Abstract
Background
Three-dimensional electroanatomic mapping (EAM) systems reduce radiation exposure when radio frequency catheter ablation (RFCA) procedures are performed by well-trained senior operators. Given the steep learning curve associated with complex RFCA, trainees and their mentors must rely on multiple imaging modalities to maximize safety and success, which might increase procedure and fluoroscopy times. The objective of the present study is to determine if 3-D EAM (CARTO and ESI-NavX) improves procedural outcomes (fluoroscopy time, radio frequency time, procedure duration, complication, and success rates) during CA procedures as compared to fluoroscopically guided conventional mapping alone in an academic teaching hospital.
Methods
We analyzed a total of 1070 consecutive RFCA procedures over an 8-year period for fluoroscopic time stratified by ablation target and mapping system. Multivariate logistic regression and adjusted odds ratios were calculated for each variable.
Results
No statistically significant differences in acute success rates were noted between conventional and 3-D mapping cases [CARTO (p = 0.68) or ESI-NavX (p = 0.20)]. Moreover, complication rates were also not significantly different between CARTO (p = 0.23) and ESI-NavX (p = 0.53) when compared to conventional mapping. Procedure, radio frequency, and fluoroscopy times were significantly longer with CARTO and ESI-NavX versus conventional mapping [fluoroscopy time: CARTO, 28.3 min; ESI, 28.5 min; and conventional, 24.3 min; p < 0.001)].
Conclusions
The use of 3-D EAM systems during teaching cases significantly increases radiation exposure when compared with conventional mapping. These findings suggest a need to develop alternative training strategies that enhance confidence and safety during catheter manipulation and allow for reduced fluoroscopy and procedure times during RFCA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background
Three-dimensional electroanatomic mapping (EAM) is an important tool utilized to perform radio frequency catheter ablation (RFCA). As compared to conventional mapping, EAM has been shown to reduce fluoroscopy time and radiation exposure and improve procedural success [1]. CARTO (Biosense, Diamond Bar, CA, USA) and EnSite NavX (St. Jude Medical, Saint Paul, MN, USA) are the most common mapping systems utilized in clinical practice. These computer-based EAM systems reconstruct and accurately identify the target composition of an intended ablation field [2, 3]. Several investigators have demonstrated that these systems can reduce radiation exposure when RFCA procedures are performed by well-trained senior operators [4–8]. Given the steep learning curve associated with complex RFCA, trainees and their mentors often rely on multiple imaging modalities to maximize patient safety and procedural success. Data demonstrating the superiority of 3-D mapping over conventional techniques for RFCA in a training setting is lacking.
Arrhythmia management has been revolutionized since introduction of RFCA procedures [9]. The combination of high procedural success and low complication rates has made RFCA the treatment of choice for most arrhythmias [10]. Conventional techniques for RFCA entail proper catheter placement guided by continuous electrogram recordings and 2-D fluoroscopic imaging. Consequently, RFCA conveys an increase in ionized radiation (IR) exposure to patients and medical staff. Interventional cardiologists are among the groups with the highest exposure to IR receiving an average dose per procedure of 0.05 mSv [11]. Cardiology trainees are exposed to doses that are significantly higher [12, 13].
We investigated whether or not EAM had an effect on fluoroscopy time and exposure to patients and staff in a large academic medical center setting. We sought to assess the differences in fluoroscopy time, procedure time, acute procedural success, and procedure time among ablation procedures in teaching cases when comparing 3-D mapping systems (CARTO and ESI-NavX) to conventional 2-D fluoroscopy-guided mapping. To our knowledge, this is the largest study to compare EAM to conventional mapping during RFCA exclusively in a teaching hospital setting. While some studies have evaluated this point among experienced electrophysiologists, there is no data exclusively among trainees at teaching hospitals, in which these procedures are routinely performed under direct expert supervision (4–8).
2 Methods
We conducted a retrospective case review of patients admitted to Montefiore Medical Center, Bronx, New York, between January 2005 and March 2013 and underwent RFCA for Wolff-Parkinson-White syndrome (WPW), atrial fibrillation (AF), atrial flutter (AFL), atrial tachycardia (AT), atrioventricular nodal reentry tachycardia (AVNRT), or ventricular tachycardia (VT). The study was approved by the Montefiore Medical Center institutional review board.
