Abstract
Background
Catheter ablation for persistent atrial fibrillation (persAF) is associated with less favorable outcomes than for paroxysmal AF. To improve success rates, left atrial (LA) substrate modification is frequently performed in addition to pulmonary vein isolation (PVI). The purpose of the study was to compare 4 different ablation approaches using radiofrequency catheter ablation (RFCA) or cryoballoon ablation (CB-A) for persAF and to evaluate the respective outcomes on a midterm follow-up of 12 months.
Methods
We did a propensity score–matched comparison of 30 patients undergoing PVI + LA posterior wall isolation (LAPWI) with CB-A, 30 patients who underwent PVI + linear ablation (roof and mitral lines) using RFCA, 60 patients with PVI alone using CB-A, and 60 patients who had PVI alone using RFCA. The endpoint was recurrence of documented atrial tachyarrhythmias (ATas) > 30 s at 1-year follow-up.
Results
After 12 months, freedom from ATas after a single procedure was 83.3% in the PVI + LAPWI group, 46.7% in the PVI + linear ablation group, 58.3% in the PVI-alone CB-A group, and 61.6% PVI-alone RFCA (p = 0.03). Moreover, freedom from ATas was significantly higher comparing the PVI + LAPWI group with each of the other groups.
Conclusions
In this propensity-matched comparison of strategies for persAF, LAPW ablation in addition to PVI with CB-A seems to improve 1-year outcome in comparison to PVI + linear ablation using RFCA and to PVI alone using RFCA or CB-A. Randomized comparisons are eagerly awaited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transcatheter ablation is a well-established treatment for paroxysmal atrial fibrillation (AF) [1]. Conversely, clinical outcomes are less encouraging when dealing with persistent atrial fibrillation (persAF) [2, 3]. In order to improve success rates, left atrial (LA) substrate modification is frequently performed in addition to pulmonary vein isolation (PVI). In this setting, various strategies have been proposed [4,5,6,7,8]. In the present study, we compared 4 different ablation approaches to PersAF and evaluated the respective outcomes on a midterm follow-up of 12 months.
2 Methods
2.1 Study population
Patients undergoing index ablation for PersAF were included in the study. We considered the following exclusion criteria: presence of paroxysmal atrial fibrillation, previous AF ablation or cardiac surgery, congenital heart disease, non-treated coronary artery disease, intracavitary thrombus, significant valvular disease, contraindications to general anesthesia, and contraindication to anticoagulation with heparin, or coumadin, direct thrombin/factor Xa inhibitors.
PersAF was defined as lasting longer than 7 days, including episodes that were terminated by cardioversion, either with drugs or by direct current cardioversion, after 7 days or more as per current guidelines [9].
A first group of 30 consecutive patients who underwent PVI + LA posterior wall isolation (LAPWI) with cryoballoon ablation (CB-A) enrolled between November 2018 and January 2019 was considered for our analysis. As from January 2013, a second group of 55 patients who underwent PVI + linear ablation (roof and mitral lines) using point by point radiofrequency catheter ablation (RFCA), a third group of 608 patients who underwent PVI with CB-A, and a fourth group of 301 patients who underwent PVI with RFCA were taken into consideration. The study was observational and retrospective in nature.
2.2 Propensity score group matching
Propensity score group matching was carried out at a 1:1 ratio for the second group and a 1:2 ratio for PVI-only groups, according to the following variables: (1) age; (2) sex; (3) CHA2DS2-VASc score; (4) LA size; (5) left ventricular ejection fraction (LVEF); and (6) mean AF duration. Therefore, a total of 30 patients formed the PVI + LAPWI group, 30 patients formed the PVI + linear ablation group, 60 patients formed PVI-alone CB-A group, and 60 patients formed PVI-alone RFCA group.
The study protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by the Declaration of Helsinki and was approved by the local ethics committee of our Institution. All patients provided written informed consent to the ablation procedure.
