Abstract
Background
Atrial fibrillation recurrence (AFR) is common after pulmonary vein isolation (PVI), and the rate does not differ between radiofrequency (RF) and cryoballoon (CB) ablation. The aim of this study was to assess the impact of the ablation modality used at the index PVI on the outcome after redo PVI in patients with paroxysmal AF.
Methods
In this prospective, single-center, non-randomized study, consecutive patients with paroxysmal AF who have undergone the index PVI with either RF ablation (RF group) or 2nd-generation CB (CB group) were included. The primary endpoint was freedom from recurrence of atrial arrhythmia lasting > 30 s.
Results
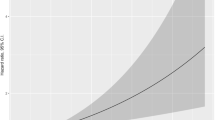
A total of 105 patients undergoing redo PVI for paroxysmal AF were included (median age 61 years; 24% female; left ventricular ejection fraction (LVEF) 57 ± 8%; left atrial volume index (LAVI) 34 ± 11 mm). Index PVI was done either with focal RF (n = 81) or with CB (n = 24) and redo PVI only with focal RF. Total procedure time (139 vs. 113 min, p = 0.10) and RF delivery time (1017 vs. 870 s, p = 0.33) of the redo PVI were not significantly different. After a median follow-up of 371 (185–470) days, there were no differences between the RF and CB groups regarding the AFR rate after the second PVI (24 vs. 23%, p = 0.89). The Kaplan-Meier analysis showed no difference between the groups regarding AFR freedom time (p = 0.81). In multivariable logistic regression, only coronary artery disease was identified as an independent long-term predictor of AFR (OR 4.15, 95% CI 1.17–14.71, p = 0.027).
Conclusions
The ablation modality used at the index PVI has no impact on long-term outcome after redo PVI in patients with paroxysmal AF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pulmonary vein isolation (PVI) by catheter ablation is well established for the treatment of paroxysmal atrial fibrillation (AF) [1]. However, atrial fibrillation recurrence (AFR) is fairly common after the index PVI ablation with rates of 20–50% and a progressive and significant decline in AFR freedom time during long-term follow-up [2,3,4]. The success rate is higher after redo procedures [1, 5]. Focal radiofrequency (RF) and cryoballoon (CB) ablation are the most widely used modalities for PVI in patients with paroxysmal AF [6, 7]. AFR rates after single PVI procedure do not differ between patients treated with focal RF and 2nd-generation CB ablation, regardless of the type of AF [7,8,9]. However, CB ablation is associated with fewer PVs reconnected and more focal reconnection gaps [10,11,12,13,14,15]. Hence, the aim of this study was to investigate the impact of ablation modality at the index PVI on the AFR rate after the redo PVI in patients with paroxysmal AF who have undergone the index PVI with either CB or RF ablation. We hypothesized that patients who underwent index PVI using CB will have lower AFR rate after a redo PVI in comparison with patients who already underwent index PVI using focal RF. Thus, the aim of this study was to assess the success rate and procedural parameters of RF-based redo PVI procedure after focal RF- or CB-based index PVI.
2 Methods
2.1 Study design and population
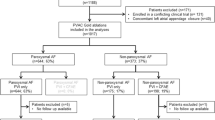
Patients enrolled in the prospective Basel Atrial Fibrillation Pulmonary Vein Isolation (BEAT-AF-PVI) registry at the University Hospital Basel between January 2010 and March 2017, were analyzed. We included in a prospective, non-randomized study all consecutive patients with symptomatic, drug-refractory paroxysmal AF. Consecutive patients with AFR after the index PVI done with either focal (point-by-point) RF ablation (RF group) or 2nd-generation CB (CB group) who underwent the redo PVI procedure using focal (point-by-point) RF were included in the study. Patients with non-paroxysmal AF at index or redo procedure, in whom additional linear lesions (box lesion, mitral isthmus line, left atrium roof line) or complex fractionated atrial electrogram (CFAE) ablation in the left atrium (LA) were performed at any of the two procedures, in whom the redo procedure was not done using focal RF ablation with 3D mapping system as well as those without Holter-ECG during follow-up were excluded from the study. Baseline demographic characteristics, medical history, and chronic medication usage were collected. Transthoracic and transesophageal echocardiogram to rule out LA thrombus and to assess LA diameter, LA volume index (LAVI), and left ventricular ejection fraction (LVEF) were performed before all procedures.
