CRT).
Methods
We reviewed patient records of all 278 patients included in two randomized controlled trials (ImagingCRT and ElectroCRT) for occurrence of heart failure (HF) hospitalization or all-cause death (primary endpoint) during long-term follow-up. Outcomes were compared between RV lead positions using adjusted Cox regression analysis. Six months after CRT implantation, we estimated left ventricular (LV) reverse remodeling by measuring LV end-systolic and end-diastolic volumes by echocardiography. Changes from baseline to 6 months follow-up were compared between RV lead positions. Device-related complications were recorded at 6-month follow-up.
Results
During median (interquartile range) follow-up of 4.7 (2.9–7.1) years, the risk of meeting the primary endpoint was similar for patients with non-apical vs. apical RV lead position (adjusted hazard ratio (HR) 0.78, 95% confidence interval (CI) 0.54–1.12, p = 0.17) and free wall vs. septal RV lead position (adjusted HR 1.03, 95% CI 0.72–1.47, p = 0.86).
Changes in LV ejection fraction and dimensions were similar with the different RV lead positions. We observed no differences in device-related complications relative to the RV lead position.
Conclusions
In patients receiving CRT, the risk of HF hospitalization or all-cause death during long-term follow-up, and LV remodeling and incidence of device-related complications after 6 months are not associated with different anatomical RV lead position as assessed by cardiac CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cardiac resynchronization therapy (CRT) reduces heart failure (HF) hospitalization and mortality in patients with symptomatic HF, reduced left ventricular (LV) ejection fraction (EF), and prolonged QRS despite optimal medical therapy [1,2,3,4]. While a substantial proportion of patients derive no measurable clinical benefit from CRT [5, 6], a favorable CRT response requires optimal LV lead positioning [7]. Only limited data are available regarding the clinical importance of right ventricular (RV) lead position, and data reported on the associations between anatomical RV lead positions and clinical outcomes including the risk of ventricular tachyarrhythmias vary widely [8,9,10,11,12,13,14,15,16]. These studies all applied fluoroscopy and/or chest radiography for documentation of the RV lead position. However, two-dimensional techniques seem inaccurate when compared to three-dimensional anatomical assessment by cardiac computed tomography (CT) and only modestly reproducible for assessment of the pacing lead position [17, 18]. Furthermore, the type and incidence of device-related complications in relation to different cardiac CT-verified RV lead positions in CRT recipients have not been described.
The aims of the present study are to evaluate the association between cardiac CT-verified anatomical RV lead position and outcomes in CRT recipients, and to report the incidence and type of device-related complications relative to the anatomical RV lead position.
2 Methods
2.1 Patient population and design
The present study is a long-term follow-up study of all patients enrolled in the ImagingCRT (empiric vs. imaging-guided LV lead placement study in CRT) and ElectroCRT (electrical vs. imaging-guided LV lead placement in CRT) trials enrolling patients between 2011–2014 and 2015–2017, respectively [19, 20]. The study designs and main results have been published [19, 20]. Briefly, in the ImagingCRT study, 182 patients were randomized to either (1) imaging-guided LV lead placement towards the optimal coronary sinus branch closest to the latest mechanically activated, non-scarred myocardial segment as defined by pre-implant CT, speckle-tracking echocardiography, and single-photon emission computed tomography or (2) routine CRT implantation without imaging guidance [19]. All patients had echocardiographic interventricular pacing delay (VVd) optimization at day 1 and 1 month post-implant [19]. In ElectroCRT, 122 patients were randomized to either (1) LV lead placement targeted towards the latest electrically activated segment combined with post-implant optimization of VVd or (2) imaging-guided LV lead implantation as performed in the ImagingCRT study [20]. In both studies, entry criteria were New York Heart Association (NYHA) functional class II–IV despite optimal medical therapy, LV EF ≤ 35%, QRS width ≥ 120 ms with left bundle branch block configuration or RV paced QRS > 180 ms, and age > 40 years. Echocardiographic LV EF and end-diastolic and end-systolic volumes (EDV and ESV) were assessed prior to CRT implantation and after 6 months of follow-up using Simpson’s biplane method. The study protocols were approved by the local ethics committee and conform to the principles of the Declaration of Helsinki. All patients gave written informed consent before enrolment.