2.3 Cryoballoon ablation
2.3.1 Pulmonary vein isolation
Our standard pre-procedural management and ablation has been previously described in detail [10, 11]. After having obtained LA access, through a steerable 15 Fr sheath (FlexCath Advance ®, Medtronic©, Minneapolis, MN, USA), an inner lumen mapping catheter (Achieve®, Medtronic©, Minneapolis, MN, USA) was advanced in the LA and was positioned in each pulmonary vein (PV) ostium. A 28-mm CB (Arctic Front Advance ™, Medtronic©, Minneapolis, MN, USA) was advanced inflated and positioned in each PV ostium with the aim of reaching an optimal vessel occlusion defined by total contrast retention and absence of backflow into the LA after a selective injection. Our ablation sequence was left superior PV (LSPV) first, followed by the left inferior PV (LIPV), right inferior PV (RIPV), and right superior PV (RSPV). After obtaining a satisfactory vein occlusion, a standard 180-s cryoapplication was started with the aim of reaching −40° within the first minute. In case the temperature did not attain this value, an extra 180-s freeze was delivered. During the right PV ablation, diaphragmatic stimulation was achieved by phrenic nerve pacing in order to avoid phrenic nerve palsy. During the whole procedure, heparin infusion was titrated to maintain an activated clotting time (ACT) value over 300 s.
2.3.2 Posterior wall ablation
In order to achieve the LAPWI, the Achieve® catheter was placed deeply in the LSPV to anchor the CB, with the distal balloon-freezing surface oriented towards the LAPW. Standard cryothermal lesions lasted 180 s. The first cryoapplication was performed very close the LSPV ostium in order to mildly overlap the lesion with the previous one created during LSPV isolation.
By a clockwise rotation and progressive “pullback” of the sheath while keeping the CB in contact with the posterior wall, consecutive freezes were applied along the LAPW in an overlapping manner. The same maneuvers were performed with the Achieve® catheter placed inside the RSPV but with a counterclockwise rotation. At the end of the procedure, entrance and exit block pacing maneuvers were used to confirm LAPWI, while the Achieve catheter was positioned in the posterior atrial wall. A multielectrode mapping catheter (Pentaray, Biosense Webster, USA) has been used to create a high-density 3D electroanatomical map (CARTO 3, Biosense Webster, USA). Areas of low voltage were defined as < 0.15 mV.
Every procedure was performed under esophageal temperature monitoring: the CIRCA’s S-CATHTM Esophageal Temperature Monitoring System (Circa Scientific, Englewood, Colorado). Cryoballoon applications were interrupted in case of recorded inner esophageal temperatures of 15 °C or less.
2.4 Radiofrequency catheter ablation
2.4.1 Pulmonary vein isolation
A double transseptal puncture was performed to gain access to LA. A circumferential mapping catheter (Lasso™, Biosense Webster©) was positioned into the LA. LA geometry was obtained using a 3D mapping system (CARTO®, Biosense Webster©). Furthermore, in the initial series of patients, multiple fluoroscopic projections were performed to meticulously delineate the PV ostia.
A 4-mm tip irrigated catheter with contact-force (CF) sensor (Thermocool, SmartTouch™, Biosense Webster©) was used to perform radiofrequency applications in a power-controlled mode with a power limit of 35 W and at a maximum temperature of 48 °C. Power was limited to 25 W on the posterior sites. Our ablation strategy consisted of the encirclement of ipsilateral PVs by creating contiguous lesions at a distance >5 mm from the ostia. The circumferential mapping catheter was used to confirm PVI and bidirectional block with a waiting time of 20 min after last application. During the whole procedure, ACT was maintained over 300 s with heparin infusion.
2.4.2 Roof and mitral lines
Ablation of LA roof was performed by creation of a linear line between the superior PVs while the mitral line was created between the posterolateral mitral annulus and the left inferior PV ostium. A total of 35 W for the mitral line and 30 W for the roof line were used as power limits for the ablation, performed using the same aforementioned 4-mm tip irrigated catheter with a long-sheath (SL-O, St. Jude Medical©). During every application, RF was applied until the electrogram amplitude was decreased by > 80% or the local potential split. Differential pacing from LA appendage versus LA posterior wall and pacing from the LA appendage versus LA septum confirmed bidirectional block across roof and mitral line. If mitral isthmus linear block was not achieved by an endocardial ablation, epicardial ablation from the coronary sinus (CS) was performed. Ablation within the CS was performed in a power-controlled mode with a power limit of max 25 W and RF deliveries of 40–120 s.
