Abstract
Purpose
The use of transvenous implantable cardioverter defibrillators (TV-ICDs) is associated with multiple risks related to the presence of the defibrillator leads within the venous system and right side of the heart, including endocarditis, venous occlusion, tricuspid regurgitation, and potential lead failure. The emergence of subcutaneous ICDs (S-ICDs) may potentially overcome the aforementioned disadvantages. However, evidence validating the safety of S-ICDs relative to TV-ICDs is limited. The present study aimed to synthesize and analyze available data from published studies to comprehensively compare transvenous and subcutaneous ICDs.
Methods
Different databases were searched for full-text publications with a direct comparison of TV- and S-ICDs. Fixed effect models were applied to pooled data, and no study-to-study heterogeneity was detected.
Results
Data from 7 studies totaling 1666 patients were pooled together. Compared to S-ICDs, the risk of suffering device-related complications was higher in patients with TV-ICDs (OR = 1.71; 95% CI: 1.23–2.38). The number of patients with an S-ICD who suffered inappropriate shocks (IS) was not significantly different than patients with a TV-ICD (OR = 0.92; 95% CI: 0.65–1.30). Subgroup analysis indicated that the TV-ICD group had a higher risk of IS due to supraventricular oversensing (OR = 3.29; 95% CI: 1.92–5.63) while T-wave oversensing tending to cause IS in the S-ICD group (OR = 0.09; 95% CI: 0.03–0.23). The risk of device-related infection in the S-ICD group was not any lower than that in the TV-ICD group (OR = 1.57; 95% CI: 0.67–3.68). The survival rate without any complications during a 1-year follow-up period was similar between the 2 groups (HR = 1.23; 95% CI: 0.81–1.86), although it was assumed that the trend leaned toward more complications in patients with a TV-ICD.
Conclusion
The present study verified the safety of S-ICDs based on pooled data. Although there were no differences between TV- and S-ICDs in the short term, fewer adverse events were found in patients with S-ICDs during long-term follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
An implantable automatic defibrillator can help to recognize and treat potentially fatal ventricular arrhythmias timely and efficiently [1]. Dating back to the 1980s, however, implantable cardioverter defibrillators (ICDs) were far different than what is currently used, particularly in that a thoracotomy is no longer necessary to place the defibrillation lead in the epicardium. Due to potential postoperative complications, operative morbidity, and mortality associated with the implantation, ICD use was limited [2].
With technological advances, a novel ICD system was developed, with transvenous defibrillation leads and a generator implanted in a subpectoral pocket, which gradually replaced the previous model, and became the currently widely used transvenous ICD (TV-ICD). However, intravenous and intracardiac implants can also cause other problems, such as cardiac perforation, tricuspid regurgitation, venous stenosis, thrombophlebitis, and endocarditis. Most of these complications are related to the transvenous defibrillation leads [3,4,5]. Additionally, some patients may not be ideal candidates for TV-ICDs due to limited vascular access (internal arteriovenous hemodialysis fistula, persistent left with absent right superior vena cava, and peripheral venous embolism), congenital cardiovascular malformation, or repeated occurrence of serious cardiac device-related infections [6,7,8,9]. Transvenous leads with abnormalities may need to be replaced, but the extraction of these leads may cause additional adverse events [9].
ICD-related risks are dependent not only on the experience of the surgeon but also on previously existing comorbidities such as diabetes, diseases requiring steroid therapy, or the presence of infection prior to device implantation [1]. Recently, subcutaneous ICDs (S-ICDs) have become a suitable alternative for patients without pacing needs. Due to the subcutaneous placement of the defibrillation leads, the risks of vascular injury, intravenous and intracardiac infection, lead extraction, and excessive radiation during fluoroscopy (especially when a lead extraction is required) may be significantly reduced compared to those for TV-ICDs [10]. Nonetheless, it remains uncertain whether S-ICDs might lead to inappropriate shocks (IS), and safety is not guaranteed without regular pacing and anti-tachycardia pacing (ATP) modes [11]. Additionally, there have been concerns about whether S-ICDs are different from TV-ICD in regard to the rate of complications.
To date, there have been only a few clinical studies on S-ICDs, and they were all small-scale designs, except for the EFFORTLESS S-ICD study by Boston Scientific, which included > 1000 patients [12]. Therefore, we felt that a comprehensive investigation to integrate the data of these existing studies would provide a profound understanding of S-ICDs. Auricchio et al. [13] summarized data from studies on TV- and S-ICDs, and the differential rate of IS between the two was analyzed by meta-regression. However, no meta-analysis has directly compared the efficacy and safety of conventional TV-ICDs and the more recently developed S-ICDs. As such, the present study aimed to synthesize and analyze the results from clinical studies with a direct comparison of TV- and S-ICDs, to comprehensively evaluate the advantages and disadvantages of S-ICDs.