All included patients gave written informed consent for the procedure as well as for participating in the BEAT-AF-PVI registry. The Hospital Ethics Committee approved the study, which was conducted in accordance with the Declaration of Helsinki.
2.2 Index PVI procedure
In the RF group, a focal 3.5-mm open-irrigated tip radiofrequency catheter (Navistar ThermoCool ST or Navistar ThermoCool SmartTouch, Biosense Webster, Diamond Bar, CA, USA) and a 20-pole variable circular mapping catheter (Lasso Nav, Biosense Webster) in combination with a 3D mapping system (CARTO3, Biosense Webster) were used to preform PVI as described in detail previously [6, 7]. No additional lesions were performed. In the CB group, PVI was performed using the 2nd-generation CB (Arctic Front Advance 23 mm or 28 mm, Medtronic Inc., Minneapolis, MN, USA) as described in detail previously [7, 16]. In brief, using a single-freeze approach, the standard freezing duration was 180 s, but could be prolonged to 240 s at the physician’s discretion. PVI was achieved with cryoablation, and no patient received RF ablation because of incomplete PVI at index day with cryoballoon. The procedural endpoint was documentation of PV entrance block using the inner lumen circular catheter (Achieve, Medtronic Inc.). CB was not used in patients with left common PV and/or early branching of right inferior PVs.
2.3 Redo PVI procedure
All redo PVI procedures were performed using focal RF ablation with the 3D mapping system (CARTO3, Biosense Webster) [7]. During the procedure, bipolar voltage mapping was done using a 20-pole variable circular mapping catheter. With a 20-pole variable circular mapping catheter positioned in the PVs, we assessed the number of reconnected PVs as well as the type of the reconnection gaps. The type of the gap was defined as focal if the PV re-isolation was achieved with up to two focal ablations or segmental if > 2 ablations were needed to achieve PV re-isolation. RF energy was delivered with 25 W at the posterior wall and 25 W or 30 W at the anterior wall for the duration of 20–50 s. The procedural endpoint was documentation of PV entrance block using a 20-pole variable circular mapping catheter (Lasso Nav, Biosense Webster). No other additional linear lesions in the LA were performed. However, the cavotricuspid isthmus (CTI) ablation and/or the isolation of the superior vena cava (SVC) could have been done at the physician’s discretion. Mostly the ration was as follows: if there was atrial flutter registered at least once after index PVI with episodes of atrial fibrillation, then, the CTI ablation was done; if there was no PV reconnection noted during redo procedure or there was only one PV reconnected or there were two PVs reconnected, but both had focal reconnection gaps, then, the isolation of the SVC was done.
2.4 Outcome and follow-up
Outcomes were measured based on the recurrence of AF during follow-up, assessing the acute success of redo PVI and the type of PV reconnection, as well as the complete redo procedural data. Episodes of AF or left atrial tachycardia lasting > 30 s occurring after a blanking period of 2 months were considered an AFR. Acute success of PVI was assessed by confirming the PV entrance block at the anatomical ostium of the PVs using a 20-pole variable circular mapping catheter (Lasso Nav, Biosense Webster). Total duration time was defined as time from puncturing the skin until removal of the sheaths. Total RF energy delivery time was defined as the sum of the ablation needed to achieve PV re-isolation. Follow-up was performed at 3, 6, and 12 months after the procedure with 12-lead ECG, 24-h and 7-day Holter-ECG monitoring, and afterwards yearly with telephone interviews and collecting data from patients’ cardiologists.
2.5 Endpoints
The primary endpoint was the success rate of a redo PVI defined as freedom from any documented recurrence of atrial tachyarrhythmia lasting > 30 s at long-term follow-up.
Secondary endpoints included procedural data: acute success of the redo PVI, total procedure time and total RF energy delivery time of the redo PVI, and the number of reconnected PVs at the redo PVI.