2.4.3 Postprocedural management and follow-up
After the procedure, patients were continuously monitored via telemetry for at least 18 h, and a TTE was performed before the discharge, to exclude possible procedure-related complications. Oral anticoagulation was started the same evening of ablation and continued for at least 3 months. The same antiarrhythmic medications that patients were used to assume were prescribed after the ablation for at least 3 months, and after that time, their discontinuation was recommended. After discharge, patients were scheduled for follow-up visits with baseline ECG and 24 h Holter recordings at 3, 6, and 12 months. After a 90-day post-ablation blanking period (BP), all atrial tachyarrhythmia (ATa) episodes > 30 s, documented with standard ECG or 24 h ECG Holter monitoring, were considered a recurrence. For all the patients complaining dysphonia, palpitations, or thoracic pain following the ablation procedure, an additional Holter monitoring was prescribed.
2.5 Statistical analysis
Continuous variables are reported as mean ± standard deviation, while categorical variables are presented as absolute numbers and percentages. Comparison of continuous data between groups was performed with the Student t test for independent samples or Mann-Whitney U test, while one-way ANOVA or Pearson χ2 was used for comparison of categorical variables. Arrhythmia-free survival curves for each group are presented as Kaplan-Meier plots, and time-to-event analysis was performed using the logrank test.
All tests were 2-sided, and a p value of 0.05 was considered statistically significant. Analyses were performed with the SPSS 23.0 statistical software (IBM Company, Chicago, IL, USA).
3 Results
3.1 Baseline characteristics
Baseline patient characteristics are reported in Table 1. The 4 groups were well-balanced, with no significant differences in patient demographics, antiarrhythmic medication before admission, and echocardiographic findings.
3.2 Procedural characteristics
At the time of ablation, 97 patients (53.9%) were in AF. Restoration of sinus rhythm during ablation occurred in 19/97 patients (19.6%), in 3/16 patients (18.8%) in the PVI + LAPWI group, in 3/15 patients (20%) in the PVI + linear ablation group, in 5/35 patients (14.3%) in the PVI-alone CB-A group, and in 8/31 patients (25.8%) in the PVI-alone RFCA group (p = 0.71). Procedure characteristics are reported in Table 2. For PVI procedures with CB-A, there were no differences in the ablation parameters between the PVI+LAPWI group and the PVI-alone group. For PVI with RFCA, characteristics were similar between the PVI + linear ablation group and the PVI-alone group.
In the PVI+LAPWI group, interruption of the application due to a luminal esophageal temperature (LET below 15 °C) occurred in 3 patients (10%), without however jeopardizing the complete LAPWI.
In the PVI + linear ablation group, a LA roof line block was successfully created in all patients; a mitral line block was adequately performed in 25/30 (83.3%); in the remaining 5 patients (16.7%), the block was completed by RFCA within the CS.
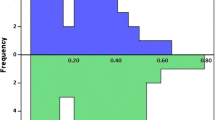
Procedure and fluoroscopy time were significantly different in the 4 groups (Fig. 1, p < 0.001); these were shorter for the PVI-alone CB-A group (59.9 ± 11.5 min and 12.8 ± 5.4 min) and for PVI + LAPWI group (93 ± 18.1 min and 20 ± 5.6 min) as compared with PVI-alone RFCA group (142.9 ± 24.6 min and 24.7 ± 7.3 min) and as compared with PVI + linear ablation group (164.9 ± 26.8 min and 37.8 ± 8.2 min) (p < 0.01 for both comparisons). In the CB-A groups, procedure and fluoroscopy time were significantly lower in the PVI-alone CB-A group (p < 0.01). Moreover, these were significantly shorter in the PVI-alone RFCA group than PVI + linear ablation group (p < 0.01).
3.3 Procedural adverse events
No deaths or cerebrovascular events occurred in the peri-procedural period and during the entire follow-up.