2 Methods
2.1 Search strategy
The present study design was stringently conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14]. A total of five databases, including PubMed, Ovid, EBSCO, Web of Science, and the Cochrane Library, were searched for keywords such as “transvenous,” “subcutaneous,” and “implantable cardioverter defibrillator” to retrieve pertinent literature published prior to July 2020.
2.2 Study selection criteria and data extraction
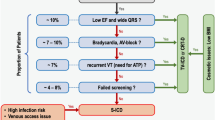
All studies were retrieved and data were collected independently by two investigators who were not informed of the protocol for the present study and who verified the quality and eligibility of the literature found. The included literature met the following criteria: English language; direct comparison between TV- and S-ICDs; full-text as opposed to abstract only; and clear definition of ICD-related complications, for example, IS or device infection requiring intervention. The causes of IS were generally divided into T-wave oversensing and supraventricular oversensing (atrial fibrillation, and atrial, sinus, or supraventricular tachycardia). Exclusion criteria included studies on TV- or S-ICDs alone; case reports, case series, and review articles; and inhospital studies, or studies with follow-up ≤ 6 months. In cases of different publications of the same study, the one with the complete data was chosen.
Important statistics such as the number of total patients and the number of patients with clearly defined events were carefully collected. Basic demographic data and follow-up duration were recorded as well. The Newcastle-Ottawa Scale was used to assess the quality of the included studies (Supplementary Table).
2.3 Data synthesis and analysis
Fixed effects models were used for data integration and to compare TV- and S-ICDs in regard to the difference in total complications, device infection requiring intervention, and IS. The results were presented as odds ratio (OR) and illustrated as forest plots. Additionally, publication bias was assessed using Begg’s adjusted rank correlation test and was shown as a funnel plot. Survival curve data from the included publications were extracted as previously described [15]. Then, these data were further processed using the previously described method to calculate the integral hazard ratio (HR) and depict the free-event survival curve [16].
3 Statistics
Statistical heterogeneity was assessed using inverse variance (I-V) statistics. Statistical analyses were performed using Stata 12.0 software (Stata Corp, College Station, TX, USA). Survival data were extracted using Engauge Digitizer 4.1 software. HR and Kaplan-Meier curves were obtained using GraphPad Prism 5 software. As a limited number of pieces of literature were included, sensitivity analysis was not necessary. Heterogeneity was calculated, and the included studies were considered to have low heterogeneity if I2 < 50% and p value > 0.05; therefore, the fixed effect model was used. The results of the heterogeneity analysis are shown in the forest plot. All p values were two-tailed, and the statistical significance was set at 0.05.
4 Results
4.1 Literature search and general description of included studies
After excluding 156 duplicates from 3513 articles to be searched, 3343 articles were also excluded for not meeting the inclusion/exclusion criteria. A total of seven articles met the eligibility requirements [17,18,19,20,21,22,23]. The flow diagram of the publication filtration is shown in Fig. 1. Data from 1666 patients with follow-up durations ranging from 6 to 48 months were pooled together, and the characteristics of the included studies can be found in Table 1. At baseline, demographic characteristics showed no significant difference between the TV- and S-ICD groups (Table 2).
4.2 Comparison of device-related complication
During the follow-up period, total implant-related complications were reported in five articles, without heterogeneity (I2 = 0). These complications included IS, pocket erosion, defibrillation threshold failure, lead failure, and device infection requiring intervention [17, 18, 20, 21, 23]. Compared to patients with S-ICDs (n = 795), the risk of suffering from total device-related complications was higher in patients with TV-ICDs (n = 782) in a fixed effect model (OR = 1.71; 95% CI: 1.23–2.38), indicating a predominance of S-ICD. Although three studies had negative results, a significant difference was obtained after pooling the data together (Fig. 2) [18, 20, 21].
Of the included studies, six articles described a number of patients with a device-related infection requiring intervention [17, 18, 20, 22, 23]. Contrary to the conventional perspective, the risk of device-related infection in the S-ICD group (n = 826) was comparable to that of the TV-ICD group (n = 826), without a significant difference (OR = 1.73; 95% CI: 0.86–3.51) from a fixed effect model (Fig. 3a). Since only one article mentioned the occurrence of infection prior to ICD placement, a risk-stratified analysis could not be carried out [18].
