Abstract
Background
Epicardial radiofrequency catheter ablation is currently considered as the therapeutic option of choice in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) and recurrent ventricular tachycardia (VT). We hypothesised that inducibility of VT may guide the ablation strategy and thereby affect long-term results. Additional epicardial ablation was only performed if VT were still inducible after thorough endocardial ablation.
Methods
The objective was to examine an inducibility-guided ablation approach by comparing the long-term results between endocardial and epi-endocardial radiofrequency catheter ablation of VT in a large cohort of patients with ARVD/C.
Results
We studied from our ARVD/C registry (70 patients in total, 48 males, age 53.2 ± 14.0) 45 patients (64.3% of all patients) who underwent catheter ablation of VT. All patients received endocardial VT ablation. After endocardial ablation, 24 patients (53.3%) remained inducible. Additional epicardial ablation was performed in 22 patients (48.9%). After ablation, non-inducibility was achieved in overall 38 patients (84.4%). During a mean follow-up of 31.1 ± 27.4 months, 13 patients in each group (59.1% after endo- and epicardial ablation, 56.5% after endocardial ablation) remained free from VT recurrence (P = 0.862).
Conclusions
An inducibility-guided catheter ablation strategy of VT in patients with ARVD/C prevents unnecessary epicardial ablation and may therefore be considered as an alternative to primary combined endo- and epicardial ablation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is a degenerative myocardial disease, associated with distinct genetic alterations. The degenerative process includes both the epicardium and the endocardium, with predominance of the epicardial RV inflow and RV outflow. Typical morphological features are RV aneurismal dilatations and dyskinesia ARVD/C. The left ventricle and the atria can also be involved [1–3].
VT and VF may occur well before the evidence of detectable macroscopic morphological changes. As VT and VF frequently provoked by exercise, ARVD/C is among the most important causes of death in athletes [4]. Exclusion from competitive sports and strenuous exercise, medication with beta-blockers, antiarrhythmic drugs and implantation of cardioverters-defibrillators (ICD) can prevent SCD. Appropriate ICD therapy is frequent in patients with ARVD/C and may save many lives. Nevertheless, ICD therapy is associated with increased morbidity and may also impair quality of life [5].
Radiofrequency catheter ablation (RFCA) is regarded as an effective therapy for patients with recurrent appropriate ICD therapy despite antiarrhythmic medication [6]. The optimal ablation strategy is unknown. In some reports, additional epicardial ablation yielded better long-term results during the follow-up than endocardial ablation alone [7–9], whereas other studies found no clear advantage of epicardial ablation [10, 11].
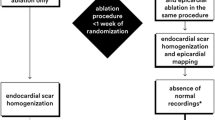
We sought to examine the long-term results of an inducibility-guided ablation strategy in a large cohort of patients with ARVD/C (Fig. 1).
Flow chart of the inducibility-guided ablation approach. VT inducibility was examined by an aggressive stimulation protocol starting with 7 beats 500 milliseconds baseline train and 1 premature extra stimulus, then advancing to maximal 3 premature extra stimuli until refractoriness with a minimum interval of 180 milliseconds. It was done first without and then with orciprenaline beginning with the right ventricular apex and—if necessary—repeated from the right ventricular outflow tract. All patients were ablated from the endocardium. Patients with inducible VT after endocardial ablation and/or likely epicardial exit underwent additional epicardial ablation. Left ventricular ablation was approached, if VT morphology indicated a left ventricular VT exit. Procedure finished if non-inducibility was achieved or if VT were still inducible after thorough endo- and epicardial ablation. VT ventricular tachycardia
2 Material and methods
2.1 Study population
From April 2006 to December 2015, 45 consecutive patients with diagnosed definite ARVD/C—according to the 2010 Revised Task Force Criteria [1]—underwent catheter ablation of VT based on an inducibility-guided strategy as depicted in Fig. 1. Table 1 displays the baseline characteristics.
2.2 Electrophysiology study, mapping and ablation
The study was conducted in agreement with the regulations of the institutional ethic committee. All patients provided written informed consent prior to electrophysiological study and catheter ablation. All patients provided written informed consent for data collection and analysis.