Adverse events occurred in 7 patients in the total population (3.9%). Cardiac tamponade occurred in 1 of 180 patients (0.6%); in the PVI + linear ablation group, instantaneous pericardiocentesis was successfully performed. One of the 180 patients had a pseudoaneurysm requiring surgical treatment (0.6%), in the PVI-alone CB-A group. In the CB-A groups (n = 90), transient phrenic palsy (5.6%) was observed in 5 patients, 2 (6.6%) occurred in the PVI + LAPWI group, and 3 (5%) in the PVI-alone CB-A group, with complete resolution before the end of the procedure.
3.4 Follow-up
3.4.1 Freedom from atrial tachyarrhythmia
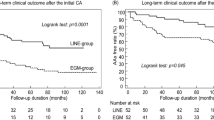
All 180 patients (100%) completed the 1-year follow-up. Overall freedom from ATas at 3, 6, and 12 months was 85%, 72.2%, and 61.7%, respectively following a single procedure. At 12 months, freedom from ATas was significantly different among the four groups (83% PVI + LAPWI; 46.7% PVI + linear ablation; 58.3% PVI-alone CB-A; 61.6% PVI-alone RFCA, log rank p = 0.03). Moreover, it was significantly higher comparing the PVI + LAPWI group with each of the other groups (vs PVI + linear ablation group log rank p = 0.003; vs PVI-alone CB-A log rank p = 0.03; vs PVI-alone RFCA log rank p = 0.04). Otherwise, there was no significant difference comparing PVI + linear ablation group, PVI-alone CB-A group, and PVI-alone RFCA group (log rank p = 0.33). Kaplan-Meier survival curves showing each group’s cumulative arrhythmia-free survival are presented in Fig. 2.
3.4.2 Atrial arrhythmia recurrences
During the BP, 67 patients (37.2%) had arrhythmia recurrences: 10 (33.3%) in the PVI + LAPWI group, 14 (46.7%) in the PVI + linear ablation group, 19 (31.7%) in the PVI-alone CB-A group, and 24 (40%) in the PVI-alone RFCA group (p = 0.51). Of these, 31/67 (46.3%) were not recorded after BP, 6/10 (60%) in the first group, 8/14 (57%) in the second group, 5/19 (26%) in the third group, and 12/24 (50%) in the fourth group. After BP, during follow-up, 69 (38.3%) patients had arrhythmia recurrences: 5 (16.7%) in the PVI + LAPWI group, 16 (53.3%) in the PVI + linear ablation group, 25 (41.7%) in the PVI-alone CB-A group, and 23 (38.3%) in the PVI-alone RFCA group (p = 0.03).
Of these, 10/69 (14.5%) were regular atrial tachycardia (RAT), and 59/69 (85.5%) were AF. RAT occurred in 2 patients (6.7%) in the PVI + LAPWI group, 5 (16.7%) in the PVI + linear ablation group, 2 (3.3%) in the PVI-alone CB-A group, and 1 (1.7%) in the PVI-alone RFCA group (p = 0.02). RAT occurrence was significantly higher in the PVI + linear ablation group in comparison to that in the PVI-alone CB-A (p = 0.03) and PVI-alone RFCA group (p = 0.007). Instead, there was no difference between PVI + linear ablation group as compared to the PVI + LAPWI group (p = 0.23). RAT occurrence in the PVI + LAPWI group was not significantly different when compared to PVI-alone CB-A (p = 0.47) and PVI-alone RFCA (p = 0.21).
The remaining arrhythmias recorded were AF (10% PVI + LAPWI group, 36.7% PVI + linear ablation group, 38.3% PVI-alone CB-A group, and 36.7% PVI-alone RFCA group, p = 0.04).
In the PVI + LAPWI group, the rate of AF recurrence was significantly lower when compared with other groups (vs PVI + linear ablation group p = 0.01; vs PVI-alone CB-A p = 0.005; vs PVI-alone RFCA p = 0.008). Otherwise, there was no significant difference comparing the other 3 groups (p = 0.98).