Patients with IS were reported by seven articles, and the proportion of patients with IS in the S-ICD group (n = 831) was similar to that in the TV-ICD group (n = 835) [17,18,19,20,21,22,23]. There was no significant difference between the two groups (OR = 0.92; 95% CI: 0.65–1.30), indicating an equal risk of IS (Fig. 3b). Of the included articles, five reported that the IS had two primary causes: T-wave oversensing and supraventricular oversensing (i.e., atrial fibrillation and atrial, sinus, or supraventricular tachycardia) [17, 18, 20, 21, 23]. Therefore, a subgroup analysis of IS was further performed to assess the distribution of causes of IS between the two groups (Fig. 4). Surprisingly, the subgroup comparison indicated that TV-ICDs had a higher risk of IS due to supraventricular oversensing (OR = 3.29; 95% CI: 1.92–5.63), while T-wave oversensing more frequently caused IS in the S-ICD group (OR = 0.09; 95% CI: 0.03–0.23).
4.3 Analysis of short-term and long-term survival with freedom from total complications
Of the four articles that presented an event-free survival curve, only one had a follow-up duration of less than 1 year [17,18,19, 21]. Therefore, survival data with a follow-up duration longer than 1 year were collected for long-term survival analysis, and the studies with a follow-up of less than a year were used to synthesize the short-term survival curve (Supplementary Fig. 1a). The complication-free survival rate for patients with an S-ICD was similar to that of those with a TV-ICD over a year-long follow-up period (HR = 1.23; 95% CI: 0.81–1.86). However, the difference between TV- and S-ICDs emerged with a long-term follow-up. The S-ICD curve entered the plateau stage at approximately 40 months postimplantation, while the TV-ICD curve showed a continuous downward trend in general (Supplementary Fig. 1b). This revealed that the probability of total complications in patient with an S-ICD was evidently less than that in patients with TV-ICDs over time (HR = 2.13; 95% CI: 1.36–3.32).
4.4 Publication bias analysis
Begg’s test was used to analyze publication bias and showed a symmetrical distribution of the included publications (p = 0.462) in a funnel plot (Supplementary Fig. 2), indicating that publication bias did not exist among the articles included in the present study.
5 Discussion
The objective of the present study was to systematically assess the complications of S-ICDs compared to TV-ICDs. Our analysis included seven independent studies, completed between 2013 and 2020. To our knowledge, this is the first meta-analysis to compare complication and event-free survival rates between patients with TV- and S-ICDs. Data from our analysis indicated that the rates of IS and device infection requiring intervention were similar between patients with TV- and S-ICDs. Subgroup analysis revealed that the primary causes of IS were supraventricular and T-wave oversensing for TV- and S-ICDs, respectively. S-ICDs were thought to present a lower risk of lead-related infection; however, without enough studies reporting corresponding data, subgroup analysis or risk stratification could not be performed. Survival analysis indicated that patients with S-ICDs had a lower risk of adverse events over a long-term follow-up when compared to patients with TV-ICDs. Contrarily, the event-free survival rate was similar between the two groups when compared over a relatively short follow-up period.
IS can cause uncomfortable feelings, although it is not life-threatening. The rate of IS was shown to gradually decrease year after year [13], and it is thought that it can even be eliminated by optimizing programming [24]. However, some recent studies found no difference in IS between patients with TV- and S-ICDs, and existing evidence suggests that patients with S-ICDs had an IS rate equal to that of patients with TV-ICDs. Additionally, there is a well-acknowledged misconception that the main advantage of S-ICDs is a lower risk of infection [1]. However, the present study showed no significant difference between TV- and S-ICDs in regard to infection. This might be a result of bias in data from different studies. Of the included publications, only one article reported the occurrence of infection prior to ICD implantation. Previously existing infection was a risk factor for device-related infection for either de novo or reimplantation of ICDs [25]. Additionally, patients with comorbidities such as diabetes and chronic kidney disease were also susceptible to infection [26], confounding factors which should be carefully considered in the study design and data analysis. TV-ICDs are related to lead adhesion and venous stenosis due to fibrosis and thrombosis induced by the intravenous implants [27, 28]. A previously published study in animals showed that the defibrillation lead of TV-ICDs was significantly related to severe venous fibrosis, thrombosis, and stenosis [29]. S-ICDs can overcome this disadvantage without degrading the efficacy of defibrillation [30]. However, S-ICDs do not offer a pacing function; therefore, they are not able to be used for anti-tachycardia pacing treatment or in potentially pacemaker-dependent patients.