2.1 Hospital setting
Montefiore Medical Center is the university hospital of the Albert Einstein College of Medicine. Approximately 80 % of RFCA cases are teaching cases performed by a team consisting of an electrophysiology fellow and one or more senior attending physicians. The decision to use the CARTO or ESI EAM systems was based on physician preference and or equipment availability.
2.2 Study data
Study data was retrieved from the Apollo Advance™ database system and from the electronic medical record system of our institution. One thousand three hundred ninety-eight consecutive catheter ablation cases were reviewed: cases where trainees were participants during the procedure and for which fluoroscopy time, procedure time, radio frequency time, acute success rate, and procedural complications were recorded. Nonteaching cases performed by attending physicians alone were excluded from the analysis.
2.3 Variables
A de-identified dataset was created which included the following variables: age, sex, date, type of mapping (conventional, CARTO, or ESI-NavX), underlying pathology (WPW, AF, AFL, AT, AVNRT, or VT), acute success, complications, fluoroscopy time, radio frequency time, and procedure time. A variable to determine the level of training was created based on the date of the procedure. Training level was divided into 6-month intervals such that procedures performed during the first half of the year could be compared to those performed during the last 6 months.
2.4 Mapping procedures
Conventional mapping was performed using multielectrode catheters to record intracardiac electrograms and 2-D fluoroscopy. Recording of sequential local activation signals was performed using real-time electrocardiography [2]. Activation sequence, entrainment, and pace mapping techniques were utilized when appropriate [14–16]. Fractionated local electrograms and voltage mapping methods were also employed when appropriate [14].
Three-dimensional EAM was performed using either a CARTO or EnSite NavX system. Both systems allow the operator to visualize and manipulate mapping catheters without the exclusive use of fluoroscopic imaging. The CARTO system utilizes a low-level magnetic field (5 × 10−6 to 5 × 10−5 T) delivered from three separate coils in a pad beneath the patient. The magnetic field strength from each coil is detected by a location sensor embedded proximal to the tip of a specialized mapping catheter. This catheter can be moved along a chamber’s surface to record local endocardial activation times for mapping, while simultaneously recording location points to generate 3-D chamber geometry [2, 17]. The EnSite NavX system creates 3-D images of the catheters, based on a low-current electrical field of 350 μA at a frequency of 5.7 kHz, generated by three pairs of nominally orthogonal skin patches in X, Y, and Z axes. The measured voltage and impedance sensed by these catheter electrodes are proportional to the distance of the electrode from the patches, thus allowing 3-D space calculations. The reference may be a surface electrode or an internal fixed electrode such as a coronary sinus catheter electrode. After impedance calibration, the position in space of each electrode can be determined for a wide range of patient body masses (34–115 kg) [3, 7].
2.5 Outcome variables
Study outcome measures are defined as follows:
-
1.
Fluoroscopy time: total duration of fluoroscopy procedure in minutes.
-
2.
Procedure time: time from patient arrival until transport out of the electrophysiology laboratory.
-
3.
Acute success: termination of arrhythmia with failure to induce the clinical arrhythmia following delivery of RF energy.
-
4.
Complications: major complications reported individually during the procedure and prior to patient discharge (MI, CVA, death, tamponade, AV block, retroperitoneal bleeding, or hematoma requiring blood transfusion, AV fistula).
-
5.
Radio frequency application time: total duration of radio frequency energy application employed for each RF ablation procedure.
2.6 Statistical analyses
Descriptive statistics are presented as means and standard deviations (SD) for continuous variables or number of cases (n) and percentages (%) for dichotomous and categorical variables. Univariate analysis was performed on outcome variables: success, complications, fluoroscopy time, procedure time, and radio frequency application time. Dichotomous outcomes were compared with Pearson’s chi-square test, and for continuous variables, Student’s t test was used when comparing two groups and analysis of variance (ANOVA) when comparing three or more groups. We used multivariate regression models to determine independent associations of mapping with outcomes, controlling for age, sex, level of training, and procedure type. For success and complication outcomes, a multivariate logistic regression was performed and adjusted odds ratios (AORs) and 95 % confidence intervals (95 % CIs) are presented. For fluoroscopy and procedure time, multivariate linear regression was performed and adjusted coefficients (mean times) and 95 % CI are presented. A p value of <0.05 was determined to be significant. All statistical procedures were done on SPSS v.21 (IBM, Chicago, IL).
