Abstract
Purpose
Ablation index (AI) is a radiofrequency lesion quality marker. The AI value that allows effective and safe pulmonary vein isolation (PVI) is still debated. We evaluated the incidence of acute and late PV reconnection (PVR) with different AI settings and its predictors.
Methods
The Ablation Index Registry is a multicenter study that included patients with paroxysmal/persistent atrial fibrillation (AF) who underwent first-time ablation. Each operator performed the ablation using his preferred ablation catheter (ThermoCool® SmartTouch or Surround Flow) and AI setting (380 posterior-500 anterior and 330 posterior-450 anterior). We divided the study population into two groups according to the AI setting used: group 1 (330–450) and group 2 (380–500). Incidence of acute PVR was validated within 30 min after PVI, whereas the incidence of late PVR was evaluated at repeat procedure.
Results
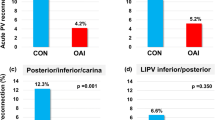
Overall, 490 patients were divided into groups 1 (258) and 2 (232). There was no significant difference in the procedural time, fluoroscopy time, and rate of the first-pass PVI between the two study groups. Acute PVR was observed in 5.6% PVs. The rate of acute PVR was slightly higher in group 2 (64/943, 6.8%, PVs) than in group 1 (48/1045, 4.6% PVs, p = 0.04). Thirty patients (6%) underwent a repeat procedure and late PVR was observed in 57/116 (49%) PVs (number of reconnected PV per patient of 1.9 ± 1.6). A similar rate of late PVR was found in the two study groups. No predictors of acute and late PVR were found.
Conclusion
Ablation with a lower range of AI is highly effective and is not associated with a higher rate of acute and late PVR. No predictors of PV reconnection were found.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ablation index (AI) (Biosense-Webster, Diamond Bar, California) is a new marker of radiofrequency (RF) lesion quality that incorporates stability, contact force, time, and power in a weighted formula. When the AI has been used in an ablation protocol respecting strict criteria for lesion contiguity [1], a high rate of first-pass pulmonary vein (PV) isolation [1,2,3,4,5] and a high single-procedure arrhythmia-free survival at 1 year [1,2,3,4,5,6] have been reported. The AI value that allows effective and safe PV isolation remains to be defined. An AI value ranging from 330 to 400 has been proposed for posterior/inferior segments of PVs and from 450 to 550 for anterior/roof segments [1, 2, 4, 7, 8].
In the present study, we compared the efficacy and the safety of two AI setting (330 for posterior/inferior segments and 450 for anterior/roof segments vs 380 for posterior/inferior segments and 500 for anterior/roof segments) during acute PV isolation (PVI) and at repeat procedure in a multicenter, prospective, and observational trial.
2 Methods
The Ablation Index Registry (AIR) (ClinicalTrials.gov Identifier: NCT03277976) is a prospective, multicenter, research study designed to evaluate the acute achievement of PV isolation with ThermoCool SmartTouch (ST) (Biosense-Webster, Diamond Bar, California) or ThermoCool SmartTouch SF (STSF) (Biosense-Webster, Diamond Bar, California) catheter using the AI Module. Enrollment started in November 2017 and ended in July 2018. The study was approved by local Ethics Committees and complied with the Declaration of Helsinki guidelines. Written informed consent was obtained from all patients.
2.1 Study population and ablation protocol
Enrollment criteria and ablation protocol has been already described [7]. Briefly, we enrolled patients with paroxysmal or persistent atrial fibrillation (AF) who underwent their first AF ablation. Each operator performed AF catheter ablation using its own ablation technique as concerning the ablation catheter (ST or STSF) and the AI setting (380 posterior-500 anterior and 330 posterior-450 anterior). No randomization was required nor was there any deviation from the clinical practice of each center and operator. Therefore, the enrolled population was divided into 4 groups: ST 330–450, ST 380–500, STSF 330–450, and STSF 380–500. For the purpose of this analysis on lesion quality, we divided the study population into two groups according to the AI setting used: group 1 (330 for posterior/inferior segments and 450 for anterior/roof segments) and group 2 (380 for posterior/inferior segments and 500 for anterior/roof segments).