2.2 CRT procedural, optimization, and image acquisition characteristics
We used a transvenous implantation approach with commercially available leads and devices in both studies. All RV leads were active fixated (screw-in). We aimed to place RV leads in a RV septal position using anteroposterior, left anterior oblique and right anterior oblique projection to document the orientation of the lead tip. The LV lead was placed according to group allocation in the ImagingCRT or ElectroCRT trials, respectively. The final RV and LV positions were verified by post-implant contrast-enhanced retrospective cardiac CT using a dual-source CT system (Siemens Somatom Definition Flash or Siemens SOMATOM Force, Siemens Healthcare, Munich, Germany) using multiplanar and 3D reconstructions (syngo.via and Multimodality workplace, Siemens Healthcare, Munich, Germany). The estimated effective radiation doses from follow-up cardiac CT in ImagingCRT and ElectroCRT were 5.7 ± 0.6 and 2.0 ± 0.8 mSv, respectively.
2.3 RV lead position
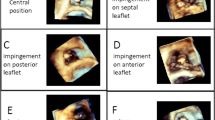
The RV lead long-axis level position was classified as either basal, mid-RV, or apical (Fig. 1) and grouped into “non-apical” (basal or mid-RV) or “apical” for analysis. The RV lead short-axis wall position was classified as either free wall or septal (Fig. 1).
Assessment and distribution of RV lead positions. RV lead positions were assessed using cardiac computed tomography and determined in the long axis (top) and short axis (bottom). Evaluation of the RV lead tip position was performed using a simple segmentation dividing the RV long axis into equal thirds (basal, mid, and apical), and the short axis into septum (area between RV and LV including the attachment of the RV free wall), and free wall (free wall except area with attachment to septum). In this example, the RV lead was located in the mid-RV free wall. LV, left ventricular; RV, right ventricular. Values are n (%)
2.4 Follow-up and endpoints
In the setting of the two randomized trials, a clinical follow-up was performed 6 months after CRT implantation including NYHA functional class assessment, 6-min walk test (6MWT), Minnesota Living with Heart Failure questionnaire (MLHFQ), electrocardiogram, and echocardiography. Moreover, we recorded all device-related complications occurring perioperatively and within the first 6 months. For the long-term clinical follow-up, we reviewed patient records for the occurrence of HF hospitalization (hospital admission > 24 h with symptoms of HF requiring intravenous administration of diuretics), ventricular tachyarrhythmias (ventricular tachycardia or ventricular fibrillation detected on remote monitoring or device follow-up), and death until December 2020. The primary endpoint was a composite of HF hospitalization or all-cause death during long-term follow-up. Secondary endpoints were occurrence of (1) HF hospitalization, (2) all-cause death, or (3) ventricular tachyarrhythmias during long-term follow-up. Outcome measures included changes from baseline in NYHA functional class, 6MWT, MLHFQ score, QRS duration, and echocardiographic LV EF, EDV, and ESV after 6 months of follow-up. These outcome measures were not available for long-term follow-up analysis.
2.5 Statistics
Continuous variables were presented as median (interquartile range (IQR)) or mean ± standard deviation (SD). Differences between groups were compared using the unpaired student’s t-test or Wilcoxon’s rank-sum test as appropriate. Categorical variables were presented as frequencies and percentages, and compared using the chi-square test for trend for ordinal variables or the Fisher exact test for unordered variables. Time to the primary composite endpoint was illustrated by the Kaplan–Meier method and compared using the log-rank test. Cox proportional regression analysis with calculation of hazard ratio (HR) with 95% confidence interval (CI) was used to assess the association between RV lead location and primary and secondary endpoints (unadjusted and adjusted for sex, ischemic vs. non-ischemic etiology, NYHA functional class, QRS duration, LV EF, EDV, and ESV). A two-tailed p-value < 0.05 was considered statistically significant. All statistical analyses were performed using Stata (StataCorp, College Station, TX, USA).
