Abstract
Purpose
The purpose of this study was to evaluate the feasibility and safety of superior vena cava (SVC) isolation in addition to standard pulmonary vein isolation (PVI) using the second-generation cryoballoon (CB) in patients with paroxysmal atrial fibrillation.
Methods
Thirty-seven consecutive patients that underwent CB ablation for paroxysmal atrial fibrillation (PAF) were prospectively enrolled in our study. After PVI the SVC was mapped for potentials. If the SVC exhibited electrical activity, isolation was achieved performing a single 180-s balloon application.
Results
Regarding SVC isolation, 180-s freeze in the SVC could be completed in 32 (86.4%) patients, and 5 patients had at least 120 s of freezing application (13.5%). Real-time recording during SVC isolation was observed in 30 (81.0%) patients. The mean time to isolation was 36.9 ± 28.7 s and the temperature at isolation was − 33 (− 15 to − 40) °C. No cases developed persistent phrenic nerve palsy (PNP) or any other complication.
Conclusions
Superior vena cava isolation proved to be safe and feasible with the second generation cryoballoon in a prospective series of patients affected by PAF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The superior vena cava (SVC) is known to be an important location of non-pulmonary vein foci triggering paroxysmal atrial fibrillation (PAF). Electrical isolation of this vessel by the means of radiofrequency (RF) is associated with improved outcome in terms of freedom from AF [1,2,3]. As published by Corrado A. et al., patients with paroxysmal atrial fibrillation who underwent pulmonary vein isolation and SVC isolation were significant less prone to recurrence with RF, after a follow-up of 12 months, compared with the group that received solely PVI [1]. In the present study, we describe a prospective experience in a cohort of patients undergoing pulmonary vein isolation (PVI) + SVC ablation with the second-generation cryoballoon (CB).
2 Methods
2.1 Study design
Consecutive patients indicated to CB ablation for PAF and SVC isolation with the CB ablation were prospectively enrolled in our study at Maria Cecilia Hospital, Cotignola. After PVI was obtained and proved by entrance and exit block, the SVC was mapped for potentials. If the SVC exhibited electrical activity, isolation was performed with a single 180-s duration cryoenergy application. A single 180-s application is known to produce a durable lesion [4]. Although virtually always reversible, phrenic nerve injury (PNI) is the most frequently observed complication during CB ablation.
To prevent the latter, phrenic nerve (PN) activity was tested during ablation of the SVC, by inserting a quadripolar catheter through the right jugular/subclavian to allow simultaneous ablation in the SVC and PN pacing.
2.2 Patient selection
Consecutive patients programmed for CB ablation for PAF were prospectively enrolled. All antiarrhythmic drugs were discontinued at least 3 days before ablation, apart from amiodarone that was stopped 1 month before. Procedures were performed under general anesthesia.
2.3 The exclusion criteria
Age younger than 18 years, left atrial diameter > 55 mm, severe valve disease, uncontrolled heart failure, contraindication to general anesthesia/deep procedural sedation, left atrial thrombus at the pre-procedural transesophageal echocardiogram (TEE).
The study was approved by the ethical committee of Maria Cecilia Hospital, Cotignola. The protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by the Declaration of Helsinki, protecting the privacy of all participants as well as the confidentiality of their personal information.
2.4 Procedure
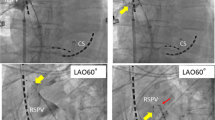
The PVI procedure was performed with a 28-mm cryoballoon advance (Arctic Front Advance™, Medtronic©) as previously described [5]. A 6F decapolar catheter was inserted in the right jugular/subclavian vein and advanced to the coronary sinus. A single trans-septal puncture was performed under fluoroscopic guidance, using the right femoral venous approach. After gaining LA access, a 70-UI/kg heparin intravenous bolus was given. A 0.32-F Emerald exchange wire (Cordis, Johnson and Johnson, Diamond Bar, CA, USA) was advanced in the left superior PV, and a steerable 15 F over-the-wire sheath (FlexCath, Cryocath) was positioned in the left atrium (LA). A 20 pole achieve recorded electrical activity throughout the procedure. Ablation of all 4 PVs was achieved. Cryoenergy applications lasted 180 s. Electrical isolation was confirmed in all 4 PVs. Effective PVI was considered to have been achieved when all PV potentials were abolished or dissociated from atrial activity. Pacing from the distal and proximal coronary sinus was performed to distinguish eventual far-field atrial signals from PV potentials recorded on the mapping catheter, respectively, for left- and right-sided PVs. After having achieved successful PVI ablation, SVC ablation was performed. The CB was retrieved to the right atrium, and the achieve catheter was advanced in the SVC. Then the CB was inflated in the right atrium and advanced toward the ostium of the SVC in order to occlude the vessel. Once total occlusion was confirmed by dye injection with total retention of contrast in the SVC (Fig. 1), cryoenergy application was started.
