Abstract
Background
Pacing the cardiac conduction system has been explored in patients with conduction system disease, but comprehensive comparisons between different pacing modalities are not well investigated.
Objective
To compare pacing characteristics and ventricular synchrony between His-bundle pacing (HBP) and left bundle branch pacing (LBBP) in patients with atrioventricular block (AVB).
Methods
Fifty pacemaker-indicated patients with AVB were enrolled. Twenty-five patients underwent HBP, and another 25 patients underwent LBBP. Success rate, procedural and fluoroscopy duration, pacing parameters, and echocardiographic data were perioperatively assessed and at 3-month follow-up.
Results
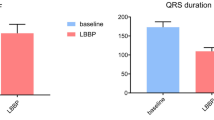
HBP was successful in 19 of 25 (76.0%) patients, whereas LBBP was successful in 22 of 25 (88.0%) patients. Compared with HBP, LBBP capture threshold was significantly lower (0.76 ± 0.25 V/0.4 ms vs. 1.27 ± 0.61 V/1.0 ms, P = 0.003) and R-wave amplitude was significantly higher with LBBP (11.7 ± 6.6 vs. 4.9 ± 2.4 mV, P < 0.001) at implant. The mean procedural time (74.3 ± 17.8 vs. 63.2 ± 12.3 min, P = 0.029) and fluoroscopy duration (10.3 ± 4.5 vs. 6.8 ± 2.2 min, P = 0.005) were significantly longer in the HBP group compared to LBBP. At 3-month follow-up, pacing capture threshold remained more stable in LBBP than in HBP group while left ventricular synchrony was similar between both groups.
Conclusion
Despite similar impact on ventricular synchrony compared with HBP, LBBP featured a significantly lower pacing capture threshold, higher R-wave amplitude, and less time to achieve similar success rate in patients with AVB. These findings indicate LBBP as a physiological pacing strategy for AVB patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Traditional right ventricular apical pacing (RVAP) is associated with increased incidence of heart failure and atrial fibrillation in pacing-dependent patients with atrioventricular block (AVB) [1, 2]. His-bundle pacing (HBP) that directly stimulates the cardiac conduction system and preserves native ventricular electrical activation synchrony is a physiological pacing modality with favorable clinical benefits [2, 3]. Despite considerably promising merits for clinical application and being well investigated, challenges remain, including a lower HBP success rate, higher and sometimes even unstable His-bundle (HB) capture threshold, especially in patients with conduction system disease [4, 5].
Huang et al. demonstrated that left bundle branch pacing (LBBP) with a capture threshold of 0.5 V could correct left bundle branch block (LBBB) in a heart failure patient with LBBB and significant improvement in heart failure was observed [6]. Later, Chen et al. reported the method of trans-ventricular septal approach to achieve LBBP implantation in bradycardia patients and the study demonstrated relatively narrow paced QRS complex, including patients with LBBB or RBBB [5]. Since then, LBBP has been under investigation, which found that LBBP generates relatively narrow QRS duration (QRSd), rapid left ventricular activation, low and stable pacing capture threshold, and a high success rate of implantation [7, 8]. The study by Li et al. demonstrated the application of LBBP in patients with AVB, while other studies demonstrated bundle branch block correction by LBBP [9]. It appears that LBBP and HBP are both suitable for AVB patients. However, direct comparisons between HBP and LBBP in patients with AVB have not been well described.
In this study, we compared the procedure, pacing, and electrophysiological characteristics and echocardiographic assessment of ventricular mechanical synchrony between HBP and LBBP in consecutive AVB patients.
2 Methods
2.1 Study patients and design
Our study prospectively recruited a series of consecutive pacemaker-indicated patients with AVB from August 2018 to April 2019 [10]. The exclusion criteria included (1) chronic atrial fibrillation, (2) cardiac resynchronization therapy or pacemaker replacement with pre-existing lead, and (3) rejection to receiving physiological pacing. Twenty-five patients were nonrandomly assigned to undergo HBP (HBP group) and compared with 25 patients who underwent LBBP (LBBP group) in a prospective fashion during the same time period. The study was approved by the Institutional Ethics Committee of Fuwai Hospital and informed consent was obtained from all patients.