2.2 Study procedures
The ablation procedure has been already described [7]. Briefly, ablation was usually performed under effective oral anticoagulation, and anti-arrhythmic drugs were usually withdrawn before the scheduled procedure. Ablation was carried out under conscious sedation or general anesthesia according to operators’ preference. One or two transseptal accesses to the left atrium were achieved using a standard approach. Then, the LASSO catheter and the ablation catheter (ST or STSF) were placed in the left atrium. Left atrium mapping was performed in sinus rhythm. Patients with AF at the beginning of the index procedure underwent electrical cardioversion. After left atrium reconstruction, the effective PV-left atrium electrical connection was checked by LASSO catheter. RF pulses were delivered using the 3.5-mm Thermocool ST or STSF Catheter in power control mode. RF power was set between 20 and 35 W depending on different left atrial sites and the catheter tip was irrigated by saline at a flow rate of 2 mL/min during mapping and of 8 mL/min (STSF) or 17 mL/min (ST) and 15 mL/min (STSF) or 30 mL/min (ST) for outputs of less than and greater than 30 W, respectively [7]. RF energy was delivered to produce a circumferential ablation around the proximal part of each PV’s ostium or around ipsilateral PVs according to the patient’s anatomy or operator’s preference. The lesion around the PV ostium was created by the sequential point-by-point application of RF energy. Real-time automated display of RF applications (Carto VISITAGTM Module, Biosense-Webster) was used with predefined settings of respiration adjustment, catheter stability (3 mm for 3 s), minimum contact force (3 g over minimum 25% of the time), with the lesion tag display size of 3 (radius of 3 mm), and AI thresholds: 450 for anterior wall and 330 for the posterior wall, for group 1, and 500 for anterior wall and 380 for the posterior wall, for group 2, respectively. In case of dislocation, a new RF application reaching the AI target was applied. Maximal interlesion distance (ILD) between 2 neighboring lesions was ≤ 6 mm [1, 9, 10]. Upon completion of circumferential ablation, a circular mapping LASSO catheter was used to confirm PV isolation (first-pass isolation). In the absence of isolation after completing the circle, LASSO-guided touch-up ablation was delivered until PV isolation was achieved. Resumption of the left atrium to PV conduction was evaluated at 30 min after ablation. The administration of adenosine/isoproterenol was not part of the protocol and was left to operators’ discretion. In case of reconnection, PVs were newly isolated targeting the points of electrical breakthrough.
All patients underwent a post-procedural ECG. Post-procedure echocardiography or other imaging was at the operators’ discretion.
2.3 Follow-up
After ablation, patients underwent a 30 days visit including a detailed history, physical examination, 12-lead standard electrocardiography, and 24-h Holter monitoring. In case of symptomatic arrhythmia recurrence, patients were treated with a repeat procedure or with anti-arrhythmic drugs, based on patients’ choice.
2.4 Repeat procedure
Repeat ablation was performed using the same modalities as the first procedures. After left atrium reconstruction, the effective PV-left atrium electrical connection was checked by LASSO catheter. In case of reconnection, PVs were newly isolated targeting the points of electrical breakthrough. In case the 4 PVs were still isolated, the operator proceeded with the search of non-PV triggers and went for a “box lesion” to isolate the posterior wall.
2.5 Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median and interquartile range according to their distribution. The normality of data distribution was tested with the Shapiro-Wilk test. Categorical variables are expressed as an absolute number with a percentage (%). Comparison among groups for continuous variables was performed by the unpaired Student t test or Mann-Whitney U test. Comparison of categorical variables among groups was performed by a chi-square test. Statistical significance was set at a 2-tailed probability level of < 0.05. All statistical analyses were performed using SPSS software (Version 24.0, IBM, Armonk, NY, US).
3 Results
3.1 Study population
A total of 490 patients were enrolled: 258 patients in group 1 and 232 in group 2. A ST catheter was used in 96/258 (37%) group 1 patients and 81/232 (35%) (p = 0.60) group 2 patients. The clinical characteristics of the study population are summarized in Table 1. Group 1 patients had a higher mean body mass index and a lower left ventricle ejection fraction. The incidence of hypertension was higher in group 2, whereas the incidence of dilated cardiomyopathy was higher in group 1.
3.2 Procedural data
Table 2 summarized the main procedural data. There was no statistically significant difference in the mean procedural time, fluoroscopy time, contact force, and impedance drop between the two study groups. Also, the rate of first-pass PV isolation was similar. A LASSO-guided touch-up ablation was required to isolate 193/1988 (9.7%) PVs (Fig. 1), with no significant differences between left or right, superior or inferior veins, nor between anterior and posterior segments. Table 3 summarized the main procedural data of PVs isolated after the first-pass isolation compared with those of PV that required a touch-up ablation. No difference in mean contact force was observed, whereas a touch-up ablation was required to isolate PVs in which lower mean power and impedance drop and longer mean RF pulse duration were used.