After the isolation of the SVC (Fig. 2), a waiting period of 15 min was taken into account, and thereafter, routine testing with adenosine was performed [6] to reveal dormant conduction, and the isolation was also confirmed with bidirectional block.
a An example of superior vena cava (SVC) potentials recorded at the ostium of the SVC during sinus rhythm prior to isolation. Shown are surface leads V1, I, II, and AVF and bipolar intracardiac electrograms recorded by circular mapping catheter (MAP 1–4). The SVC potentials can be distinguished from the right atrial (RA) electrogram by their sharp appearance. b Example of electrical isolation of the SVC as measured by the circular mapping catheter (yellow arrow)
2.5 Phrenic nerve monitoring
Prior to ablation of the right-sided PVs and the SVC, a 6F decapolar catheter that was inserted in the right jugular/subclavian vein and placed in the coronary sinus was placed distally in the SVC, and diaphragmatic stimulation was achieved by pacing the ipsilateral phrenic nerve with a 1000-ms cycle and a 20-mA output. Phrenic nerve pacing started once the temperature reached − 20 °C in order to avoid balloon dislodgement due to diaphragmatic contraction in the first phase of cryoenergy application. Pacing was continued throughout the entire duration of cryoenergy delivery. In cases of phrenic nerve palsy, the freeze was immediately aborted with a “double stop” technique [7] and observed for recovery.
2.6 Post-procedural management
Post-procedural management was performed as standard clinical practice. The next day, the patients underwent a transthoracic echocardiogram (TTE) and a chest X-ray. During the chest X-ray, a “sniff test” was performed to assess PN function. The patients were monitored under telemetry for 18 h after ablation.
Clinical follow-up including regular cardiological consultations and 24-h Holter ECG monitoring was performed as standard clinical practice at 1, 6, and 12 months.
2.7 Statistical analysis
All statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were reported as absolute and relative frequencies. Continuous variables were evaluated for parametric distribution using Kolmogorov-Smirnov test. Continuous variables with parametric distributions were reported as a mean ± standard deviation and were compared using non-paired Student t test. Continuous variables with non-parametric distributions and discrete variables were reported as median (interquartile range) and compared using Mann-Whitney test. A significance cut-off for p value of less than 0.05 (two sided) and a 95% confidence interval (CI) was used.
3 Results
A total of 37 patients were prospectively included in the study: 27 (72.9%) were males, with a mean age of 54.6 ± 10.7 years and a mean body mass index of 27.6 ± 3.5 kg/m2. Incidence of comorbidities, echocardiographic parameters, and chronic medication are reported in Table 1. All patients had PAF with a median duration of symptoms of 12 (11–26) months. Procedural details are reported in Table 2.
All patients are presented with potentials in the SVC. Two patients exhibited spontaneous triggering arising from the SVC.
Regarding SVC isolation, a 180-s freeze in the SVC was completed in 32 (86.4%) patients, and 5 patients had at least 120 s of freezing application (13.5%). Real time recording during SVC isolation was observed in 30 (81.0%) patients. The mean time to isolation was 36.9 ± 28.7 s, and the temperature at isolation was − 33 (− 15 to − 40) °C. The mean time to reach − 40 °C was 41.6 ± 11.7 s, and the temperature at 60 s was − 39 (− 29 to − 43) °C, while minimum reached temperature was − 41.3 ± 8.1 °C. Mean SVC diameter was 21.8 ± 4.0 mm. None of the ablation parameters differed with the presence or absence of real-time recording during SVC isolation. None of the ablation parameters correlated with SVC diameter. SVC diameter was higher in patients with real-time recordings in SVC during ablation, although not statistically significant (p = 0.09, OR = 1.44, 95%CI = 0.95–1.91). During SVC ablation, 2 patients developed transient PNP during cryotherapy. Resumption of PN activity occurred before the end of the procedure. Additionally, 3 patients experienced impending PN damage with reduction of diaphragmatic contraction. Interruption of the freeze immediately led to full resumption of phrenic activity. None of the patients presented PNP during the right-sided veins ablation. SVC isolation was achieved in all patients with one application. No patient expired minor or major complication related with the procedure including persistent phrenic nerve paralysis and access site problems. Also, there was no sinus node injury.