The patients were sedated with propofol, midazolam and fentanyl during the procedures. Twelve-lead ECG electrodes were placed according to standard recommendations [12]. The clinical VT was (were) defined by comparison between 12-lead ECG documentation of the spontaneous clinical and the through electrophysiological study induced VT. In the absence of 12-lead ECG documentation, the clinical VT was assumed as probable if cycle lengths of the VT in the ICD interrogation and during the electrophysiological study were similar.
After femoral vascular access, a quadripolar (Inquiry, Irvine Biomedical Inc., St. Jude Medical, Irvine, California, USA) catheter was placed in the RV apex. This catheter was used for programmed ventricular stimulation. It was also used as a reference for activation mapping in Carto electroanatomical mapping system (Biosense Webster, Inc, Diamond Bar, California, USA). A decapolar catheter (Irvine Biomedical Inc., St. Jude Medical, Irvine, California, USA) was placed in the coronary sinus. In order to induce VT programmed RV apical stimulation (S1: 500, 430, 370, and 330 ms) with up to three premature extra stimuli (S4 only with S1: 500 ms) was done until refractoriness or a minimum interval of 180 ms was reached. The quadripolar catheter was moved from the RV apex into the RV outflow tract if no VT initially was inducible. Then, the entire stimulation protocol was repeated. Orciprenaline was given if still no VT was inducible, aiming a baseline heart frequency above 100 per minute. Then, the stimulation protocol from the RV apex and eventually from the RV outflow tract was repeated. The entire stimulation protocol was repeated after endocardial, after epicardial ablation and at the end of the procedure (Fig. 1).
Three-dimensional electroanatomical mapping with Carto electroanatomical mapping system (Biosense Webster, Inc, Diamond Bar, California, USA) was used all patients. Substrate mapping aimed to define the scar area. Locations with diastolic, fractionated and late electrograms were tagged. Scar was defined as areas with bipolar local electrogram ≤0.5 mV. Normal myocardium was defined as areas with a bipolar local electrogram ≥1.5 mV. Epicardial scar was defined as areas with a bipolar local electrogram ≤1.5 mV. Entrainment-mapping was performed—if possible—to plot the reentry circuit, especially the protected isthmus. Catheter ablation was guided by activation-, substrate-, and pace-mapping. The 3.5-mm irrigated-tip catheter (Thermocool©, Biosense Webster, Diamond Bar, CA, USA) was used for mapping and ablation. A steerable sheath (Agilis©, St. Jude Medical, St. Paul, MN, USA) was employed to facilitate catheter steering for endocardial and epicardial ablation. The standard ablation setting consisted of a pre-selected catheter tip temperature of 43 °C, a power of 30–50 W and a irrigation rate of 30 ml/min. After initial endocardial ablation programmed RV (apical and/or outflow), ventricular stimulation (see above) was done to assess the effect of catheter ablation. Epicardial ablation was only chosen if VT remained inducible after thorough ablation from the endocardium and/or an epicardial exit was likely.
The epicardial approach was obtained through an anterior subxyphoid puncture guided by a 45° left anterior oblique (LAO) fluoroscopic projection as previously described [13].
The desired end point of the ablation procedure was non-inducibility of any monomorphic VT (Fig. 1). Acute complete success was defined as the lack of inducibility of any VT by programmed ventricular stimulation at the end of the procedure. Partial success was defined as non-inducibility of the clinical VT, and ≥1 non-clinical VT remaining inducible at the end of catheter ablation procedure. Unsuccessful catheter ablation was defined as inducibility of clinical VT at the end of the ablation procedure [14–16].
2.3 Follow-up
ICDs were routinely interrogated every 3 months after catheter ablation. The slowest programmed VT detection zone included the slowest documented VT. Data were retrospectively collected from the intra-hospital data system and by telephone inquiries. VT recurrence and appropriateness of ICD interventions were confirmed by experienced electrophysiologists (A.M.; A.A.).
2.4 Statistical analysis
Statistical analysis was performed with SPSS 20.0 (SPSS Inc., Chicago, Il., USA). Descriptive statistics for continuous variables with normal distribution were analysed as mean and standard deviation. For categorical variables, we reported absolute frequencies and percentages. Between-group comparisons were done with the unpaired Student’s t test, the χ 2 test or Fisher’s exact test where appropriate. The time to first VT recurrence plotted according to the Kaplan-Meier method. Probability of VT recurrence based on the time to first arrhythmia recurrence between different groups was determined by the Kaplan-Meier analysis with log-rank test. Multivariate analysis—adjusted for relevant confounders—was performed by Cox regression survival analysis. A value of P < 0.05 was considered statistically significant.