3 Results
We reviewed 1398 consecutive cases, of which 1306 had recorded procedure and fluoroscopy times, and excluded another 233, as these procedures were performed solely by attending physicians, for a total of 1070 cases. Procedural statistics for our sample stratified by arrhythmia type are listed in Table 1. Mean age was 52 ± 19 years old and 59.8 % were male. A similar number of procedures were performed during the first and second levels of training (first half n = 505 vs. second half n = 565). Conventional mapping was most commonly utilized for RFCA of WPW (92.5 %) and AVNRT (78.1 %). Conversely, EAM was most commonly used for VT (94 %) and exclusively used among AF cases (100 %). The overall acute success for all cases in this study was 92 %. AVNRT had the highest acute success rate (96.8 %) and AT the lowest (80 %). Complications were reported in 1.2 % of cases. The highest complication rates were reported for VT cases (4.7 %). Mean procedure time was 270.2 ± 137.9 min, being longest during AF (432.6 ± 97.2) and shortest during AVNRT (191.0 ± 77.0 min). Mean fluoroscopy time was 27.9 min (SD ± 16.1), and mean RF application time was 24.5 min (SD ± 28.5).
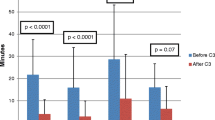
In univariate analysis among the pooled sample (Table 2), acute success rates were better among conventional mapping (conventional 94.2 %; CARTO 88.2 %; ESI 91.1 %; p = 0.016). Conversely, complication rates (CARTO, 1.7 %; ESI, 2.3 %; conventional, 0.4 %; p = 0.037), procedure time (CARTO, 349.8 min; ESI, 314.8 min; conventional, 209.1 min; p < 0.001), fluoroscopy time (CARTO, 30.7 min; ESI, 29.9 min; conventional, 25.4 min; p < 0.001), and RF time (CARTO, 37.8 min; ESI, 34.2 min; conventional, 15.4 min; p < 0.001) were significantly higher in cases where 3-D EAM was utilized. Acute success rate, procedure time, and fluoroscopy time did not differ with level of training. Complications were most common during the first 6 months of training (first half, 2 % vs. second half, 0.5 % p = 0.03 %). When analyzing SVT cases (Table 3) and excluding complex cases (AF, VT), considerably longer procedure time (CARTO, 262.4 min; ESI, 266.1 min; conventional, 206.8 min; p < 0.001) and RF application time (CARTO, 20.9 min; ESI, 23.1 min; conventional, 13.6 min; p < 0.001) were observed when 3-D EAM was utilized. Fluoroscopy time was slightly longer with the use of CARTO EAM (CARTO, 28.8 min; ESI, 26.2 min; conventional, 26.6 min; p < 0.035). Furthermore, significantly longer fluoroscopy time (focal 25.9 min vs re-entry 32.5 min, p = 0.039) and RF application time (focal 15.8 min vs re-entry 44.9 min, p = <0.001) were noted when VT cases were differentiated for the target substrate (focal vs re-entry) (Table 4). Among individual underlying arrhythmias, there was no difference in acute success or complication rates as well as fluoroscopy time by the different mapping types (Figs. 1, 2, and 3). Procedure time was only longer during AT ablation using CARTO and ESI (p < 0.039, Fig. 4), whereas a statistically significant increase in radio frequency time was noted for AFL cases when 3-D EAM was used (Table 5).