4 Discussion
The main findings of our 1-year follow-up study of ablation strategy for persAF are (1) PVI using CB-A in conjunction with LAPWI proved to be superior to all the other groups in terms of clinical outcome guaranteeing freedom from any ATas in 83% of patients; (2) PVI alone is comparable to additional linear ablation in terms of ATas recurrence; and (3) procedure and fluoroscopy times are significantly shorter in CB-A groups than that in RFCA groups.
LAPW may play a non-negligible role in the initiation and maintenance of AF. In this regards, LAPW and its myocardial extensions into the PVs share similar embryologic origins and intrinsic pacemaker activity has been demonstrated in these myocardial sleeves [12, 13]. Moreover, the significant heterogeneity in myocardial fiber orientation within the PV antra and posterior wall, creates anisotropic conduction, favoring local reentry [14, 15]. Consequently, the LAPWI with the CB-A seems to offer interesting clinical outcome in the setting of persAF, and several studies have reported satisfying results [6, 7].
In a prospective study, Kuniss et al. [7] technically described the creation of a roof line along with its beneficial effect on the treatment of PersAF; in their study, freedom from AF appeared to be close to 80% at 1-year follow-up, better than PVI only. Moreover, electroanatomical maps following ablation showed that the lesions were extended well beyond the roof; data confirmed also in the interesting paper by Nanbu et al. [16] in which the mean isolation area, after cryoballoon roofline, covered about 70% of the whole LAPW. Similarly, Aryana et al. [6] showed that LAPWI offers additional benefits over PVI alone using CB-A in the treatment of persAF. The authors indicated that all atrial arrhythmias were lower in LAPWI group comparing to PVI group at 1 year of follow-up. A similar finding appears also in a recent paper by Barbhaiya et al. [17] in which the comparison between LAPWI and a stepwise ablation technique approach in non-paroxysmal AF, although showing a similar incidence of any atrial arrhythmia, reported higher incidence of persistent arrhythmias and repeat ablations in the stepwise approach group. Our results seem to be in line with the aforementioned studies, since neither in our data performing additional linear ablations was associated with a better outcome.
Up to now, only Aryana et al. [18] provided insights by the means of a prospective randomized trial. In their work, the authors prospectively evaluated the short-term and long-term outcomes of PVI versus PVI + LAPWI using the cryoballoon in patients with persAF. One group received only cryo-PVI, while the other group received cryo-PVI + LAPWI. All the patients underwent also RF cavo-tricuspid isthmus (CTI) ablation. After a 1-year follow-up, the incidence of recurrent atrial fibrillation was significantly lower with PVI + LAPWI (25.5% vs. 45.5%).
In accordance with these early findings, our data show rates of freedom of ATas of around 80%.
Noteworthy, in the same direction, the ongoing PLEA trial by Romero et al. [19] will provide, in the close future, data about the comparison between only PVI versus PVI + LAPWI (alone or in addition to coronary sinus isolation and left atrial appendage isolation).
The ideal ablation strategy for persAF is still unclear, and numerous techniques have been proposed.
Despite some studies reporting satisfactory outcomes following substrate modification during AF ablation in this patient population [4, 5], the STAR AF 2 trial [20] reported that creating additional lines or eliminating complex fractionated atrial electrograms in adjunction to PVI does not offer any benefit. In our data, performing additional linear ablation was not associated with a better outcome.
The reason why additional ablations are not associated with a more favorable outcome is still unclear. A potential explanation might lie in the difficulty of creating a permanent linear block. Reports in the literature state that late reconnection rates for roof line can occur in up to 40–60% and in 60–90% for mitral lines, in patients undergoing redo ablations for clinically overt AF recurrences [21,22,23,24]. This aspect, combined with more extensive ablation, might create substrate areas for arrhythmias [25, 26] and could explain the high rate of ATas in this specific group in our study. In addition, the (non-significant) longer duration of AF prior to the ablation may have influenced recurrence rate. Although in line with previous randomized data [20], our finding that 1-year outcome after PVI alone is comparable to PVI with additional linear ablation should be interpreted cautiously given the limited sample size and abovementioned aspects.