2.6 Statistical analysis
Categorical variables are presented as absolute values and percentages. Categorical variables were compared with the chi-square test. Continuous data are expressed as means and standard deviations or median with corresponding interquartile range (IQR). For continuous variables, comparisons were made using Student’s t test, or Mann-Whitney U test, as appropriate. Predictors of AFR were assessed in a univariate and multivariate Cox regression analysis. Univariable and two different multivariable Cox proportional-hazard models using a stepwise forward selection approach were constructed to assess the associations between all variables of interest and the AFR rate. In the “crude” model, we corrected for age, sex, and BMI only. In a more detailed model, we corrected in addition for hypertension, diabetes mellitus, smoking, and coronary artery disease (CAD), as well as for LA diameter in parasternal long axis (PLAX) and LVEF. The Kaplan-Meier analysis with log-rank test was used to compare the probability of freedom of AFR. p values < 0.05 were considered significant. The statistical analysis was done using SPSS Version 20 (IBM SPSS Statistics, New York, USA).
3 Results
A total of 956 patients with AF were included in the BEAT-AF PVI registry between January 2010 and March 2017. After application of inclusion and exclusion criteria, 105 patients with paroxysmal AF undergoing a redo PVI were included in the study. Mean age of the study population was 60 ± 10 years (median 61 years); 24% were female. Patients were divided into two groups according to the ablation modality used at the index PVI procedure: RF group (n = 81) and CB group (n = 24). Median follow-up after the redo PVI was 371 (IQR 185–470) days. There were no significant differences between the study groups regarding baseline characteristics (Table 1).
3.1 Redo procedural data
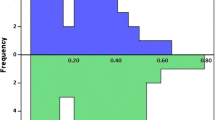
Acute procedural success of redo PVI was achieved in all patients. The total redo procedure time in the RF and CB groups was similar (139 ± 42 vs. 113 ± 31 min; p = 0.10). A total of 225 PVs in 105 patients were reconnected at the redo procedure which corresponds to a mean 2.15 ± 1.1 (median 2, IQR 1–3) PVs per patient. There was no difference between the two groups in the total number of reconnected PVs (RF 2.16 ± 1.1 vs. CB 2.08 ± 1.14 PVs, p = 0.77), in the type of the reconnection gaps (focal vs. segmental), nor in the location of the gaps regarding the specific pulmonary vein (Fig. 1). One, two, three, and four PVs were reconnected in 21, 41, 22, and 14 patients, respectively, without significant differences between the study groups (p = 0.99). Seven patients (7%) were found to have chronically isolated all four PVs at the redo procedure (RF 6.1 vs. CB 8.3% of patients, p = 0.89). The total RF delivery time at the redo procedure did not differ between the two groups (1017 ± 628 vs. 870 ± 554 s, p = 0.33). Additional lesions on the right side were made in 29 patients (27.6%), out of which isolation of the superior vena cava in 12 and cavotricuspid isthmus (CTI) ablation in 17 patients. There were no differences between the study groups regarding additional lesions (SVC isolation 7 vs. 5 patients; p = 0.14; CTI ablation 15 vs. 2 patients, p = 0.35). Also, there was no difference between the groups regarding the RF delivery time when we made a distinction of RF delivery time needed to achieve PV re-isolation (RF 794 ± 508 vs. 741 ± 559 s, p = 0.49) and to achieve additional lesions (202 ± 409 vs. 92 ± 168 s, p = 0.86).
3.2 Outcome and follow-up
During the follow-up, all patients were off antiarrhythmic drugs (AADs). Eighty-nine patients (93%) had a complete 6-month follow-up, and 7 were lost to any follow-up. After a median follow-up of 371 (IQR 185–470) days, 23% (23/98) of the patients experienced another AF recurrence. Overall, the freedom of AF after the redo PVI was 76% in the RF group and 77% in the CB group (p = 0.53). There were no significant differences between the RF and CB groups regarding AFR rate (23.7 vs. 22.7%, p = 0.89). In addition, there was no difference regarding the AFR rate between the patients with and without additional ablation done on the right side during the redo procedure (25 vs. 7 patients, p = 0.80).