2.2 Equipment and devices
Pacing parameters were intraprocedurally measured with a pacing system analyzer (PSA, Model 2290, Medtronic Inc., Minnesota, USA). A twelve-lead electrogram (ECG) and intracardiac electrogram (EGM) were recorded by a multichannel electrophysiologic monitor (Bard Electrophysiology, Lowell, MA, USA). The fluoroscopic mode was CARDIO EP, Extremely Low, 3.75 f/s (General Electric medical system, MA, USA).
2.3 Implantation procedure
2.3.1 HBP under conventional fluoroscopy
Under local anesthesia, the left subclavian vein was punctured and a short 8F sheath (Model HLS-1008 M, Medtronic, Minneapolis, MN, USA) was advanced over a guidewire to obtain venous access. The C315 HIS (Medtronic, Minneapolis, MN, USA) was advanced into the right atrium (RA) via the guidewire. Then, the Select Secure pacing lead (Model 3830–69 cm, Medtronic, Minneapolis, MN, USA) was advanced to the tip of sheath with its tip just beyond the distal part of the sheath for HB potential recording and unipolar pacing. Unipolar connections were achieved between the tip of the lead and subcutaneous tissue. Under the fluoroscopic right anterior oblique (RAO) 30°, the sheath with the pacing lead was carefully moved around the HB region to find a clear HB potential. Once an optimal HB potential was recorded, or HB was directly recruited by pace mapping, the lead was then screwed in this position by 4 to 5 clockwise rotations (Fig. 1a). If an acceptable HB capture (≤ 2.5 V/1.0 ms) could not be achieved after a His lead fluoroscopy time (HL-FT) of 20 min, the lead was then placed in RV mid septum.
ECG and EGM changes before and after HBP (a)/LBBP (b and c). (A1) Patient’s 12-lead ECG before HBP: the baseline QRSd was 88 ms; (A2) a His potential was recorded: HV interval was 44 ms; (A3) NS-HBP: the HB threshold was 1.1 V/1.0 ms; (A4): RV myocardial pacing: the threshold was 0.5 V/1.0 ms; (B1) baseline QRS (104 ms) before LBBP implantation; (B2) QRS complex morphology by endocardial pacing (arrow shows the mid-notch in lead V1); (B3 and B4) with the lead further screwing, the notch (arrow) in the paced QRS complex in lead V1 migrated towards the end of the QRS wave; (B5) development of a terminal R wave in lead V1 (red circle) in LBBP, the paced QRSd was 128 ms; (B6) the LBB potential (*) recorded; c the LBBP lead-tip entering in the ventricular septum, which was visualized by the fluoroscopic imaging with contrast injection (white arrow). ECG, electrocardiogram; EGM, electrogram; HBP, His-bundle pacing; LBBP, left bundle branch pacing; QRSd, QRS duration; RBBB, right bundle branch block; LBB, left bundle branch; HB, His-bundle; RV, right ventricle; NS-HBP, non-selective His-bundle pacing; LAO, left anterior oblique
2.3.2 LBBP under conventional fluoroscopy
LBBP was performed also using Select Secure 3830 lead and C315 sheath. After the delivery sheath was inserted to the RV, the pacing lead was advanced through the sheath in the RAO 30°. Following the successful mapping of HB potential, the lead-tip was coursing approximately 1–2 cm in the RV septum along the line between HB region mapped and RV apex. If HB potential was failed to be mapped, the sheath was moved to the high RV septum. The ideal initial site was defined where the paced QRS complex in lead V1 displayed a “W” morphology with a mid-notch. The lead was then screwed with 6 to 8 clock-wise rotations under fluoroscopic left anterior oblique (LAO) 30°. The notch in the paced QRS complex in lead V1 moved towards the end of the QRS. Another 2 to 3 rotations were attempted until lead V1 presented with development of a terminal R wave in lead V1, and a narrowed QRS duration was observed (Fig. 1b). At this point, fluoroscopic imaging with contrast injection can be used to confirm the distal part of the pacing lead entering in the ventricular septum (Fig. 1c). If LBBP could not be achieved after a left bundle branch (LBB) lead fluoroscopy time of 20 min, the lead was also placed in RV mid septum.
2.4 Definition of successful HBP or LBBP
The success of HBP was defined as [1] HB capture was ≤2.5 V/1.0 ms, [2] HL-FT ≤ 20 min, and [3] pacing response was categorized as selective (isoelectric interval between stimulus and paced QRS on EGM, and paced morphology is the same as the native QRS morphology) or non-selective HBP (no isoelectric interval between stimulus and paced QRS on EGM, and paced QRSd will usually be longer than the native QRS duration by the His-QRS interval) on the basis of HBP collaborative working group’s recommendations [11].