3.3 Thirty-minute PV reconnection
Resumption of the left atrium to PV conduction 30 min after ablation was observed in 5.6% PVs (Fig. 2), with no significant differences between left or right, superior or inferior veins, nor between anterior and posterior segments. The rate of 30-min PV reconnection was slightly higher in group 2 (64/943, 6.8%, PVs) as compared to group 1 (48/1045, 4.6% PVs, p = 0.04). Table 4 summarized the main procedural data of PVs that showed a 30-min reconnection compared with those of PVs that did not. No difference in mean contact force, impedance drop, mean power, and mean RF pulse duration was observed.
3.4 Repeat procedure and late PV reconnection
Thirty patients (6%) underwent repeat procedure for symptomatic arrhythmia recurrence (Table 5): in 9/30 (30%) (5 in group 1 and 4 in group 2), a durable PVI was observed, whereas in the others with PVR (n = 21), the mean number of reconnected veins was 1.9 ± 1.6. Overall, 57/116 (49%) PVs were reconnected and reconnections were more likely in the right veins and in the anterior segments (Fig. 3). No difference was found in the rate of reconnection between groups 1 and 2 (72% vs 68%; p = 0.6). Compared to patients with PVR, patients with 4 isolated veins had similar first procedural and RF characteristics (RF time, force time integral, power, contact force, or impedance drop) (Table 6). The time to arrhythmia recurrence was longer in patients with durable PVI compared to those with reconnections (4.8 ± 4.7 months vs 10.2 ± 6.6 months, p = 0.05) (Fig. 4). Furthermore, the AI setting did not influence the time to recurrence in patients with PVR (4.6 ± 4.8 months vs 5.1 ± 4.8, p = 0.8; in group 1 and group 2 respectively).
3.5 Complications
A complication (4 pericardial effusions, 2 transient phrenic nerve palsy, 1 cardiac tamponade, 1 pneumonia) was observed in 8 (1.6%) patients without any difference among the two study groups (1.5% vs 1.7%, p = 0.88).
4 Discussion
4.1 Main findings
This study, evaluating the impact of two AI settings on the rate of first-pass PV isolation and acute and late PV reconnection, shows that lower AI values were as safe and effective as higher ones. With both AI settings, a high rate of first-pass PV isolation and a low rate of acute PV reconnection were achieved irrespective of patient and operator characteristics. The probability of finding 4 isolated veins at the repeat procedure was 30%. No predictors of acute and late PVR were found.
4.2 AI setting in acute PV isolation
The AI is a novel marker of RF application quality that incorporates stability, contact force, time, and power in a weighted formula, and has shown to accurately estimate lesion depth and diameter [11]. Not well defined are the best AI values that allow effective, safe, and durable PV isolation. Das et al. [8] studied the relationship between the AI and PVR at repeat electrophysiology study. From receiver operating characteristic (ROC) curve analysis, optimal cutoff points (Youden index) were calculated. For AI, the optimal cutoff for anterior/roof segments was 376 (sensitivity 63.6%, specificity 77.8%, and positive predictive value 97.2%) and for posterior/inferior segments was 340 (sensitivity 52.9%, specificity 94.3%, and positive predictive value 98.2%). No late reconnection was seen in anterior/roof segments where the minimum AI value was ≥ 480 or in posterior/inferior segments where the minimum AI value was ≥ 370. El Haddad et al. [12] studied acute and late PV reconnection. By ROC curve analysis, they found the highest (90%) specificity to predict durable PV isolation with an AI > 550 on the anterior wall and > 417 on the posterior wall. Based on an initial survey within the 25 centers involved in our registry, the two most used AI settings (330 posterior/450 anterior and 380 posterior/500 anterior) were compared regarding the rate of first-pass PV isolation, rate of acute and late PV reconnection, and safety. No differences were observed between the two AI settings. Our findings that a higher AI setting is not acutely superior than a lower one might be justified by Ullah et al. [11] series. They showed that ablation beyond 430 AI provides minimal additional biophysical efficacy, suggesting an upper limit to use for clinical ablation. Recently, Lee et al. [13] evaluated the optimal AI threshold for avoiding acute PV reconnection. AI values of ≥ 450 at the anterior/roof segments and of ≥ 350 at the posterior/inferior/carina segments were identified as the optimal AI thresholds for avoiding acute PV reconnection, confirming present results.