3 Results
3.1 Study population and distribution of RV lead position
Of the 304 patients included in the ImagingCRT and ElectroCRT trials, 278 patients with available data on the RV lead position contributed to the outcome analysis. Data were missing in 26 (9%) patients due lack of cardiac CT follow-up (ImagingCRT, n = 3; ElectroCRT, n = 23). Overall mean age was 71 ± 9 years, 65 (23%) were female, 140 (50%) had ischemic cardiomyopathy, mean LV EF was 27 ± 7%, QRS width was 167 ± 21 ms, and all received optimal medical HF therapy. Median (IQR) follow-up was 4.7 (2.9–7.1) years with no patients lost to follow-up. A total of 273/278 patients attended the 6-month follow-up.
The RV lead position was non-apical in 126 (45%) and apical in 152 (55%) patients in the long-axis view, and free wall in 138 (50%) and septal in 140 (50%) patients in the short-axis view (Fig. 1). Baseline characteristics in relation to RV lead position are presented in Table 1. Patients with non-apical RV lead position were less likely to be male and receive angiotensin converting enzyme inhibitors/angiotensin receptor blockers than patients with an apical RV lead (Table 1). More patients with a free wall RV lead position than patients with a septal RV lead were classified as NYHA III or IV (Table 1). Separate baseline characteristics and RV lead positions from the two studies are presented in Supplementary information, Table S1.
3.2 The RV lead position and outcomes at 6-month follow-up
From baseline to 6-month follow-up, we observed significant improvements in NYHA functional class, MLHFQ score, 6MWT, QRS duration, LV EF, LV EDV, and LV ESV (all p < 0.01) independently of RV lead position. We observed no statistically significant difference in improvement in functional parameters or in LV reverse remodeling from baseline to 6-month follow-up between non-apical vs. apical RV lead position (Table 2 and Fig. 2a) or between free wall vs. septal RV lead position (Table 3 and Fig. 2b). Device-related complications did not vary with anatomical RV lead positions (Supplementary information, Table S2).
Comparison of the LV reverse remodeling (i.e., absolute changes in LV EF (%), EDV (ml) and ESV (ml)) between patients with a apical vs. non-apical RV leads, or b septal or free wall RV lead positions. EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricular; RV, right ventricular
3.3 Relationship between RV lead position and long-term outcomes
Overall, 130 (47%) patients met the primary composite endpoint (98 (55%) from ImagingCRT and 32 (32%) from ElectroCRT). The proportion of patients meeting the primary composite endpoint was lower in those with a non-apical RV lead position than among those with an apical RV position (49 (38%) vs. 81 (53%) patients). However, the predefined adjusted risk of HF hospitalization or all-cause death was not statistically significantly different between non-apical and apical RV lead position (adjusted HR 0.78, 95% CI 0.54–1.12, p = 0.17) (Figs. 3 and 4). Comparing patients with free wall and septal RV lead position, similar proportions of patients met the primary endpoint (61 (44%) vs. 56 (40%), p = 0.55, adjusted HR 1.03, 95% CI 0.72–1.47, p = 0.86) (Figs. 3 and 5).
Forest plot comparing non-apical vs. apical RV lead positions and free wall vs. septal RV lead positions in term of risk of all-cause mortality, HF hospitalization, or ventricular tachyarrhythmia. HR adjusted for sex, ischemic cardiomyopathy, NYHA, QRS duration, LV EF, LV EDV, and LV ESV. CI, confidence interval; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; HF, heart failure; HR, hazard ratio; LV, left ventricular; NYHA, New York Heart Association; RV, right ventricular
The risks of meeting the secondary endpoints of HF hospitalization, all-cause death, or ventricular tachyarrhythmia alone did not differ significantly between non-apical vs. apical and free wall vs. septal RV lead positions (Fig. 3).