Freedom from AF rates at 1, 6, and 12 months was 100, 97.3, and 91.9%, respectively. Recurrence was in the form of AF in 2 cases, atypical left atrial flutter in 1 case, solved with re-isolation of the right inferior PV, and a roofline, respectively. In univariate Cox survival regression, none of the SVC ablation parameters, presence of real-time recording, or SVC diameter predicted AF recurrence. Remarkably, in all 3 patients who underwent a repeat ablation procedure, the SVC was still isolated.
4 Discussion
To the best of our knowledge, this is the largest study with the longest follow-up that prospectively assessed the safety and feasibility of a novel CB ablation technique for SVC isolation after PVI.
Pulmonary vein isolation is the basis in today’s everyday practice for atrial fibrillation ablation; it removes the majority of the triggers responsible for the beginning and maintenance of AF. Nevertheless, a substantial percentage of the patients will recur with atrial arrhythmias subsequent to this procedure. Up to 28% of AF patients might exhibit non-PV foci. Elimination of the latter in addition to PVI might be an important step to improve success rates and freedom from arrhythmia recurrence. Among the non-PV foci, the SVC was described in being the most common source of ectopy [8]. The arrhythmogenic properties of the SVC, as one of the most common source of non-PV triggers, might be explained by the common embryologic origin of the sinus node [9]. Various approaches such as ablation of the SVC especially, when triggers arise from it, were proposed as adjunctive techniques to PVI. However, the results are debatable, and the optimal ablation approach that might lead to significantly improved outcomes still needs to be determined. In addition, most studies published in the literature on this issue addressed SVC isolation by the means of RF energy. Isolation of this structure with the cryoballoon has been only reported in one prospective study with 30 patients with 6 months follow-up after ablation. Different from the latter, our study includes only PAF. In addition, all SVC were isolated with a single freeze. This might be beneficial for the patients in reducing the risk of adverse effects. Finally, our study reported results on 12 months follow-up. Thus, to the best of our knowledge, this is the largest prospective study conducted in a consecutive series of patients affected exclusively by PAF assessing the feasibility and safety of the second generation CB ablation in the SVC.
According to recent publications, SVC isolation might improve clinical outcomes [1, 2, 8, 10,11,12]. The ablation of the SVC can be problematic to accomplish due to the vicinity of the PN and sinus node. The injury of the right phrenic nerve is the major concern and is one of the most common complications related to CB ablation technique [4, 13,14,15]. Point by point ablation allows to verify the position of phrenic nerve by local high energy pacing and to avoid with ablation just that site. The latter can give RF technique a theoretical advantage to avoid PNI.
Different techniques such as palpation of the diaphragmatic excursion, diaphragmatic compound motor action potentials (CMAPs) monitoring, and the “double stop” strategy have been established to avoid PNI [16]. In our study, we performed the most common technique, consisting in the palpation of the strength of diaphragmatic excursion, during ablation on both the right-sided PVs and the SVC, with a decapolar catheter positioned distally in the SVC, and diaphragmatic stimulation was achieved by pacing the ipsilateral phrenic nerve with a 1000-ms cycle and a 20-mA output. One advantage of pacing the PN via subclavian access is to guarantee the total occlusion of the SVC with the CB, thus avoiding possible gaps caused by the presence of a pacing catheter used to monitor phrenic nerve function coming through the right atrial, which might reduce the durability of SVC isolation. [17] In our study, all patients exhibiting PNI during the ablation of the SVC had complete resumption of diaphragmatic contraction before the end of the procedure.
Additional safety concern is the inappropriate sinus node tachycardia (ISNT) [18]. As already reported, CB ablation can cause acute parasympathetic denervation in the heart, and the vagal denervation of the sinus node might be the cause of ISNT [19, 20]. The ablation of the SVC might add some degree of denervation in the epicardial ganglionated plexi 1, between the superior vena cava and the aortic root just above the right upper pulmonary vein [21], and might contribute to a more extensive parasympathetic denervation; however, none of the 37 patients presented ISNT during the period of 1-year follow-up.