The first S-ICD implantation occurred in 2008, initiating subsequent clinical studies of S-ICDs. The IDE study, including 330 patients, was aimed at evaluating the efficacy and safety of S-ICDs [30]. The EFFORTLESS S-ICD study, including approximately 1000 patients, was carried out to assess the long-term complications of S-ICDs [12]. Despite the previously mentioned studies having prospective and multicenter designs, they also had drawbacks, because there was no direct comparison of TV- and S-ICDs. Additionally, the MADIT S-ICD study is an ongoing study designed to verify the hypothesis that post-myocardial infarction patients with the comorbidity of diabetes and a relatively preserved ejection fraction have a survival benefit from S-ICD implantation [31]. Exclusion of pacing dependence should be a prerequisite of S-ICD implantation, or as an alternate, and a backup pacing lead should be placed. As such, adverse consequences (death, syncope, hospital admission, and subsequent implantation of pacemaker) caused by a lack of anti-tachycardia and backup pacing function in S-ICDs could not be evaluated.
The PRAETORIAN study, with a direct comparison of TV- and S-ICDs, was designed to further investigate the efficacy and safety of S-ICDs [32]. Recently, results of the PRAETORIAN investigation have been published, which update our ideas. In the PRAETORIAN study, appropriate ICD therapy (including anti-tachycardia pacing), death from any cause, major adverse cardiac events, hospitalization for heart failure, and crossover between the assigned devices as secondary end points were all reported. This well-designed, randomized control study suggested a very low incidence of bradycardia, which requires pacemaker intervention in the S-ICD group. With a large sample size (876 patients), the PRAETORIAN study results indicated that only 5 of the 426 patients in the S-ICD group underwent subsequent implantation of a transvenous pacing lead for the treatment of bradycardia [23]. Therefore, the risk of requiring a pacemaker should be acceptable, if a careful evaluation of the indications and contraindications for S-ICD was performed before the procedure.
As far as we are aware, the present review was the first meta-analysis to systematically and directly compare TV- and S-ICDs regarding device-related complications. In the present meta-analysis, S-ICDs were proven to have fewer complications than TV-ICDs. However, results from the present study should be updated if more prospective, large sample size, randomized, and multicenter clinical trials are published. Finally, cumulative experimentation for S-ICD implantation is highly needed in the future.
6 Limitations
The present meta-analysis included seven studies, all of which were designed as case controls. As some of the included articles had deficits, such as no randomization, a retrospective design, or a small scale, the present meta-analysis had these deficits as well. Furthermore, only a few articles were eligible based on the selection criteria; thus, sensitivity analysis was not possible. Only one article mentioned the occurrence of infection prior to ICD implantation. Additionally, patients who received steroids or had comorbidities such as diabetes and chronic kidney disease were also susceptible to infection, and these confounding factors were not presented in the included studies. Therefore, risk-stratified analysis could not be performed. Consequently, large-scale, prospective, multicenter, and randomized clinical trials are still needed to clearly explore confounding factors.
7 Conclusion
The present study quantitatively and comprehensively analyzed the differences in complications between TV- and S-ICDs via meta-analysis. Compared to TV-ICDs, the risk of suffering from total device-related complications was lower in patients with S-ICDs. The proportion of patients who experienced IS in the S-ICD group was similar to that of the TV-ICD group. Subgroup analysis indicated that patients with TV-ICDs had a higher risk of IS due to supraventricular oversensing, while T-wave oversensing was the primary cause of IS in S-ICD patients. The risk of device-related infection in the S-ICD group was no lower than that for the TV-ICD group. The complication-free survival rate was similar between the TV- and S-ICD groups over a 1-year follow-up period. The probability of complication occurrence was lower in the S-ICD group. As a result, S-ICDs could be a viable alternate to TV-ICDs, chosen to decrease the long-term risk of device-related complications.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Khatib SM, Friedman P, Ellenbogen KA. Defibrillators: selecting the right device for the right patient. Circulation. 2016;134(18):1390–404. https://doi.org/10.1161/circulationaha.116.021889.
Reddy RK, Bardy GH. Unipolar pectoral defibrillation systems. Pacing Clin Electrophysiol. 1997;20(2 Pt 2):600–6. https://doi.org/10.1111/j.1540-8159.1997.tb06213.x.