4 Discussion
In the present analysis of data from CRT recipients included in one of two randomized controlled trials investigating targeted LV lead placement, we assessed anatomical RV lead positions using cardiac CT. During long-term follow-up, we demonstrated that risk of all-cause death, HF hospitalization, or ventricular tachyarrhythmias did not differ significantly between patients with different anatomical RV lead positions after median 4.7 years of follow-up. Consistent with this, we observed no differences in measures of LV reverse remodeling or device-related complications between different RV lead positions after 6 months of follow-up.
To the best of our knowledge, previous studies evaluating the clinical impact of RV lead position in CRT recipients applied fluoroscopy or chest radiography [9,10,11,12,13,14,15,16, 21, 22]. These methods are inaccurate and only modestly reproducible for assessment of pacing site compared with cardiac CT [17, 18]. A previous study demonstrated that the majority of cardiac CT-verified apical or free wall RV lead position were misclassified by fluoroscopy as basal/mid-RV or septal position, respectively [17]. This methodological problem using fluoroscopy or chest radiography may at least partly explain why previous studies assessing the importance of RV lead position have yielded conflicting results. We observed that 50% of RV leads was positioned in the free wall despite targeting septal placement during implantation. This is in accordance with a previous study demonstrating 11 out of 18 RV leads positioned in the anterior RV wall using cardiac CT, which is classified as free wall in this study [23]. The heart can be aligned exactly according to its long-axis using cardiac CT, while fluoroscopy cannot account for an unpredictable position of the heart due to cardiac dilatation and rotation in HF patients [17]. This observation supports the evidence that the standard fluoroscopic definitions of septum do not reflect anatomic reality. However, we acknowledge that challenges in assessing CRT lead positions also may occur with cardiac CT due to artifacts from the lead tips.
Sub-studies of REVERSE (resynchronization reverses remodeling in systolic LV dysfunction) and MADIT-CRT (multicenter automatic defibrillator implantation trial-CRT) are in agreement with our findings, showing no statistical differences in the risk of HF hospitalization or all-cause death when comparing non-apical and apical RV lead positions [9, 11, 14]. We did observe a point estimate of 0.78 with respect to the main endpoint in patients with non-apical RV lead positions, where the Kaplan Meier curves for the two groups diverged after approximately 3 years, and later converged (Fig. 4). Whether this is a signal that would have reached statistical significance in a larger study cohort is unknown. This was an observational study, and the fact that significantly more patients were females in the non-apical group, and female gender is associated with improved CRT response [24], may have played a role.
To our knowledge, no prior studies compared septal RV leads to free wall RV leads, possibly because of difficulties of distinguishing the free wall from septal RV lead positions in fluoroscopy and chest radiography. We were not able to demonstrate a lower risk of HF hospitalization or all-cause death with any of the lead positions. Currently, routine methods for placing the RV lead septal or free wall are not accurate or reproducible [17, 18]. Therefore, it is not likely that this question will be answered in a well-powered randomized controlled trial unless an accurate and reproducible method is applied.
It appears that the RV lead position is not a major determinant of outcome in CRT. It could be hypothesized that the optimal RV lead location also may be tailored to the LV position. A previous study investigating the relation between LV-RV inter-lead distance and LV lead electrical delay found that both parameters were acting synergistically in predicting LV reverse remodeling [25]. Another study demonstrated favorable clinical outcome in patients with a longer electrical inter-lead distance [8]. Efforts in optimizing both parameters may therefore potentially improve CRT outcomes.
Patients with HF and prolonged ventricular activation are at high risk of ventricular tachyarrhythmia despite treatment with CRT, and several factors have been suggested in order to reduce this risk including different ventricular lead positions [15]. In our study, we found no difference between non-apical vs. apical RV lead position or free wall vs. septal RV lead position in that regard. This conflicts with a sub-study of MADIT-CRT, reporting higher risk of ventricular tachyarrhythmias with non-apical RV pacing [9], and a larger registry study reporting a higher incidence of appropriate-ICD therapy in patients with apical RV lead position [15]. However, our findings are accordant with those of a sub-study of SPICE (septal positioning of ventricular implantable cardioverter-defibrillator electrodes), which reported similar risk between septal and apical RV position [10]. The mechanisms of arrhythmia in CRT patients may relate more to the presence of scar tissue and specific types of cardiomyopathy than to the exact RV lead positions.