The success of LBBP was defined as (1) paced QRS morphology was shown as the development of a terminal R wave in lead V1, (2) paced QRSd was less than 130 ms, (3) LBBP lead fluoroscopy time ≤ 20 min, and (4) pacing response was categorized as selective (isoelectric interval between stimulus and paced QRS on EGM, and paced QRS morphology was characterized as completely development of a terminal R wave in lead V1) or non-selective LBBP (no isoelectric interval between stimulus and paced QRS on EGM, and paced QRS morphology was characterized as incompletely development of a terminal R wave in lead V1) [5, 12].
2.5 Device programming
Pacing parameters were measured in VVI mode (2290, Medtronic, Minneapolis, Minnesota). Ventricular safety pacing and auto capture management were activated. Atrioventricular (AV) delay programming was individualized, taking into consideration AV conduction times and the presence of heart block. The automatic AV search function was routinely activated inpatients among patients with intact AV conduction. For HBP patients, the pulse width was set at 1.0 ms during the procedure and at 0.4 ms at follow-up.
2.6 Data collection and follow-up
Baseline data including demographic characteristics and indication for implantation were collected at enrolment. Twelve-lead ECG, pacing parameters, EGM, and procedure-related complications were recorded during implantation and at 3-month follow-up. Echocardiographic parameters using the Vivid E95 ultrasound system (GE Vingmend Ultrasound, Horten Norway), including left atrial diameter (LAD), left ventricular end-diastolic diameter (LVEDD), and left ventricular ejection fraction (LVEF), were measured pre-implantation and at 3-month follow-up. Two-dimensional speckle tracking electrocardiographic strain imaging was analyzed using commercially available software (EchoPAC 201; GE Vingmed Ultrasound). We defined contraction duration as the time from the onset of Q/R on the electrocardiogram to peak negative longitudinal strain in 17 LV segments, and mechanical dispersion was calculated as the strain dispersion of contraction durations from 17 LV segments using apical four-chamber, two-chamber, and long-axis views. Mechanical dispersion (peak strain dispersion, PSD) was used for synchrony evaluation. The bull’s-eye displays the PSD in a color scheme in which green indicates normal contraction, blue indicates early contraction, and yellow to red indicates late contraction.
2.7 Statistical analysis
Continuous variables were reported as mean ± SD and categorical variables are presented as proportions (%). Fisher’s exact probability test (categorical variables) and Student’s t test (continuous variables) were used to determine differences between groups. Baseline echocardiogram characteristics, intra-procedural pacing parameters, and that at 3-month follow-up were compared using paired Student’s t test. Analyses were performed with SPSS 22.0 software (SPSS, Inc., IBM, Armonk, New York) for Windows. All two-sided P < 0.05 was considered to indicate statistical significance.
3 Results
3.1 Baseline characteristics
A comparison of baseline characteristics between the HBP and LBBP group are summarized in Table 1. Of all 50 patients, 31 patients (62.0%) were complete AVB. In HBP group, 36.0% patients were atrioventricular (AV) nodal block while 64.0% patients were infranodal block. Baseline LBBB and RBBB were present in 22.0% (11/50) and 16.0% (8/50) of the patients, respectively. Paroxysmal atrial fibrillation was diagnosed in 18.0% (9/50) of the patients. Of note, baseline characteristics were similar between the two groups.
3.2 Implant outcome
A comparison of implant outcome is shown in Table 2. The success rate of HBP was 76.0% (19/25): non-selective HBP (NS-HBP) was achieved in most patients (73.7%). HBP was unsuccessful in 6 patients: HB potential could not be recorded or HB was not captured in 4 patients, the HB was captured with unacceptable threshold (> 2.5 V/1.0 ms) in 2 patients. In the LBBP group, successful LBBP was obtained in 22 of 25 patients (88.0%): non-selective LBBP in 10 patients (45.5%). In 3 patients unsuccessfully undergoing LBBP, implant failure resulted from an inability to capture the LBB (the paced QRSd or paced morphology did not meet the success criterial of LBBP). The success rate was comparable between HBP and LBBP groups (76.0% vs. 88.0%, P = 0.462). The mean procedure time (HBP: 74.3 ± 17.8 vs. LBBP: 63.2 ± 12.3 min, P = 0.029) and His/LBB lead fluoroscopy time (HBP: 10.3 ± 4.5 vs. LBBP: 6.8 ± 2.2 min, P = 0.005) were significantly longer in the HBP group compared to that in the LBBP group.