3 Results
3.1 Baseline clinical and demographic characteristics
The ARVD/C registry of the Heart Centre Leipzig currently contains 70 patients with the definite diagnosis ARVD/C (48 men, mean age 53.2 ± 14.0 years). In our ARVD/C registry, 14 patients have been diagnosed with PKP2 mutation. Three patients were DSG2 mutation positive and in one patient, a DSP mutation was found. From our ARVD/C registry, we selected all 45 consecutive patients (30 men, age 53.4 ± 14.2 years) treated by inducibility-guided RFCA strategy for frequent VT. They cumulatively underwent 92 RFCA procedures (range 1–6; 26 epicardial RFCA). All patients had evidence of a monomorphic VT morphology by 12-lead ECG documentation or in the ICD interrogation despite either antiarrhythmic drugs and/or beta-blocker. Table 1 shows the baseline characteristics.
3.2 Radiofrequency catheter ablation
Ninety-two ablation procedures, including 26 with epicardial access and ablation, were done during the study period (median 1.0/patient, mean 2.04/patient). All clinical and inducible non-clinical VTs were targeted for ablation. During electrophysiology study and catheter ablation procedure, 1–6 VTs were inducible in all but two patients (mean 2.2 ± 1.4 VT/patient, clinical VT cycle length 349 ± 73 ms; range 230–500 ms). The two patients with non-inducible VT underwent substrate-based RFCA because of frequent ICD therapy. The total procedure and fluoroscopy times were 181.2 ± 58.1 and 35.9 ± 23.3 min, respectively. Table 1 displays baseline and procedural data. Acute procedural success with ablation of all clinical and non-clinical VTs could be achieved in 38 patients (84.4%). Acute complete procedural success was not achieved in six patients. Three of them underwent endocardial ablation only due to long procedure time. The other three patients without acute complete procedural success underwent endo- and epicardial ablation. Five major complications occurred in 92 ablation procedures (5.4%) including one transient ischemic attack, two acute pericardial effusions and two pulmonary thromboembolisms (one lethal) later during the hospital stay.
3.3 Follow-up and the predictors of freedom from VT
Among 45 patients with previous RFCA between April 2006 and December 2015, 26 patients (57.8%) had no recurrence of VT based on regular ICD interrogations and clinical visits after a mean of 2 ablations procedures per patient (range 1–6) (Fig. 2a). The mean follow-up time was 31.1 ± 27.4 months (range 3–114 months). Univariate analysis revealed acute complete success of catheter ablation, shorter procedure time and younger age were associated with long-term freedom from VT (Table 1). Twenty-five of 38 patients (65.8%) with acute complete success, i.e. non-inducibility of any VT at the end of the procedure, and only 1 out of 7 patients (14.3%) with partial success, i.e. with inducible non-clinical VTs at the end of the procedure, remained free from VT recurrence during the follow-up period.
a Kaplan-Meier curve representing freedom from ventricular tachycardia and implantable cardioverter defibrillator therapies. b Kaplan-Meier curve representing freedom from ventricular tachycardia and appropriate implantable cardioverter defibrillator therapies, depicting results after epicardial and epi-endocardial ablation. The P value was generated using Cox regression survival analysis
Endocardial ablation was performed in 23 of 45 patients (51.1%). Additional epicardial ablation was performed in 22 patients (48.9%). This ablation approach achieved non-inducibility of all VT in 38 of 45 patients (84.4%) at the end of the procedure performed. Left ventricular ablations were performed in 8 patients (17.8%). Left ventricular ablation was approached, if VT morphology indicated a left ventricular VT exit. Left ventricular ablation was not associated with VT free survival, but was more commonly performed in patients with endo- and epicardial access, suggesting a more extensive substrate (Tables 1 and 2).
At last follow-up, 7 patients (15.6%) (4 patients from the group with VT recurrences and 3 patients from the group without VT recurrences) were without beta-blockers and without antiarrhythmic therapy. Twenty-one patients (46.7%) were treated with beta-blockers only. Seventeen patients (37.8%) were treated with class 1 or 3 antiarrhythmic drugs. Four patients (8.9%) were on beta-blockers and class I or III antiarrhythmic drug therapy.