Prior studies investigated the extent of LV reverse remodeling between different RV lead positions [11, 13]. A meta-analysis reported overall similar LV remodeling between apical and non-apical RV lead location, which was also confirmed in our study [22]. Furthermore, the QRS duration was independent of RV lead positions as previously published [10, 11]. Therefore, RV lead position seems to be of less importance in relation to short-term echocardiographic response.
One may expect a higher risk of lead perforation and pericardial effusion in patients with free wall RV leads. This was not confirmed in our analysis, as none of these complications were observed in this cohort of moderate size.
The limitations of the ImagingCRT and ElectroCRT trials have been described previously [19, 20]. The inclusion of two randomized controlled trials in one observational study may have an influence on the overall results. However, the study population in both studies were homogenous, and the long-term follow-up study of the ImagingCRT demonstrated no significant difference on clinical outcomes between groups [26], and ElectroCRT demonstrated non-inferioriry of the electrical-guided LV lead pacement to the imaging-guided LV lead placement on short-term [20]. We acknowledge the inherent limitations of single-center studies, each with a moderate sample size. However, the data quality is excellent, and follow-up was done in a protocolled, meticulous setup. The sample size is moderate, and signals of less magnitude may have been overlooked. Furthermore, patients underwent different VVd optimization according to study protocol and potentially during the follow-up period, which may influence on our results. However, we had large numbers of primary endpoints, and there was consistency among the neutral findings in the echocardiographic results after 6 months and the long-term clinical outcomes. This analysis is observational, and patients did not undergo randomization to one of more RV-lead positions. Therefore, causality cannot be investigated.
In conclusion, increased risk of HF hospitalization, all-cause death, or ventricular tachyarrhythmia during long-term follow-up is not significantly associated with certain anatomical RV lead positions. There is no association between LV remodeling, changes in QRS duration, NYHA functional class, quality of life or 6MWT, or incidence of device-related complications and anatomical RV lead position as defined by cardiac CT during 6 months follow-up.
References
Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur heart J. 2021.
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53.
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50.
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49.
Kronborg MB, Mortensen PT, Kirkfeldt RE, Nielsen JC. Very long term follow-up of cardiac resynchronization therapy: clinical outcome and predictors of mortality. Eur J Heart Fail. 2008;10:796–801.
Yu CM, Sanderson JE, Gorcsan J 3rd. Echocardiography, dyssynchrony, and the response to cardiac resynchronization therapy. Eur Heart J. 2010;31:2326–37.
Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765–73.
Miranda RI, Nault M, Johri A, Simpson CS, Michael KA, Abdollah H, et al. Maximal electric separation-guided placement of right ventricular lead improves responders in cardiac resynchronization defibrillator therapy. Circ Arrhythm Electrophysiol. 2012;5:927–32.
Kutyifa V, Bloch Thomsen PE, Huang DT, Rosero S, Tompkins C, Jons C, et al. Impact of the right ventricular lead position on clinical outcome and on the incidence of ventricular tachyarrhythmias in patients with CRT-D. Heart Rhythm. 2013;10:1770–7.
Asbach S, Lennerz C, Semmler V, Grebmer C, Solzbach U, Kloppe A, et al. Impact of the right ventricular lead position on clinical end points in CRT recipients–a subanalysis of the multicenter randomized SPICE trial. Pacing Clin Electrophysiol. 2016;39:261–7.
Thébault C, Donal E, Meunier C, Gervais R, Gerritse B, Gold MR, et al. Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the REVERSE trial. Eur Heart J. 2012;33:2662–71.
Leclercq C, Sadoul N, Mont L, Defaye P, Osca J, Mouton E, et al. Comparison of right ventricular septal pacing and right ventricular apical pacing in patients receiving cardiac resynchronization therapy defibrillators: the SEPTAL CRT Study. Eur Heart J. 2016;37:473–83.
Khan FZ, Salahshouri P, Duehmke R, Read PA, Pugh PJ, Elsik M, et al. The impact of the right ventricular lead position on response to cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011;34:467–74.