3.3 Pacing parameters at implantation and follow-up
As is summarized in Table 2, a HB potential was recorded in 84.2% patients, whereas a LBB potential was recorded in 40.9% patients (P = 0.012). The interval from LBB potential to QRS onset (25.0 ± 2.2 ms) was significantly shorter than that of HV interval (72.7 ± 67.7 ms) (P = 0.048). The paced QRSd was similar between HBP and LBBP groups (122.8 ± 20.1 vs. 115.1 ± 10.1 ms, P = 0.142). Besides, three patients (15.8%) presented a left axis of paced QRS in HBP group whereas 9 patients in LBBP (40.9%) group (P = 0.078). At 3-month follow-up, the HB capture thresholds changed from 1.27 ± 0.61 V/1.0 ms to 1.22 ± 0.89 V/0.4 ms in HBP group (P = 0.531). LBB capture threshold significantly improved in LBBP group (from 0.76 ± 0.25 V/0.4 ms to 0.65 ± 0.20 V/0.4 ms; P < 0.001). Three patients (15.8%) in the HBP group had HB capture thresholds >2.5 V/0.4 ms at 3-month follow-up (two patients with LBBB and one patient with RBBB, the maximum of capture threshold was 3.0 V/0.4 ms), but there was no need for lead revision. Besides, the R-wave amplitude in both groups remained unchanged during the follow-up.
Compared with HBP, LBBP capture threshold was significantly lower at implant (0.76 ± 0.25 V/0.4 ms vs. 1.27 ± 0.61 V/1.0 ms, P = 0.003) and at 3-month follow-up (0.65 ± 0.20 V/0.4 ms vs. 1.22 ± 0.89 V/0.4 ms, P = 0.015). In addition, R-wave amplitude was significantly higher in LBBP than in HBP at implant (11.7 ± 6.6 vs. 4.9 ± 2.4 mV, P < 0.001) and at 3-month follow-up (12.0 ± 5.8 vs. 5.0 ± 2.2 mV, P < 0.001) (Fig. 2).
a Post-HBP implant transthoracic echocardiogram showing the HBP lead, the distance from the lead-tip to the root of TVA is approximately 4.6 mm. b Post-LBBP implant transthoracic echocardiogram showing the LBBP lead, the distance from the lead-tip to the root of TVA is approximately 34.5 mm. HBP, His-bundle pacing; LBBP, left bundle branch pacing; TVA, tricuspid valve annulus
3.4 Complications and echocardiogram characteristics after implantation
In the LBBP group, two patients had acute perforation of the ventricular septum during the LBBP procedure. Lead re-position in these two patients was performed without further issue. No thrombosis was observed in LBBP group patients. One patient had lead dislodgement at follow-up. Lead revision was attempted on day 26 but failed. The lead was then placed in RV septal. In the HBP group, one patient with paroxysmal atrial fibrillation in HBP group developed pocket hematoma and secondary infection due to inappropriate dosage of warfarin post-implant, with the device removed on postoperative day 83.
The PSD at baseline and at 3-month follow-up was available in 18 patients receiving HBP and 21 patients receiving LBBP. Mechanical dispersion (PSD value) was slightly improved in the HBP group (55.6 ± 18.0 vs. 48.2 ± 10.9 ms, P = 0.018) and the LBBP (51.4 ± 11.8 vs. 45.2 ± 8.9 ms, P < 0.001) group after 3-month follow-up when compared with that before the implantation, with the LBBB patients at baseline being the foremost (Figs. 3 and 4). However, the overall improvement of the PSD did not differ between the two groups (HBP: − 7.4 ± 11.9 vs. LBBP: − 6.1 ± 6.7 ms, P = 0.699) (Fig. 5). Besides, subgroup analysis showed that for AVB patients with bundle branch block (BBB) (N = 14), mechanical dispersion was significantly improved after 3-month follow-up when compared with that before the implantation (48.4 ± 7.8 vs. 63.6 ± 11.3 ms, P < 0.001). However, for AVB patients without BBB (N = 25), ventricular dyssynchrony at follow-up was not improved compared with that at baseline but a trend towards superior was observed (45.6 ± 10.7 vs. 47.5 ± 13.6 ms, P = 0.200). Compared to baseline, there was worsening of tricuspid valve regurgitation at least 1 grade in 3 patients (2 in HBP group and 1 in LBBP group) but without observation of the abnormal valve leaflet motion caused by the lead. Besides, ten patients (6 in HBP group and 4 in LBBP group) had an improvement at least 1 grade in tricuspid regurgitation.