4.3 First-pass PV isolation
The most common technique for PVI is creating circular RF lesions in a point-by-point manner around the PV ostia [14]. The introduction of the AI to guide the RF delivery greatly improved the rate of first-pass isolation from about 50% [15, 16] to above 90% [1,2,3]. In particular, in a previous analysis of the multicenter AIR Registry, we observed that PVI guided by AI allows every operator to achieve a rate of first-pass PV isolation ≥ 84%, thus concluding that the AI improves the “operator variable” by generalizing and standardizing physicians’ skills and experience applied to the AF ablation procedure. Few data are available on the site of persistent atrium-PV connection that requires a touch-up ablation until PV isolation is achieved. In Hussein et al. series [2], first-pass PV isolation with wide area circumferential ablation (WACA) circle was achieved in the AI group in 173/178 (97%) circles, of the 5 (3%) WACA circles in which first-pass isolation failed to occur, 4 (80%) were related to the right ipsilateral PVs and required further RF delivery. In the Dhillon et al. series [4], first-pass isolation occurred in 82% WACAs, with first-pass isolation occurring in both left and right WACAs in 68% (34/50) of patients. Further ablation was required on the inter-venous ridge between the PVs in order to isolate them in 16% (16/100) WACAs. In our series, we observed that a touch-up ablation was required to isolate 193/1988 (9.7%) PVs, with no significant differences between left or right, superior or inferior veins, nor between anterior and posterior segments. No difference in mean contact force was observed, whereas a touch-up ablation was required to isolate PVs in which lower mean power and impedance drop and longer mean radiofrequency pulse duration were used. This finding suggests a higher impact of RF biophysics over anatomical characteristics in achieving first-pass PV isolation.
4.4 Acute PV reconnection
More data are available on the sites of acute PV reconnection. In the Hussein et al. series [2], either spontaneous or adenosine-induced PV reconnection was identified in 11 (6%) circles in the AI group (3 on the left and 8 on the right). In the Das et al. [8] series, acute reconnection was seen after the minimum 20-min waiting period in 28 (6%) segments (14 spontaneous and 14 adenosine induced) in 20 (50%) patients, affecting 21 (26%) WACA circles. In El Haddad et al. series [12], acute PV reconnection occurred in 11/48 (23%) patients, 14/96 (15%) circles, and 25/980 (2.6%) PV segments. In the Lee et al. series [11], in the AI-guided PV isolation group, 4.2% of PV segments showed acute PV reconnection with a mean of 0.6 ± 0.9 segments per patient. Similarly, in our series, resumption of the left atrium to PV conduction 30 min after ablation was observed in 5.6% PVs, with no significant differences between left or right, superior or inferior veins, nor between anterior and posterior segments. No predictors of acute PV reconnection were found.
4.5 Late PV reconnection
Prior studies investigated the durability of PVI after a CF-guided ablation protocol aiming to enclose the veins with stable, contiguous, and optimized RF lesions. De Pooter et al. [17] reported a likelihood of finding 4 isolated veins at repeat ablation of 62% and no clinical and procedural differences between patients with or without PV reconnections and also, time to AF recurrence from the first PVI was similar. However, this was a single-center study, and all the PVI procedures were performed by highly skilled operators. Recently, Duytschaever et al. [6] reported the data of the multicenter prospective VISTAX study and described a 41.2% rate of durable PVI that reflects some variability across different sites. In our series of patients, we found a durable PVI at 30%. The inclusion of persistent AF patients (37%) in our case series may partly explain this lower rate compared to the VISTAX study; however, there are other possible reasons. First, we cannot exclude the resumption of dormant PV conduction [18], as the adenosine/isoproterenol was not given in all patients at the end of the first procedure. Nonetheless, a recent meta-analysis by Chen [19] demonstrated that the adenosine test does not reduce the rate of AF recurrence. Second, the persistence of PV potential at the carina that reflects the connections between ipsilateral veins may be the cause of PV reconnection [20]. In our study in patients requiring touch-up ablations, the choice to perform ablation at the carina was left to each operator. However, the data about the efficacy of this approach are still controversial, as the study by Higashiya demonstrated no clear benefit [21].
As for the predictors of durable PVI, similarly to De Pooter et al., we did not find any distinct clinical nor procedural characteristics in patients with 4 isolated veins at repeat ablation.