Sunderland N, Kaura A, Murgatroyd F, Dhillon P, Scott PA. Outcomes with single-coil versus dual-coil implantable cardioverter defibrillators: a meta-analysis. Europace. 2018;20(3):e21–e9. https://doi.org/10.1093/europace/euw438.
Chang JD, Manning WJ, Ebrille E, Zimetbaum PJ. Tricuspid valve dysfunction following pacemaker or cardioverter-defibrillator implantation. J Am Coll Cardiol. 2017;69(18):2331–41. https://doi.org/10.1016/j.jacc.2017.02.055.
Akbarzadeh MA, Mollazadeh R, Sefidbakht S, Shahrzad S, Bahrololoumi Bafruee N. Identification and management of right ventricular perforation using pacemaker and cardioverter-defibrillator leads: a case series and mini review. J Arrhythm. 2017;33(1):1–5. https://doi.org/10.1016/j.joa.2016.05.005.
Alonso P, Osca J, Rueda J, Cano O, Pimenta P, Andres A, et al. Conventional and right-sided screening for subcutaneous ICD in a population with congenital heart disease at high risk of sudden cardiac death. Ann Noninvasive Electrocardiol. 2017;22(6). https://doi.org/10.1111/anec.12461.
Bordachar P, Marquié C, Pospiech T, Pasquié JL, Jalal Z, Haissaguerre M, et al. Subcutaneous implantable cardioverter defibrillators in children, young adults and patients with congenital heart disease. Int J Cardiol. 2016;203:251–8. https://doi.org/10.1016/j.ijcard.2015.09.083.
Vachharajani TJ, Salman L, Costanzo EJ, Mehandru SK, Patel M, Calderon DM, et al. Subcutaneous defibrillators for dialysis patients. Hemodial Int. 2018;22(1):4–8. https://doi.org/10.1111/hdi.12577.
Wazni O, Wilkoff BL. Considerations for cardiac device lead extraction. Nat Rev Cardiol. 2016;13(4):221–9. https://doi.org/10.1038/nrcardio.2015.207.
Lewis GF, Gold MR. Safety and efficacy of the subcutaneous implantable defibrillator. J Am Coll Cardiol. 2016;67(4):445–54. https://doi.org/10.1016/j.jacc.2015.11.026.
Lewis GF, Gold MR. Clinical experience with subcutaneous implantable cardioverter-defibrillators. Nat Rev Cardiol. 2015;12(7):398–405. https://doi.org/10.1038/nrcardio.2015.56.
Burke MC, Gold MR, Knight BP, Barr CS, Theuns D, Boersma LVA, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol. 2015;65(16):1605–15. https://doi.org/10.1016/j.jacc.2015.02.047.
Auricchio A, Hudnall JH, Schloss EJ, Sterns LD, Kurita T, Meijer A, et al. Inappropriate shocks in single-chamber and subcutaneous implantable cardioverter-defibrillators: a systematic review and meta-analysis. Europace. 2017;19(12):1973–80. https://doi.org/10.1093/europace/euw415.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. https://doi.org/10.1136/bmj.g7647.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. https://doi.org/10.1186/1745-6215-8-16.
Wu XL, Tu Q, Faure G, Gallet P, Kohler C, de Carvalho Bittencourt M. Diagnostic and prognostic value of circulating tumor cells in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Sci Rep. 2016;6:20210. https://doi.org/10.1038/srep20210.
Honarbakhsh S, Providencia R, Srinivasan N, Ahsan S, Lowe M, Rowland E, et al. A propensity matched case-control study comparing efficacy, safety and costs of the subcutaneous vs. transvenous implantable cardioverter defibrillator. Int J Cardiol. 2017;228:280–5. https://doi.org/10.1016/j.ijcard.2016.11.017.
Mithani AA, Kath H, Hunter K, Andriulli J, Ortman M, Field J, et al. Characteristics and early clinical outcomes of patients undergoing totally subcutaneous vs. transvenous single chamber implantable cardioverter defibrillator placement. Europace. 2018;20(2):308–14. https://doi.org/10.1093/europace/eux026.
Pettit SJ, McLean A, Colquhoun I, Connelly D, McLeod K. Clinical experience of subcutaneous and transvenous implantable cardioverter defibrillators in children and teenagers. Pacing Clin Electrophysiol. 2013;36(12):1532–8. https://doi.org/10.1111/pace.12233.