NS-HBP and echocardiographic mechanical dispersion imaging. a, c Twelve-lead ECGs of a patient with baseline complete AVB (asterisk shows the atrial impulse failed to reach the ventricle) and NS-HBP are shown. b, d Representative mechanical dispersion imaging demonstrates an improvement in overall PSD with NS-HBP. e, g Twelve-lead ECGs of a patient with baseline LBBB and NS-HBP with LBBB recruitment are shown (red circle means ventricular stimulation signal: bipolar fashion). f, h Representative mechanical dispersion imaging demonstrates a significant improvement in overall PSD with NS-HBP. NS-HBP, non-selective His-bundle pacing; ECGs, electrocardiograms. AVB, atrioventricular block; LBBB, left bundle branch block; PSD, peak strain dispersion
LBBP and echocardiographic mechanical dispersion imaging. a, c Twelve-lead ECGs of a patient with baseline second degree AVB (asterisk shows the atrial impulse failed to reach the ventricle) and LBBP are shown. b, d Representative mechanical dispersion imaging demonstrates an improvement in overall PSD with LBBP. e, g Twelve-lead ECGs of a patient with baseline LBBB and LBBP with LBBB recruitment are shown (red circle means ventricular stimulation signal: bipolar fashion). f, h Representative mechanical dispersion imaging demonstrates a significant improvement in overall PSD with LBBP. LBBP, left bundle branch pacing; ECGs, electrocardiograms, AVB, atrioventricular block; LBBB, left bundle branch block, PSD, peak strain dispersion
Comparisons of PSD between baseline and 3-month follow-up. a Mechanical dispersion was slightly improved in both HBP and LBBP patients after 3-month follow-up when compared with that at baseline; b the overall improvement of synchrony did not differ between the two groups. NS-HBP, non-selective His-bundle pacing; LBBP, left bundle branch pacing; PSD, peak strain dispersion
4 Discussion
The present study investigated the two pacing modalities in patients with AVB and had the following major findings: (1) among patients with AVB, LBBP featured a significantly lower pacing threshold and higher R-wave amplitude at implant and 3-month follow-up compared with HBP. (2) LBBP resulted in similar paced QRSd, success rate with HBP. However, LBBP was observed having shorter procedure and fluoroscopy time. (3) Both LBBP and HBP could improve left ventricular (LV) synchrony, and the overall improvement of synchrony did not differ between the two groups. These results demonstrate the equal clinical feasibility and efficacy of HBP and LBBP for AVB patients.
4.1 Advantages and challenges with HBP
Traditional RVAP was associated with the increasing risk of atrial fibrillation and heart failure in AVB patients [13, 14]. Recently, HBP has emerged as a promising approach to delivering physiological pacing, maintaining long-term ventricular synchrony [2]. Vijayaraman et al. have reported that routine HBP in patients with AVB was feasible and safe. Abdelrahman et al. further demonstrated that HBP was associated with favorable long-term prognosis compared with RVAP [3, 4]. The HBP could result in physiological ventricular activation and was considered as physiological pacing [2]. However, challenging issues regarding this pacing modality still remain, including the unstable pacing threshold, lower R-wave amplitude, and unpredictable success rate, especially for patients with AVB [15]. Bhatt et al. showed that the success rate in those with AVB was only 56% [16]. Besides, Abdelrahman et al. demonstrated that about 14% of patients had HB capture threshold >2.5 V at follow-up [3].
4.2 Characteristics of LBBP
A first LBBP case described by Huang et al. in 2017 indicated that pacing more distally in the conduction system beyond the site of block could result in a narrow paced QRSd, low threshold and large R waves [6]. Besides, Chen et al. demonstrated the clinical feasibility of LBBP by using the trans-ventricular septal approach [5]. The emergence of LBBP raised another type of physiological pacing. Vijayaraman et al. showed that the LBBP threshold at implant was 0.6 ± 0.4 V/0.5 ms and R waves were 10 ± 6 mV and remained stable during follow-up [7]. Li et al. reported their experience of LBBP in 33 AVB patients. With a high success rate of 90.9%, LBBP yielded a lower, stable threshold, and preserved LV synchrony with few complications [9]. More importantly, Hou and colleagues demonstrated that LBBP preserved better electrical and LV mechanical synchrony compared with RV septal pacing [8]. The underlying assumption was that LBBP further enriched the physiological pacing or might even become more applicable in AVB patients [2].