There were no significant differences between the patients with and without AFR after a redo procedure, except for hypertension, HAS-BLED, and CHA2DS2-VASc score (Table 2). In univariate Cox regression analysis, hypertension and coronary artery disease (CAD), a CHA2DS2-VASc score of 0, and LA diameter (in PLAX) before the redo procedure were significantly correlated to AFR, but ablation modality used at the index PVI was not (Table 3). In multivariable Cox regression analysis corrected for age, gender, and BMI, CAD was identified as the sole significant predictor of AFR after redo PVI procedure (Table 3). In a more detailed multivariable Cox proportional-hazard model with stepwise approach, corrected in addition for hypertension, diabetes mellitus, smoking, CAD, LA diameter in PLAX, and LVEF, no variable was found to be a significant risk factor for AFR (Table 3). The Kaplan-Meier analysis regarding AFR freedom time showed no significant differences between the RF and CB groups (p = 0.81).
4 Discussion
To the best of our knowledge, this is the first study investigating the impact of the ablation modality used at the index PVI procedure on the long-term success after a redo procedure. The main findings of this study are the following. Ablation modality used at the index PVI had (1) no impact on AFR rate after a redo PVI procedure; (2) no impact on the time of freedom from AFR after a redo PVI procedure; and (3) no impact on the total procedure and RF delivery time of the redo PVI procedure.
The long-term AFR rate after ablation procedures varies from 20 to 50%, meaning a large proportion of AF patients have an indication for a redo procedure [17,18,19]. In line with earlier findings in populations off any AADs, the total AFR rate in our study was 23% after the redo PVI [17,18,19,20]. In previous studies, AFR did not differ between patients treated by focal RF and CB ablation at the index PVI, currently most used ablation techniques [1, 6,7,8]. However, several studies indicated that the use of CB may result in fewer and/or more focal reconnection gaps, which could have an impact on the procedural parameters of the redo PVI [10,11,12,13,14,15]. Our results show that the ablation modality used at the index PVI did not influence the redo procedural data and most importantly did not impact the long-term outcome after the redo PVI. It could be argued that the results would have been different if the modality used at the index PVI was used at a redo procedure. However, nowadays, almost exclusively focal RF in combination with the 3D mapping system is used for redo procedures, which proved to be significantly better in these circumstances [1, 20,21,22].
Additional lesions on the right side (SVC isolation and CTI ablation) had no impact on long-term outcome, which is in line with the previous studies and one meta-analysis [23, 24]. However, the question is whether electrophysiologists only make these lesions to the patients who are, for some reason, considered to be at a higher risk for AFR after a redo procedure or because they have clinical documentation of atrial flutter. Consequently, there could be a bias in these patients that affects the long-term outcome [25].
Hypertension, coronary artery disease (CAD), CHA2DS2-VASc score of 0, and LA diameter were proved to be significantly correlated to AFR, but ablation modality used at the index PVI was not. All of mentioned are well-known risk factors for AF occurrence as well as of AFR after the index PVI and herein after redo PVI [26,27,28,29,30]. However, in a detailed multivariate analysis, no variable was proved to be a significant risk factor in our study population.
So far, there have been no similar studies to address the specific issue of predicting AFR after the redo PVI procedure and the potential impact of ablation modality. Moreover, given that the recurrence rate after the index PVI is fairly high, even in patients with paroxysmal AF, and that the redo procedure is expected to be performed in more and more patients, it may be necessary to focus on the search for risk factors of AFR after a redo procedures. Larger, prospective, clinical studies with different combinations of the ablation modalities and procedural endpoints should be conducted to determine more effective procedural setups in preventing long-term AF recurrence after redo PVI procedure/s.
4.1 Limitations
The results of the present study should be interpreted in the light of several limitations. Firstly, this was a single-center experience performed in a small group of patients with a disproportion of the number of patients in the study groups according to the ablation modality used during index PVI. Secondly, the results refer to a relatively specific group of patients with paroxysmal AF that underwent index PVI with either focal RF or 2nd-generation CB and consequently, conclusions cannot be drawn to a wider population with different types of AF. However, focal RF and 2nd-generation CB are the most used modalities in PVI ablation, and symptomatic patients with paroxysmal AF are the majority of those undergoing PVI procedure. Thirdly, we did not apply stringent procedural endpoints; i.e., more lesions around the PV were allowed even if all PVs were already isolated. Fourthly, despite standardized follow-up including visits with assessing symptoms and 24-h Holter-ECG at 3 and 6 months and a 7-day Holter-ECG at 12 months, the non-continuous nature of monitoring could underestimated AF recurrence.