Freedom from VT during the follow-up was achieved in 13 patients (56.5%) with previous endocardial ablation and in 13 patients (59.1%) with previous endo- and epicardial ablation. VT recurred in 10 patients (43.5%) with previous endocardial ablation and in 9 patients (40.9%) with previous epi- and endocardial ablation (P = 0.862).
The inducibility-guided ablation strategy yielded similar long-term freedom from VT after endocardial and after combined epi- and endocardial ablation (Fig. 2b, log-rank 0.550).
4 Discussion
4.1 Major findings
VT free survival was 57.8% during the follow-up period of 31.1 ± 27.4 months (Fig. 2a). Similar success rates have been reported by previous reports.
The inducibility-guided ablation strategy yielded similar long-term freedom from VT after endocardial and after combined epi- and endocardial ablation, thereby preventing unnecessary epicardial access in half of the patients (Fig. 2b).
4.2 Previous studies
Several studies have shown improved long-term VT free survival for epicardial ablation. However, to our knowledge, the ablation strategy has been chosen upon the discretion of the treating physicians and no randomised study has compared endocardial and combined epi-endocardial ablation thus far.
Berruezo and colleagues reported results after endocardial and epicardial mapping and catheter ablation in 11 patients with ARVD/C. The end point of the ablation procedures was scar dechanneling and the abolition of all inducible VTs. Seven of 11 patients additionally received sotalol after catheter ablation procedure. During a median follow-up of 11 months (6–24 months), only 1 (9%) patient had a VT recurrence [9].
Philips et al. studied 87 patients with ARVD/C who underwent 175 RFCA at 80 different electrophysiology centres (1992–2011) with a mean follow-up of 88.3 ± 66 months. Freedom from VT was 47, 21 and 15%, after 1, 5, and 10 years, respectively. However, following epicardial RFCA the cumulative freedom from VT recurrence was 64 and 45% at 1 and 5 years, respectively, which was significantly better than after endocardial-alone RFCA (P = 0.021) [8].
Bai et al. studied 49 ARVD/C patients from seven different centres undergoing catheter ablation of VT. Twenty-three patients underwent endocardial and 26 patients endocardial and epicardial ablation. The majority of the patients from endo- and epicardial ablation group (14 from 26) had failed a previous endocardial ablation approach. After a follow-up of >3 years, freedom from VT was 52.2% (12/23) in the endocardial group and 84.6% (22/26) in the endo- and epicardial ablation group (P = 0.029), with 21.7% (5/23) and 69.2% (18/26) patients off antiarrhythmic medications, respectively (P < 0.001) [7].
Santangeli and colleagues recently published long-term results of 62 patients with ARVD/C referred for VT ablation. Similar to our approach, epicardial ablation was performed when VT recurred or remained inducible after endocardial ablation. Thus, adjuvant epicardial ablation was performed in 39 patients (63%). VT-free survival was 71% during a follow-up of 56 ± 44 months. At the last follow-up, 36% of patients were treated with class 1 or 3 antiarrhythmic drugs, whereas 64% of patients were treated with beta-blockers or completely remained without antiarrhythmic drug therapy. The results of this study support the concept that primary endocardial ablation with adjuvant epicardial ablation only if needed may result in good long-term control of recurrent VT recurrence in patients with ARVD/C [10].
Kumatsu et al. examined whether epicardial substrate ablation from the endocardium was feasible [11]. Forty-six patients with different cardiomyopathies (ischemic cardiomyopathy, non-ischaemic dilated cardiomyopathy, ARVD/C) with sustained VT underwent combined endo- and epicardial mapping. All patients received endocardial ablation, followed by epicardial ablation if needed. Endocardial ablation was able to eliminate epicardial substrate at least partially in 15 patients (83%) with ischemic cardiomyopathy, and 11 patients (73%) with ARVD/C but only in 2 patients (13%) with non-ischaemic dilated cardiomyopathy. The authors concluded that the elimination of epicardial substrate by endocardial ablation was feasible and had maximum benefit in patients with ARVD/C and ischemic cardiomyopathy where the epicardial substrate is located in a thin myocardial wall [11].