The design of the study was to demonstrate the safety and feasibility of the procedure with the second-generation CB. Nevertheless, after a short follow-up of 12 months, 91.9% of the patients are free from any arrhythmia. Although conducted on a limited number of patients, our study seems to convey the message that the SVC can be isolated with the second-generation CB. Future randomized studies are required to conclude that adding the isolation of the SVC to PVI will increase the clinical results for AF ablation.
4.1 Study limitations
This study has several limitations. It was a single center, with a relatively small population and no control group. SVC stenosis was not actively investigated in our study. Nevertheless, no patients suffered from any symptom potentially related to SVC stenosis clinics during follow-up. Although in our center the PN pacing is done with high output, the best practice guide for CB ablation recommends pacing output doubles the capture threshold [22]. No isoproterenol test was performed to search non-PV triggers. Our study results should be consolidated in a prospective large study.
5 Conclusion
Superior vena cava isolation proved to be safe and feasible with the second-generation cryoballoon in a prospective series of patients affected by PAF.
Abbreviations
- CMAPs:
-
Compound motor action potentials
- CI:
-
Confidence interval
- CB:
-
Cryoballoon
- ISNT:
-
Inappropriate sinus node tachycardia
- LA:
-
Left atrium
- PAF:
-
Paroxysmal atrial fibrillation
- PN:
-
Phrenic nerve
- PNP:
-
Phrenic nerve palsy
- PVI:
-
Pulmonary vein isolation
- RF:
-
Radiofrequency
- SVC:
-
Superior vena cava
References
Corrado A, Bonso A, Madalosso M, Rossillo A, Themistoclakis S, Di Biase L, et al. Impact of systematic isolation of superior vena cava in addition to pulmonary vein antrum isolation on the outcome of paroxysmal, persistent, and permanent atrial fibrillation ablation: results from a randomized study. J Cardiovasc Electrophysiol. 2010;21(1):1–5. https://doi.org/10.1111/j.1540-8167.2009.01577.x.
Arruda M, Mlcochova H, Prasad SK, Kilicaslan F, Saliba W, Patel D, et al. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol. 2007;18(12):1261–6. https://doi.org/10.1111/j.1540-8167.2007.00953.x.
Fukumoto K, Takatsuki S, Kimura T, Nishiyama N, Tanimoto K, Aizawa Y, et al. Electrophysiological properties of the superior vena cava and venoatrial junction in patients with atrial fibrillation: relevance to catheter ablation. J Cardiovasc Electrophysiol. 2014;25(1):16–22. https://doi.org/10.1111/jce.12271.
Mugnai G, De Asmundis C, Ciconte G, Irfan G, Saitoh Y, Velagic V, et al. Incidence and characteristics of complications in the setting of second-generation cryoballoon ablation: a large single-center study of 500 consecutive patients. Heart Rhythm. 2015;12(7):1476–82. https://doi.org/10.1016/j.hrthm.2015.04.001.
Osório TG, Iacopino S, Coutiño HE, Ströker E, Sieira J, Salghetti F, et al. Evaluation of the luminal esophageal temperature behavior during left atrium posterior wall ablation by means of second-generation cryoballoon. J Interv Card Electrophysiol. 2019;55:191–6. https://doi.org/10.1007/s10840-019-00523-0.
Chierchia GB, Di Giovanni G, Sieira-Moret J, De Asmundis C, Conte G, Rodriguez-Manẽro M, et al. Initial experience of three-minute freeze cycles using the second-generation cryoballoon ablation: acute and short-term procedural outcomes. J Interv Card Electrophysiol. 2014;39(2):145–51. https://doi.org/10.1007/s10840-013-9855-x.
Ghosh J, Sepahpour A, Chan KH, Singarayar S, McGuire MA. Immediate balloon deflation for prevention of persistent phrenic nerve palsy during pulmonary vein isolation by balloon cryoablation\. Heart Rhythm. 2013;10(5):646–52. https://doi.org/10.1016/j.hrthm.2013.01.011.
Yamaguchi T, Tsuchiya T, Miyamoto K, Nagamoto Y, Takahashi N. Characterization of non-pulmonary vein foci with an EnSite array in patients with paroxysmal atrial fibrillation. Europace. 2010;12(12):1698–706. https://doi.org/10.1093/europace/euq326.