4.3 HBP vs. LBBP
To the best of our knowledge, this is the first study comparing pacing parameters and echocardiogram characteristics between HBP and LBBP in patients with AVB. From our results, compared with HBP, LBBP featured a significantly lower pacing threshold and higher R-wave amplitude at implant and 3-month follow-up. The pacing threshold and R-wave amplitude in LBBP were acceptable, alleviating the potential of loss of capture and sensing problems during short-term follow-up. Therefore, satisfactory pacing parameters could be relatively easily achieved in AVB patients by LBBP. In addition, LBBP resulted in similar paced QRSd, success rate compared with HBP. Moreover, LBBP was observed to have shorter procedure and fluoroscopy times. Unlike HB, LBB, coving the subendocardium of the LV, run through the LV septum and fan out to form a wider target for pacing location [17]. Thus, the pacing lead could be easily screwed into the interventricular septum to pace the LBB when compared with HBP. Besides, LV synchrony was slightly improved in both HBP and LBBP patients over the short-term follow-up, but with no significant difference between two pacing modalities. LBB is a continuation of the main branch of the HB and was divided into left anterior branch and left posterior branch [17]. Thus, both the HB and LBB are ideal pacing targets for physiological pacing. LV synchrony could be preserved or even improved by pacing either HB or LBB. The results of subgroup analysis demonstrated that HBP and LBBP had no detrimental impact on ventricular mechanical synchrony. Therefore, permanent HBP and LBBP yielded a preserved left ventricular synchrony in AVB patients without BBB but an improved left ventricular synchrony in AVB patients with BBB at short-term follow-up. Catanzariti et al. demonstrated that HBP could help to maintain long-term LV synchrony. Whether LBBP could preserve long-term synchrony needs to be further evaluated [18]. Besides, previous study showed that the abnormal paced QRS axis maybe a predictor of pacing induced left ventricular dysfunction in RVAP [19]. In our study, the proportions of patients presenting left axis deviation were higher in LBBP group than those in HBP group. Long-term follow-up will be required to evaluate whether the paced QRS axis deviation in LBBP group affects cardiac synchrony.
4.4 Recommendations of physiological pacing in AVB patients
Although LBBP have some merits such as favorable procedural pacing parameters, and less complexity in procedure, more data are needed to evaluate long-term clinical outcomes of LBBP [2]. Besides, the risks of LBBP have not been well investigated including septal perforation, difficulties in lead extraction, and thrombosis formation [12, 20]. In our study, two patients developed septal perforations during the LBBP procedure. The importance of assessing ventricular septal thickness by Echo before implantation and close monitoring pacing parameters have been recommended [12, 21]. Further observations are required to explore the long-term safety of LBBP [12]. Thus, before LBBP safety profile is well documented, HBP for patients with AVB may remain the first choice while LBBP can be an alternative to HBP in AVB patients unsuccessfully undergoing HBP.
4.5 Study limitations
A few limitations of this study should be emphasized. This was a prospective, observational, nonrandomized study involving a limited number of AVB patients in a single center, so inevitable is the selection bias. To further compare the merits between HBP and LBBP, large randomized and multi-center studies are required. Besides, the present study had a short follow-up period, and long-term follow-up is needed to make comparison between HBP and LBBP.
5 Conclusion
Compared with HBP, LBBP resulted in similar paced QRSd, success rate, and LV synchrony but had significantly lower pacing threshold and higher R-wave amplitude in patients with AVB. Besides, LBBP was associated with shorter procedure time and fluoroscopy duration. Our preliminary results indicate that LBBP holds promise as an attractive physiological pacing strategy for AVB patients but long-term clinical outcomes and safety need further evaluation.
References
Nahlawi M, Waligora M, Spies SM, Bonow RO, Kadish AH, Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. 2004;44(9):1883–8.
Sharma PS, Vijayaraman P, Ellenbogen KA. Permanent his bundle pacing: shaping the future of physiological ventricular pacing. Nat Rev Cardiol. 2020;17(1):22–36.
Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun H, et al. Clinical outcomes of his bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. 2018;71(20):2319–30.