To conclude, results of studies comparing endocardial versus endo-epicardial ablation show heterogeneous results. Whereas Bai [7] and Philips [8] and report improved results by epicardial ablation, the results of our study and of Santangeli [10] suggest an inducibility-guided endocardial-first ablation strategy provides reasonable results and spares many patients the added risk of epicardial ablation. More definitive proof requires a randomised controlled clinical trial.
4.3 Limitations
This is a retrospective, observational study with a limited number of patients. Selection bias may have affected the results.
4.4 Conclusion
Endocardial ablation of VT seems to be sufficient to supress VT inducibility in about half of patients with ARVD/C and frequent VT. An inducibility-guided ablation strategy yielded similar long-term results after endocardial and after combined endo- and epicardial ablation. It may therefore be a reasonable strategy to avoid unnecessary epicardial ablation. Randomised, prospective studies are needed to further clarify this issue.
References
Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J. 2010;31:806–14.
Rizzo S, Pilichou K, Thiene G, Basso C. The changing spectrum of arrhythmogenic (right ventricular) cardiomyopathy. Cell Tissue Res. 2012;348:319–23.
Murray B. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C): a review of molecular and clinical literature. J Genet Couns. 2012;21:494–504.
Finocchiaro G, Papadakis M, Robertus JL, Dhutia H, Steriotis AK, Tome M, et al. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J Am Coll Cardiol. 2016;67:2108–15.
Bhonsale A, James CA, Tichnell C, Murray B, Gagarin D, Philips B, et al. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol. 2011;58:1485–96.
Arbelo E, Josephson ME. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2010;21:473–86.
Bai R, Di Biase L, Shivkumar K, Mohanty P, Tung R, Santangeli P, et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011;4:478–85.
Philips B, Madhavan S, James C, Tichnell C, Murray B, Dalal D, et al. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:499–505.
Berruezo A, Fernández-Armenta J, Mont L, Zeljko H, Andreu D, Herczku C, et al. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012;5:111–21.
Santangeli P, Zado ES, Supple GE, Haqqani HM, Garcia FC, Tschabrunn CM, et al. Long-term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8:1413–21.
Komatsu Y, Daly M, Sacher F, Cochet H, Denis A, Derval N, et al. Endocardial ablation to eliminate epicardial arrhythmia substrate in scar-related ventricular tachycardia. J Am Coll Cardiol. 2014;63:1416–26.
Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, et al. Recommendations for the standardization and interpretation of the electrocardiogram: part I: the electrocardiogram and its technology a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2007;49:1109–27.
Sosa E, Scanavacca M, d’Avila A, Oliveira F, Ramires JA. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol. 2000;35:1442–9.
Müssigbrodt A, Dinov B, Bertagnoli L, Sommer P, Richter S, Breithardt OA, et al. Precordial QRS amplitude ratio predicts long-term outcome after catheter ablation of electrical storm due to ventricular tachycardias in patients with arrhythmogenic right ventricular cardiomyopathy. J Electrocardiol. 2015;48:86–92.
Arya A, Eitel C, Bollmann A, Wetzel U, Sommer P, Gaspar T, et al. Catheter ablation of scar-related ventricular tachycardia in patients with electrical storm using remote magnetic catheter navigation. Pacing Clin Electrophysiol. 2010;33:1312–8.
Arya A, Bode K, Piorkowski C, Bollmann A, Sommer P, Gaspar T, et al. Catheter ablation of electrical storm due to monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy: acute results and its effect on long-term survival. Pacing Clin Electrophysiol. 2010;33:1504–9.
Acknowledgements
We thank all patients for providing their information and all physicians that helped us to obtain follow-up data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted in agreement with the regulations of the institutional ethic committee. All patients provided written informed consent prior to electrophysiological study and catheter ablation. All patients provided written informed consent for data collection and analysis.
Funding sources
None
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Andreas Müssigbrodt and Elena Efimova contributed equally and share first authorship.
Rights and permissions
About this article
Cite this article
Müssigbrodt, A., Efimova, E., Knopp, H. et al. Should all patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergo epicardial catheter ablation?. J Interv Card Electrophysiol 48, 193–199 (2017). https://doi.org/10.1007/s10840-016-0209-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-016-0209-3