Agarwal SC, Bittinger L, Tang AS. Importance of superior vena cava isolation in successful ablation of persistent atrial fibrillation in patient with partial anomalous pulmonary vein. PACE Pacing Clin Electrophysiol. 2013;36(5):e146–9. https://doi.org/10.1111/j.1540-8159.2012.03508.x.
Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102(1):67–74. https://doi.org/10.1161/01.CIR.102.1.67.
Lin W-S, Tai C-T, Hsieh M-H, Tsai C-F, Lin Y-K, Tsao H-M, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non–pulmonary vein ectopy. Circulation. 2003;107(25):3176–83. https://doi.org/10.1161/01.CIR.0000074206.52056.2D.
Wei HQ, Li J, Sun Q, Guo XG, Wang HY, Du Yang J, et al. Safety and efficacy of superior vena cava isolation using the second-generation cryoballoon ablation in a canine model. J Cardiol. 2019. https://doi.org/10.1016/j.jjcc.2019.08.013.
Sacher F, Jais P, Stephenson K, O’Neill MD, Hocini M, Clementy J, et al. Phrenic nerve injury after catheter ablation of atrial fibrillation. Indian Pacing Electrophysiol J. 2007;7(1):1–6. https://doi.org/10.1016/j.jacc.2006.02.050.
Sánchez-Quintana D, Cabrera JA, Climent V, Farré J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005;16(3):309–13. https://doi.org/10.1046/j.1540-8167.2005.40759.x.
Martins RP, Hamon D, Césari O, Behaghel A, Behar N, Sellal J-M, et al. Safety and efficacy of a second-generation cryoballoon in the ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2014;11(3):386–93. https://doi.org/10.1016/j.hrthm.2014.01.002.
Osório TG, Coutiño H-E, Brugada P, Chierchia G-B, De Asmundis C. Recent advances in cryoballoon ablation for atrial fibrillation. Expert Rev Med Devices. 2019:1–10. https://doi.org/10.1080/17434440.2019.1653181.
Ng B, Ilsar R, McGuire MA, Singarayar S. Atrial fibrillation resulting from superior vena cava drivers addressed with cryoballoon ablation: late reconnection at the site of phrenic nerve pacing catheter. HeartRhythm Case Rep. 2019;5(1):10–4. https://doi.org/10.1016/j.hrcr.2018.09.010.
Sucu M, Aytemir K, Yorgun H. Inappropriate sinus tachycardia after superior vena cava isolation in addition to pulmonary veins isolation of paroxysmal atrial fibrillation cryoballoon ablation. J Atr Fibrillation. 2015;8(2):14–6. https://doi.org/10.4022/jafib.1270.
Osório TG, Coutiño HE, Iacopino S, Sieira J, Ströker E, Martín-Sierra C, et al. Quantification of acute parasympathetic denervation during cryoballoon ablation by using extracardiac vagal stimulation. J Cardiovasc Med (Hagerstown). 2019;20(3):107–13. https://doi.org/10.2459/JCM.0000000000000760.
Guiot A, SavourÉ A, Godin B, Anselme F. Collateral nervous damages after cryoballoon pulmonary vein isolation. J Cardiovasc Electrophysiol. 2012. https://doi.org/10.1111/j.1540-8167.2011.02219.x.
Osório TG, Paparella G, Stec S, Chierchia GB, de Asmundis C. Cardiac parasympathetic modulation in the setting of radiofrequency ablation for atrial fibrillation. Arch Med Sci (Figure 1). 2019:1–6. https://doi.org/10.5114/aoms.2019.84717.
Su W, Kowal R, Kowalski M, Metzner A, Svinarich JT, Wheelan K, et al. Best practice guide for cryoballoon ablation in atrial fibrillation: the compilation experience of more than 3000 procedures. Heart Rhythm. 2015;12(7):1658–66. https://doi.org/10.1016/j.hrthm.2015.03.021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethical committee of Maria Cecilia Hospital, Cotignola. The protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by the Declaration of Helsinki, protecting the privacy of all participants as well as the confidentiality of their personal information.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iacopino, S., Osório, T.G., Filannino, P. et al. Safety and feasibility of electrical isolation of the superior vena cava in addition to pulmonary vein ablation for paroxysmal atrial fibrillation using the cryoballoon: lessons from a prospective study. J Interv Card Electrophysiol 60, 255–260 (2021). https://doi.org/10.1007/s10840-020-00740-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00740-y