Vijayaraman P, Naperkowski A, Ellenbogen KA, Dandamudi G. Electrophysiologic insights into site of atrioventricular block: lessons from permanent his bundle pacing. JACC Clin Electrophysiol. 2015;1(6):571–81.
Chen K, Li Y, Dai Y, Sun Q, Luo B, Li C, et al. Comparison of electrocardiogram characteristics and pacing parameters between left bundle branch pacing and right ventricular pacing in patients receiving pacemaker therapy. Europace. 2019;21(4):673–80.
Huang W, Su L, Wu S, et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33(12):1736 e1731–1736 e1733.
Vijayaraman P, Subzposh FA, Naperkowski A, Panikkath R, John K, Mascarenhas V, et al. Prospective evaluation of feasibility, electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019;16(12):1774–82.
Hou X, Qian Z, Wang Y, Qiu Y, Chen X, Jiang H, et al. Feasibility and cardiac synchrony of permanent left bundle branch pacing through the interventricular septum. Europace. 2019;21(11):1694–702.
Li X, Li H, Ma W, Ning X, Liang E, Pang K, et al. Permanent left bundle branch area pacing for atrioventricular block: feasibility, safety, and acute effect. Heart Rhythm. 2019;16(12):1766–73.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and Antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):e350–408.
Vijayaraman P, Dandamudi G, Zanon F, Sharma PS, Tung R, Huang W, et al. Permanent his bundle pacing: recommendations from a multicenter his bundle pacing collaborative working group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm. 2018;15(3):460–8.
Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16(12):1791–6.
Tse HF, Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol. 1997;29(4):744–9.
Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107(23):2932–7.
Su L, Wu S, Wang S, Wang Z, Xiao F, Shan P, et al. Pacing parameters and success rates of permanent his-bundle pacing in patients with narrow QRS: a single-Centre experience. Europace. 2019;21(5):763–70.
Bhatt AG, Musat DL, Milstein N, Pimienta J, Flynn L, Sichrovsky T, et al. The efficacy of his bundle pacing: lessons learned from implementation for the first time at an experienced electrophysiology center. JACC Clin Electrophysiol. 2018;4(11):1397–406.
Elizari MV. The normal variants in the left bundle branch system. J Electrocardiol. 2017;50(4):389–99.
Catanzariti D, Maines M, Manica A, Angheben C, Varbaro A, Vergara G. Permanent his-bundle pacing maintains long-term ventricular synchrony and left ventricular performance, unlike conventional right ventricular apical pacing. Europace. 2013;15(4):546–53.
Kim SH, Oh YS, Nam GB, Choi KJ, Park JS, Park SW, et al. Paced qrs axis as a predictor of pacing-induced left ventricular dysfunction. J Interv Card Electrophysiol. 2014;41(3):223–9.
Zhang S, Zhou X, Gold MR. Left bundle branch pacing: JACC review topic of the week. J Am Coll Cardiol. 2019;74(24):3039–49.
Chen K, Li Y. How to implant left bundle branch pacing lead in routine clinical practice. J Cardiovasc Electrophysiol. 2019;30(11):2569–77.
Acknowledgments
The authors thank Mrs. Jiangtao Wang (General Electric healthcare, Beijing, China) for her assistance of echocardiographic data analysis.
Author information
Authors and Affiliations
Contributions
Yiran Hu: design and drafting article; Min Gu and Wei Hua: pacemaker implantation (Dr. Hua as the operator and Dr. Gu as the assistant); Nixiao Zhang, Xuhua Chen, and Xi Liu: data collection and data analysis; Hui Li: echo data collection; Cuihong Hou and Hongxia Niu: critical revision of article; Xiaohong Zhou: language grammar check; Shu Zhang: approval of article.
Corresponding author
Ethics declarations
The study was approved by the Institutional Ethics Committee of Fuwai Hospital and informed consent was obtained from all patients.
Conflict of interest
Yiran Hu, Hui Li, Min Gu, Wei Hua, Hongxia Niu, Nixiao Zhang, Xi Liu, Xuhua Chen, Cuihong Hou, and Shu Zhang declare that they have no conflict of interest. Xiaohong Zhou is an employee of Medtronic, Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, Y., Li, H., Gu, M. et al. Comparison between his-bundle pacing and left bundle branch pacing in patients with atrioventricular block. J Interv Card Electrophysiol 62, 63–73 (2021). https://doi.org/10.1007/s10840-020-00869-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00869-w