5 Conclusion
The use of focal radiofrequency ablation or cryoballoon ablation has an influence neither on the AF recurrence rate after the redo PVI in patients with paroxysmal AF, nor on the procedural data of the redo PVI procedure. Moreover, it did not have any influence on redo PVI procedural data. Coronary artery disease was identified as an independent significant long-term risk factor of AFR after the redo PVI procedure.
References
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–78.
Kis Z, Muka T, Franco OH, Bramer WM, De Vries LJ, Kardos A, et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev. 2017;13:199–208.
Sivasambu B, Balouch MA, Zghaib T, Bajwa RJ, Chrispin J, Berger RD, et al. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J Cardiovasc Electrophysiol. 2018;29:239–45.
Takarada K, Overeinder I, de Asmundis C, Stroker E, Mugnai G, de Regibus V, et al. Long-term outcome after second-generation cryoballoon ablation for paroxysmal atrial fibrillation - a 3-years follow-up. J Interv Card Electrophysiol. 2017;49:93–100.
Mujovic N, Marinkovic M, Lenarczyk R, Tilz R, Potpara TS. Catheter ablation of atrial fibrillation: an overview for clinicians. Adv Ther. 2017;34:1897–917.
Reissmann B, Metzner A, Kuck KH. Cryoballoon ablation versus radiofrequency ablation for atrial fibrillation. Trends Cardiovasc Med. 2017;27:271–7.
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–45.
Cardoso R, Mendirichaga R, Fernandes G, Healy C, Lambrakos LK, Viles-Gonzalez JF, et al. Cryoballoon versus radiofrequency catheter ablation in atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2016;27:1151–9.
Knecht S, Sticherling C, von Felten S, Conen D, Schaer B, Ammann P, et al. Long-term comparison of cryoballoon and radiofrequency ablation of paroxysmal atrial fibrillation: a propensity score matched analysis. Int J Cardiol. 2014;176:645–50.
Galand V, Pavin D, Behar N, Auffret V, Feneon D, Behaghel A, et al. Localization of gaps during redo ablations of paroxysmal atrial fibrillation: preferential patterns depending on the choice of cryoballoon ablation or radiofrequency ablation for the initial procedure. Arch Cardiovasc Dis. 2016;109:591–8.
Godin B, Savoure A, Gardey K, Anselme F. Lessons from radiofrequency redo-procedure after cryoballoon pulmonary vein isolation for paroxysmal atrial fibrillation. Circ J. 2013;77:2009–13.
Ciconte G, Velagic V, Mugnai G, Saitoh Y, Irfan G, Hunuk B, et al. Electrophysiological findings following pulmonary vein isolation using radiofrequency catheter guided by contact-force and second-generation cryoballoon: lessons from repeat ablation procedures. Europace. 2016;18:71–7.
Kuck KH, Albenque JP, Chun KJ, Fürnkranz A, Busch M, Elvan A, et al. Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE AND ICE trial. Circ Arrhythm Electrophysiol. 2019;12:e007247.
Glowniak A, Tarkowski A, Fic P, Wojewoda K, Wojcik J, Wysokinski A. Second-generation cryoballoon ablation for recurrent atrial fibrillation after an index procedure with radiofrequency versus cryo: different pulmonary vein reconnection patterns but similar long-term outcome-results of a multicenter analysis. J Cardiovasc Electrophysiol. 2019;30:1005–12.
Aryana A, Singh SM, Mugnai G, de Asmundis C, Kowalski M, Pujara DK, et al. Pulmonary vein reconnection following catheter ablation of atrial fibrillation using the second-generation cryoballoon versus open-irrigated radiofrequency: results of a multicenter analysis. J Interv Card Electrophysiol. 2016;47:341–8.