With regard to outcomes in the PVI-alone groups, we did not find differences in terms of freedom from ATas, which corroborates with previous studies [1, 10], reporting freedom of ATas in approximately 60%. These collective findings fuel the need for randomized comparisons on outcomes after different ablation strategies for persAF. In terms of procedural and fluoroscopy times, our data underscore that the shorter procedural times and lower exposition to fluoroscopy in the CB-A groups were not associated with worse outcomes.
Finally, data in the literature shows an incidence of recurrent atrial tachyarrhythmias within 3 months of approximately 40% and approximately half of these patients with early recurrence do not continue to have atrial tachyarrhythmias [27, 28]. Our study confirmed these findings. It could be explained by the potentially reversible mechanisms of arrhythmia induced by the ablation procedure. The consequent inflammatory response to ablation and the modifications of the cardiac autonomic nervous system, such as acute adrenergic tone, can cause an early and potentially reversible proarrhythmic substrate [29, 30]. Although more extensive atrial ablation should provide a greater arrhythmogenic substrate in the first few weeks, we do not find a significant difference between the four groups.
5 Limitations
The study was a non-prospective, non-randomized trial conducted in a relatively limited number of patients. Furthermore, it reports a single-center experience. Although the use of a propensity score matching is done in order to better balance the four groups, the potential for residual confounding factors or uncontrolled selection bias together with a possible temporal bias cannot be excluded. Some of the procedures were carried out a few years ago (from January 2013) when some technological innovations, currently available, were not yet ready for use. Moreover, the fact of having used multiple fluoroscopic projections in a proportion of patients in the RF groups might explain the increased fluoroscopy times in these groups. Since the absence of use of long-term ECG monitoring (loop-recorder or 7 day Holter), the real arrhythmia recurrence rate may be underestimated. In addition, the number of regular atrial tachycardias is relatively low (n = 10 in the whole population) to reach clear conclusions; the data should be considered hypothesis-generating. Finally, since no esophageal esophagogastroduodenoscopy (EGDS) was performed after ablation, iatrogenic esophageal lesions might be underdiagnosed.
6 Conclusions
In the setting of persAF, LAPWI in addition to PVI seems to improve outcome if compared to the PVI-only strategy using CB-A or RFCA and if compared to PVI plus linear ablation (roof and mitral lines) using point by point RFCA, on a midterm follow-up. Prospective randomized controlled trials are warranted in order to confirm our findings.
References
Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160.
Jannik LP, Gunnar HG, Jim H, et al. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: a nationwide Danish cohort study. Eur Heart J. 2018;39:442–9.
Perino AC, Leef GC, Cluckey A, et al. Secular trends in success rate of catheter ablation for atrial fibrillation: the SMASH-AF cohort. Am Heart J. 2019;208:110–9.
Willems S, Klemm H, Rostock T, et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur Heart J. 2006;27:2871–8.
Yao Y, Zheng L, Zhang S, et al. Stepwise linear approach to catheter ablation of atrial fibrillation. Heart Rhythm. 2007;4:1497–504.
Aryana A, Baker JH, Espinosa Ginic MA, et al. Posterior wall isolation using the cryoballoon in conjunction with pulmonary vein ablation is superior to pulmonary vein isolation alone in patients with persistent atrial fibrillation: a multicenter experience. Heart Rhythm. 2018;15:1121–9.
Kuniss M, Greiß H, Pajitnev D, et al. Cryoballoon ablation of persistent atrial fibrillation: feasibility and safety of left atrial roof ablation with generation of conduction block in addition to antral pulmonary vein isolation. Europace. 2017;19:1109–15.
Salih M, Darrat Y, Ibrahim AM, et al. Clinical outcomes of adjunctive posterior wall isolation in persistent atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2020;31(6):1394-1402.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–78.
Ciconte G, Baltogiannis G, de Asmundis C, et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace. 2015;17:559–65.
Bisignani A, Overeinder I, Kazawa S, et al. Posterior box isolation as an adjunctive ablation strategy with the second-generation cryoballoon for paroxysmal atrial fibrillation: a comparison with standard cryoballoon pulmonary vein isolation. J Interv Card Electrophysiol. 2020. https://doi.org/10.1007/s10840-020-00812-z.