4.6 Limitations
First, this is a non-randomized study. No deviation from the clinical practice of each center and operator was required. This might justify the clinical and procedural differences in the two study groups. Nevertheless, the high and comparable rate of first-pass PV isolation that we observed in the two study groups, with different tools and operators, is a strong point of our study. Second, the target ILD during the ablation procedure was ≤ 6 mm [1, 6], because the study was conducted before the publication of the randomized trial by Hoffmann et al. [22] that demonstrated that the target ILD should be 3–4 mm. Third, after the circumferential ablation after a waiting period of 30 min, the decision whether to administrate adenosine/isoproterenol was left to each operator; thus, we cannot exclude that the cause of the PV reconnection was the resumption of dormant conduction.
5 Conclusions
In conclusion, lower AI (330–450) values were as safe and effective as higher ones (380–500) in obtaining acute PV isolation in patients with paroxysmal/persistent AF who underwent first catheter ablation. With both AI settings, a high rate of first-pass PV isolation and a low rate of acute and late PV reconnection were achieved irrespective of patient and operator characteristics. No predictors of acute and late PV reconnection were found.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Taghij P, El Haddad M, Philips T, Wolf M, Knecht S, Vandekerckhove Y, et al. Evaluation of a strategy aiming to enclose the pulmonary vein with contiguous and optimized radiofrequency lesion in paroxysmal atrial fibrillation. JACC Clin Electrophysiol. 2018;4:99–108.
Hussein A, Das M, Chaturvedi V, Asfour IK, Daryanani N, Morgan M, et al. Prospective use of ablation index targets improves clinical outcomes following ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:1037–47.
Solimene F, Schillaci V, Shopova G, Urraro F, Arestia A, Iuliano A, et al. Safety and efficacy of atrial fibrillation ablation guided by ablation index module. J Interv Card Electrophysiol. 2019;54:9–15.
Dhillon G, Ahsan S, Honarbakhsh S, Lim W, Baca M, Graham A, et al. A multicentered evaluation of ablation at higher power guided by ablation index: establishing ablation targets for pulmonary vein isolation. J Cardiovasc Electrophysiol. 2019;30:357–65.
Stabile G, Lepillier A, De Ruvo E, Scaglione M, Anselmino M, Sebag F, et al. Reproducibility of pulmonary vein isolation guided by the ablation index: 1-year outcome of the AIR registry. J Cardiovasc Electrophysiol. 2020;31:1694–701.
Duytschaever M, Vijgen J, De Potter T, Scherr D, Van Herendael H, Knecht S, et al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: the multicentre VISTAX trial. Europace. 2020;euaa157.
Solimene F, Lepillier A, De Ruvo E, Scaglione M, Anselmino M, Sebag FA, et al. Reproducibility of acute pulmonary vein isolation guided by the ablation index. Pacing Clin Electrophysiol. 2019;42:874–81. https://doi.org/10.1111/pace.13710.
Das M, Loveday JJ, Wynn GJ, Gomes S, Saeed Y, Bonnett LJ, et al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace. 2017;19:775–83.
Stabile G, Di Donna P, Schillaci V, Di Monaco A, Iuliano A, Caponi D, et al. Safety and efficacy of pulmonary vein isolation using a surround flow catheter with contact force measurement capabilities: a multicenter registry. J Cardiovasc Electrophysiol. 2017;28:762–7.
Park CI, Lehrmann H, Keyl C, Schiebeling J, Allgeier J, Schurr P, et al. Mechanisms of Pulmonary Vein Reconnection After Radiofrequency Ablation of Atrial Fibrillation: The Deterministic Role of Contact Force and Interlesion Distance. Journal of Cardiovascular Electrophysiology. 2014;25(7):701–8.
Ullah W, Hunter RJ, Finlay MC, McLean A, Dhinoja MB, Sporton S, et al. Ablation index and surround flow catheter irrigation impedance-based appraisal in clinical ablation. J Am Coll Cardiol EP. 2017;3:1080–8.
El Haddad M, Taghji P, Phlips T, Wolf M, Demolder A, Choudhury R, et al. Determinants of acute and late pulmonary vein reconnection in contact force-guided pulmonary vein isolation: identifying the weakest link in the ablation chain. Circ Arrhythm Electrophysiol. 2017;10(4):e004867. https://doi.org/10.1161/CIRCEP.116.004867.
Lee SR, Choi EK, Lee EJ, Choe WS, Cha MJ, Oh S. Efficacy of the optimal ablation index-targeted strategy for pulmonary vein isolation in patients with atrial fibrillation: the OPTIMUM study results. J Interv Card Electrophysiol. 2019;55:171–81. https://doi.org/10.1007/s10840-019-00565-4.