Köbe J, Reinke F, Meyer C, Shin DI, Martens E, Kääb S, et al. Implantation and follow-up of totally subcutaneous versus conventional implantable cardioverter-defibrillators: a multicenter case-control study. Heart Rhythm. 2013;10(1):29–36. https://doi.org/10.1016/j.hrthm.2012.09.126.
Brouwer TF, Yilmaz D, Lindeboom R, Buiten MS, Olde Nordkamp LR, Schalij MJ, et al. Long-term clinical outcomes of subcutaneous versus transvenous implantable defibrillator therapy. J Am Coll Cardiol. 2016;68(19):2047–55. https://doi.org/10.1016/j.jacc.2016.08.044.
Boveda S, Chalbia TE, Jacob S, Combes S, Combes N, Cardin C, et al. Duration of hospital admission, need of on-demand analgesia and other peri-procedural and short-term outcomes in sub-cutaneous vs. transvenous implantable cardioverter-defibrillators. Int J Cardiol. 2018;258:133–7. https://doi.org/10.1016/j.ijcard.2017.11.104.
Knops RE, Olde Nordkamp LRA, Delnoy PHM, Boersma LVA, Kuschyk J, El-Chami MF, et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383(6):526–36. https://doi.org/10.1056/NEJMoa1915932.
Friedman PA, Bradley D, Koestler C, Slusser J, Hodge D, Bailey K, et al. A prospective randomized trial of single- or dual-chamber implantable cardioverter-defibrillators to minimize inappropriate shock risk in primary sudden cardiac death prevention. Europace. 2014;16(10):1460–8. https://doi.org/10.1093/europace/euu022.
Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17(5):767–77. https://doi.org/10.1093/europace/euv053.
Guha A, Maddox WR, Colombo R, Nahman NS Jr, Kintziger KW, Waller JL, et al. Cardiac implantable electronic device infection in patients with end-stage renal disease. Heart Rhythm. 2015;12(12):2395–401. https://doi.org/10.1016/j.hrthm.2015.08.003.
Lickfett L, Bitzen A, Arepally A, Nasir K, Wolpert C, Jeong KM, et al. Incidence of venous obstruction following insertion of an implantable cardioverter defibrillator. A study of systematic contrast venography on patients presenting for their first elective ICD generator replacement. Europace. 2004;6(1):25–31. https://doi.org/10.1016/j.eupc.2003.09.001.
Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace. 2007;9(5):328–32. https://doi.org/10.1093/europace/eum019.
Yu Z, Wu Y, Qin S, Wang J, Chen X, Chen R, et al. Comparison of single-coil lead versus dual-coil lead of implantable cardioverter defibrillator on lead-related venous complications in a canine model. J Interv Card Electrophysiol. 2018;52(2):195–201. https://doi.org/10.1007/s10840-018-0312-8.
Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128(9):944–53. https://doi.org/10.1161/circulationaha.113.003042.
Kutyifa V, Beck C, Brown MW, Cannom D, Daubert J, Estes M, et al. Multicenter automatic defibrillator implantation trial-subcutaneous implantable cardioverter defibrillator (MADIT S-ICD): design and clinical protocol. Am Heart J. 2017;189:158–66. https://doi.org/10.1016/j.ahj.2017.04.014.
Olde Nordkamp LR, Knops RE, Bardy GH, Blaauw Y, Boersma LV, Bos JS, et al. Rationale and design of the PRAETORIAN trial: a Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am Heart J. 2012;163(5):753–60.e2. https://doi.org/10.1016/j.ahj.2012.02.012.
Author information
Authors and Affiliations
Contributions
HT and LS contributed to the study conception and design. LS, JG, and YH collected the data and performed the data analysis. HT and LS contributed to the interpretation of the data and the completion of figures and tables. All authors contributed to the drafting of the article and final approval of the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figure 1.
Kaplan-Meier curve showing the complication-free survival rates for short-term (A) and long-term follow-up (B), respectively. (PNG 225 kb).
Supplementary Figure 2.
Begg’s test showed a symmetrical distribution of the included publications (p = 0.462), which indicated that there was not a publication bias among the articles included in the present study. (PNG 197 kb).
Supplementary Table 1
(DOCX 15 kb).
Rights and permissions
About this article
Cite this article
Su, L., Guo, J., Hao, Y. et al. Comparing the safety of subcutaneous versus transvenous ICDs: a meta-analysis. J Interv Card Electrophysiol 60, 355–363 (2021). https://doi.org/10.1007/s10840-020-00929-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00929-1