Knecht S, Kuhne M, Osswald S, Sticherling C. Quantitative assessment of a second-generation cryoballoon ablation catheter with new cooling technology-a perspective on potential implications on outcome. J Interv Card Electrophysiol. 2014;40:17–21.
Duytschaever M, Demolder A, Phlips T, Sarkozy A, El Haddad M, Taghji P, et al. PulmOnary vein isolation With vs. without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39:1429–37.
Duytschaever M, O’Neill M, Martinek M. Increasing the single-procedure success rate of pulmonary vein isolation. Arrhythm Electrophysiol Rev. 2017;6:217–21.
Das M, Wynn GJ, Morgan M, Ronayne C, Waktare JE, Todd DM, et al. Reablated sites of acute reconnection after pulmonary vein isolation do not predict sites of late reconnection at repeat electrophysiology study. J Cardiovasc Electrophysiol. 2016;27:381–9.
De Regibus V, Iacopino S, Abugattas JP, Mugnai G, Moran D, Stroker E, et al. Repeat procedures using the second-generation cryoballoon for recurrence of atrial fibrillation after initial ablation with conventional radiofrequency. J Interv Card Electrophysiol. 2017;49:119–25.
Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016;37:2858–65.
Pokushalov E, Romanov A, Artyomenko S, Baranova V, Losik D, Bairamova S, et al. Cryoballoon versus radiofrequency for pulmonary vein re-isolation after a failed initial ablation procedure in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:274–9.
Mesquita J, Ferreira AM, Cavaco D, Carmo P, Madeira M, Freitas P, et al. Impact of prophylactic cavotricuspid isthmus ablation in atrial fibrillation recurrence after a first pulmonary vein isolation procedure. Int J Cardiol. 2018;259:82–7.
Sharma SP, Sangha RS, Dahal K, Krishnamoorthy P. The role of empiric superior vena cava isolation in atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. 2017;48:61–7.
Li JY, Jiang JB, Zhong GQ, Ke HH, He Y. Comparison of empiric isolation and conventional isolation of superior vena cava in addition to pulmonary vein isolation on the outcome of paroxysmal atrial fibrillation ablation. Int Heart J. 2017;58:500–5.
Sciacqua A, Perticone M, Tripepi G, Tassone EJ, Cimellaro A, Mazzaferro D, et al. CHADS2 and CHA2DS2-VASc scores are independently associated with incident atrial fibrillation: the Catanzaro Atrial Fibrillation Project. Intern Emerg Med. 2015;10:815–21.
D’Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase L, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int J Cardiol. 2013;167:1984–9.
Lin KJ, Cho SI, Tiwari N, Bergman M, Kizer JR, Palma EC, et al. Impact of metabolic syndrome on the risk of atrial fibrillation recurrence after catheter ablation: systematic review and meta-analysis. J Interv Card Electrophysiol. 2014;39:211–23.
Sultan A, Luker J, Andresen D, Kuck KH, Hoffmann E, Brachmann J, et al. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German Ablation Registry. Sci Rep. 2017;7:16678.
Khaykin Y, Oosthuizen R, Zarnett L, Essebag V, Parkash R, Seabrook C, et al. Clinical predictors of arrhythmia recurrences following pulmonary vein antrum isolation for atrial fibrillation: predicting arrhythmia recurrence post-PVAI. J Cardiovasc Electrophysiol. 2011;22:1206–14.
Author information
Authors and Affiliations
Contributions
IZ: Concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article; SK: concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article, statistics; FS: data analysis/interpretation, drafting article, approval of article; TR: data analysis/interpretation, critical revision of article, approval of article; BS: data analysis/interpretation, critical revision of article, approval of article; SO: data analysis/interpretation, critical revision of article, approval of article; CS: concept/design, data analysis/interpretation, critical revision of article, approval of article; MK: concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Hospital Ethics Committee approved the study, which was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeljkovic, I., Knecht, S., Spies, F. et al. Paroxysmal atrial fibrillation recurrence after redo procedure-ablation modality impact. J Interv Card Electrophysiol 57, 77–85 (2020). https://doi.org/10.1007/s10840-019-00694-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-019-00694-w