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. https://doi.org/10.1016/j.hrthm.2017.05.012.
Hocini M, Sanders P, Jaïs P, Weerasoriya R, Scavée C, Takahashi Y, et al. Prevalence of pulmonary vein disconnection after anatomical ablation for atrial fibrillation: consequences of wide atrial encircling of the pulmonary veins. Eur Heart J. 2005;26:696–704.
Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Y, et al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace. 2018;20(FI_3):f419–27. https://doi.org/10.1093/europace/eux376.
De Pooter J, Strisciuglio T, El Haddad M, Wolf M, Phlips T, Vandekerckhove Y, et al. Pulmonary vein reconnection no longer occurs in the majority of patients after a single pulmonary vein isolation procedure. JACC Clin Electrophysiol. 2019;5(3):295–305.
Arentz T, Macle L, Kalusche D, Hocini M, Jais P, Shah D, et al. Dormant pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004;15:1041–7.
Chen C, Li D, Ho J, Liu T, Li X, Wang Z, et al. Clinical implications of unmasking dormant conduction after circumferential pulmonary vein isolation in atrial fibrillation using adenosine: a systematic review and meta-analysis. Front. Physiol. 2019;9:1861.
Rajappan K, Kistler PM, Earley MJ, Thomas G, Izquierdo M, Sporton SC, et al. Acute and chronic pulmonary vein reconnection after atrial fibrillation ablation: a prospective characterization of anatomical sites. Pacing Clin Electrophysiol. 2008;31:1598–605.
Higashiya S, Yamaji H, Murakami T, Hina K, Kawamura H, Murakami M, et al. Adjunctive interpulmonary isthmus ablation has no added effects on atrial fibrillation recurrence. Open Heart. 2017;4:e000593.
Hoffmann P, Ramirez ID, Baldenhofer G, Stangl K, Mont L, Althoff TF. Randomized study defining the optimum target interlesion distance in ablation index-guided atrial fibrillation ablation. Europace. 2020;22:1480–6.
Author information
Authors and Affiliations
Contributions
Concept/design: Solimene F., Lepillier A., Anselmino M., Bertaglia E., Stabile G.
Data analysis/interpretation: Solimene F., Lepillier A., De Ruvo E., Scaglione M., Anselmino M., Sebag F.A., Pecora D., Gallagher M.M., Rillo M., Viola G., Pisanò E., Abbey S., Lamberti F., Pani A., Zucchelli G., Sgarito G., De Simone A., Bertaglia E., Strisciuglio T., Stabile G.
Drafting article: Solimene F., Lepillier A., Anselmino M., Gallagher M.M., Rillo M., Viola G., De Santis V., Landolina M., De Simone A., Bertaglia E., Strisciuglio T., Stabile G.
Critical revision of article: Solimene F., Lepillier A., De Ruvo E., Scaglione M., Anselmino M., Sebag F.A., Pecora D., Gallagher M.M., Rillo M., Viola G., Pisanò E., Abbey S., Lamberti F., Pani A., Zucchelli G., Sgarito G., De Simone A., Bertaglia E., Strisciuglio T., Stabile G.
Approval of article: Solimene F., Lepillier A., De Ruvo E., Scaglione M., Anselmino M., Sebag F.A., Pecora D., Gallagher M.M., Rillo M., Viola G., Pisanò E., Abbey S., Lamberti F., Pani A., Zucchelli G., Sgarito G., De Simone A., Bertaglia E., Strisciuglio T., Stabile G.
Statistics: Bertaglia E., Strisciuglio T., Stabile G.
Data collection: Solimene F., Lepillier A., De Ruvo E., Scaglione M., Anselmino M., Sebag F.A., Pecora D., Gallagher M.M., Rillo M., Viola G., Pisanò E., Abbey S., Lamberti F., Pani A., Zucchelli G., Sgarito G., De Simone A., Bertaglia E., Strisciuglio T., Stabile G.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interest.
Ethics approval and consent to participate
As this is a multicenter study, the ethical committee of each center approved the study. Before inclusion in the study, every patient signed the informed consent.
Consent for publication
All authors consent to the publication of the manuscript in JICE.
Code availability
NA.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lepillier, A., Strisciuglio, T., De Ruvo, E. et al. Impact of ablation index settings on pulmonary vein reconnection. J Interv Card Electrophysiol 63, 133–142 (2022). https://doi.org/10.1007/s10840-021-00944-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-021-00944-w