Daimee UA, Klein HU, Giudici MC, Zareba W, McNitt S, Polonsky B, et al. Right ventricular lead location, right-left ventricular lead interaction, and long-term outcomes in cardiac resynchronization therapy patients. J Interv Card Electrophysiol. 2018;52:185–94.
Kronborg MB, Johansen JB, Haarbo J, Riahi S, Philbert BT, Jørgensen OD, et al. Association between implantable cardioverter-defibrillator therapy and different lead positions in patients with cardiac resynchronization therapy. Europace. 2018;20:e133–9.
Kronborg MB, Johansen JB, Riahi S, Petersen HH, Haarbo J, Jørgensen OD, et al. Association between right ventricular lead position and clinical outcomes in patients with cardiac resynchronization therapy. Europace. 2018;20:629–35.
Sommer A, Kronborg MB, Nørgaard BL, Gerdes C, Mortensen PT, Nielsen JC. Left and right ventricular lead positions are imprecisely determined by fluoroscopy in cardiac resynchronization therapy: a comparison with cardiac computed tomography. Europace. 2014;16:1334–41.
Rickard J, Ingelmo C, Sraow D, Wilkoff BL, Grimm RA, Schoenhagen P, et al. Chest radiography is a poor predictor of left ventricular lead position in patients undergoing cardiac resynchronization therapy: comparison with multidetector computed tomography. J Interv Card Electrophysiol. 2011;32:59–65.
Sommer A, Kronborg MB, Norgaard BL, Poulsen SH, Bouchelouche K, Bottcher M, et al. Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Eur J Heart Fail. 2016;18:1365–74.
Stephansen C, Sommer A, Kronborg MB, Jensen JM, Nørgaard BL, Gerdes C, et al. Electrically vs. imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Europace. 2019;21:1369–77.
Benz AP, Vamos M, Erath JW, Bogyi P, Duray GZ, Hohnloser SH. ICD lead type and RV lead position in CRT-D recipients. Clin Res Cardiol. 2018;107:1122–30.
Zografos TA, Siontis KC, Jastrzebski M, Kutyifa V, Klein HU, Zareba W, et al. Apical vs. non-apical right ventricular pacing in cardiac resynchronization therapy: a meta-analysis. Europace. 2015;17:1259–66.
Moore P, Coucher J, Ngai S, Stanton T, Wahi S, Gould P, et al. Imaging and right ventricular pacing lead position: a comparison of CT, MRI, and echocardiography. Pacing Clin Electrophysiol. 2016;39:382–92.
Rickard J, Michtalik H, Sharma R, Berger Z, Iyoha E, Green AR, et al. Predictors of response to cardiac resynchronization therapy: a systematic review. Int J Cardiol. 2016;225:345–52.
Merchant FM, Heist EK, Nandigam KV, Mulligan LJ, Blendea D, Riedl L, et al. Interlead distance and left ventricular lead electrical delay predict reverse remodeling during cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2010;33:575–82.
Fyenbo DB, Sommer A, Nørgaard BL, Kronborg MB, Kristensen J, Gerdes C, et al. Long-term outcomes in a randomized controlled trial of multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy. Europace. 2022. https://doi.org/10.1093/europace/euab314.
Funding
This work was supported by Aarhus University, the Danish Heart Foundation (grant number R140-A9482-B2407), Health Research Foundation of Central Denmark Region (grant number R64-A3194-B1667), and Gangstedfonden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JCN is supported by grants from the Novo Nordisk Foundation (grant number NNF16OC0018658, NNF17OC0029148). HKJ is supported by grants from the Novo Nordisk Foundation, Denmark (NNF18OC0031258 and NNF20OC0065151), and received lecture fees from Abbott Denmark and Biosense Webster, Europe. All other declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fyenbo, D.B., Sommer, A., Stephansen, C. et al. Cardiac computed tomography-verified right ventricular lead position and outcomes in cardiac resynchronization therapy. J Interv Card Electrophysiol 64, 783–792 (2022). https://doi.org/10.1007/s10840-022-01193-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01193-1