Perez-Lugones A, McMahon JT, Ratliff NB, et al. Evidence of specialized conduction cells in human pulmonaryveins of patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14(8):803–9.
Jongbloed MRM, Schalij MJ, Poelmann RE, et al. Embryonic conduction tissue: a spatial correlation with adult arrhythmogenic areas. J Cardiovasc Electrophysiol. 2004;15(3):349–55.
Suenari K, Chen YC, Kao YH, et al. Discrepant electrophysiological characteristics and calcium homeostasis of left atrial anterior and posterior myocytes. Basic Res Cardiol. 2011;106(1):65–74.
Ehrlich JR, Cha T, Zhang L, et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol. 2003;551(Pt 3):801–13.
Nanbu T, Yotsukura A, Suzuki G, et al. Important factors in left atrial posterior wall isolation using 28-mm cryoballoon ablation for persistent atrial fibrillation-block line or isolation area? J Cardiovasc Electrophysiol. 2020;31(1):119–27.
Barbhaiya CR, Knotts RJ, Beccarino N, et al. Multiple procedure outcomes for nonparoxysmal atrial fibrillation: left atrial posterior wall isolation versus stepwise ablation. J Cardiovasc Electrophysiol. 2020;31(12):3117–23.
Aryana A, Allen SL, Pujara DK, et al. Concomitant pulmonary vein and posterior wall isolation using cryoballoon with adjunct radiofrequency in persistent atrial fibrillation. JACC: Clinical Electrophysiology. Published online October 28, 2020.
Romero J, et al. Systematic evaluation of ablation techniques for non-paroxysmal atrial fibrillation. ClinicalTrials.gov Identifier: NCT04216667.
Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22.
Kim TH, Park J, Uhm JS, et al. Challenging achievement of bidirectional block after linear ablation affects the rhythm outcome in patients with persistent atrial fibrillation. J Am Heart Assoc. 2016;5:e003894.
Anousheh R, Sawhney NS, Panutich M, et al. Effect of mitral isthmus block on development of atrial tachycardia following ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:460–8.
Rostock T, O’Neill MD, Sanders P, et al. Characterization of conduction recovery across left atrial linear lesions in patients with paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:1106–11.
Sawhney N, Anousheh R, Chen W, et al. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:243–8. 20.
Shah AJ, Jadidi A, Liu X, et al. Atrial tachycardias arising from ablation of atrial fibrillation: a proarrhythmic bump or an antiarrhythmic turn? Cardiol Res Pract. 2010;2010:950763.
Matsuo S, Wright M, Knecht S, et al. Perimitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm. 2010;7:2–8.
Andrade JG, Macle L, Khairy P, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol. 2012;23:1295–301.
Themistoclakis S, Schweikert RA, Saliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm. 2008;5:679–85.
Liang JJ, Dixit S, Santangeli P. Mechanisms and clinical significance of early recurrences of atrial arrhythmias after catheter ablation for atrial fibrillation. World J Cardiol. 2016 Nov 26;8(11):638–46.
Mugnai G, de Asmundis C, Hünük B, et al. Second-generation cryoballoon ablation for paroxysmal atrial fibrillation: predictive role of atrial arrhythmias occurring in the blanking period on the incidence of late recurrences. Heart Rhythm. 2016;13:845–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SB is a consultant for Medtronic, Boston Scientific, and Microport; PB reports consulting fees and speaker honoraria from Medtronic; CdA reports speaker fees for Medtronic, Biotronik, Biosense Webster, Abbott, and Boston Scientific; teaching honoraria from Medtronic, Biotronik, Abbott and Boston Scientific; and proctoring honoraria from Medtronic, Abbott, and Biotronik; GBC reports speaker fees for Medtronic, Biotronik, Biosense Webster, and Abbott; teaching honoraria from Medtronic and Biotronik; and proctoring honoraria from Medtronic.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bisignani, A., Cecchini, F., Mugnai, G. et al. Single procedural outcomes in the setting of percutaneous ablation for persistent atrial fibrillation: a propensity-matched score comparison between different strategies. J Interv Card Electrophysiol 64, 9–16 (2022). https://doi.org/10.1007/s10840-021-00968-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-021-00968-2