Acute success rate by type of mapping stratified by procedure type. Chi-square test was used to determine statistical differences between groups. WPW Wolff-Parkinson-White, AF atrial fibrillation, AFL atrial flutter, AT atrial tachycardia, AVNRT atrioventricular node reentry tachycardia, VT ventricular tachycardia
Complication rate by type of mapping stratified by procedure type. Chi-square test was used to determine statistical differences between groups. WPW Wolff-Parkinson-White, AF atrial fibrillation, AFL atrial flutter, AT atrial tachycardia, AVNRT atrioventricular node reentry tachycardia, VT ventricular tachycardia
Fluoroscopy time by type of mapping stratified by procedure type. Error bars represent 95 % confidence interval of the mean. Analysis of variance (ANOVA) test was used to determine statistical differences between groups. WPW Wolff-Parkinson-White, AF atrial fibrillation, AFL atrial flutter, AT atrial tachycardia, AVNRT atrioventricular node reentry tachycardia, VT ventricular tachycardia
Procedure time by type of mapping stratified by procedure type. Error bars represent 95 % confidence interval of the mean. Analysis of variance (ANOVA) test was used to determine statistical differences between groups. WPW Wolff-Parkinson-White, AF atrial fibrillation, AFL atrial flutter, AT atrial tachycardia, AVNRT atrioventricular node reentry tachycardia, VT ventricular tachycardia
When multivariate logistic regression analysis was performed (Table 6), controlling for age, sex, level of training, and underlying pathology, adjusted odds ratios (AORs) of acute success were not statistically significant for CARTO (p = 0.68) or ESI (p = 0.20) when compared to conventional mapping. Likewise, the difference seen in univariate analysis in terms of complication rates was no longer appreciated (CARTO p = 0.23 or ESI p = 0.25). In multivariate linear regression analysis, procedure time was significantly longer with EAM versus conventional mapping (CARTO, 408.3 min; ESI, 373.7 min; conventional, 268.2 min; p < 0.001). Fluoroscopy time was significantly longer in cases utilizing EAM as compared to conventional mapping (CARTO, 28.3 min; ESI, 28.5 min; conventional, 24.3 min; p < 0.001).
4 Discussion
The use of EAM systems has been widely adopted, as they provide precise three-dimensional tagging for laborious and complex electrophysiological procedures. The use of EAM in less complex procedures can significantly reduce IR dose [6, 8, 18]. Conflicting results regarding procedural outcomes of nonfluoroscopic techniques are documented in the literature. Some authors have described a reduction in fluoroscopy and radiation exposure rates with “near-zero fluoroscopy exposure” or recently “zero fluoroscopy exposure” using both ultrasound and 3-D mapping techniques to perform RFCA of supraventricular tachyarrhythmia [6, 19, 20]. Others have reported significantly higher fluoroscopy and radiation exposure while using EAM during complex procedures such as pulmonary vein isolation in AF [5]. In a meta-analysis of 13 prospective randomized clinical studies involving 1292 patients assessing fluoroscopy time during RFCA of different tachyarrhythmia, only AFL (P < 0.0001) and AVNRT (P = 0.02) demonstrated a statistically significant decrease in fluoroscopy time. This was not observed for RFCA of AF, AT, VT, or AVRT cases. Similarly, no additional benefit was conferred in other study outcomes such as acute success, procedure duration, and complication rates [8].
In a small randomized prospective study that included teaching cases, the use of EAM during RFCA demonstrated a significant reduction in fluoroscopy time when compared to conventional mapping [4]. This study did not exclusively apply to cases performed in a fellowship or teaching setting. EAM does not provide real-time correlation of catheter placement and heart border motion. Although intracardiac echocardiography (ICE) and catheter force contact sensors can provide valuable real-time information, these technologies can be cost prohibitive and should not be used routinely in all RFCA cases [21, 22]. While supervising trainees, fluoroscopic imaging is often critical to assess catheter contact and position in addition to ensure patient safety [23, 24].
To our knowledge, this is the largest study to assess the role of EAM exclusively while training fellows. Our findings demonstrated that EAM use does not reduce exposure to IR and is in contrast to the findings of others in nonacademic settings. EAM was associated with a significant increment in fluoroscopy exposure as compared to procedures where conventional mapping was utilized. However, EAM was often used when a complex case was anticipated possibly accounting for this discrepancy. Higher IR exposure during interventional cardiovascular teaching cases has been described in one large prospective study aiming to evaluate fluoroscopy time (FT) as well as IR exposure in 3400 diagnostic cardiac procedures. The investigators noted significantly higher IR exposure in cases performed by trainees as compared to those performed by experienced senior operators [13]. We considered that this effect might be related to the constant supervision and interaction dictated by the current master/apprentice training model applied by the vast majority of training programs, leading to the recurrent necessity of fluoroscopic correlation for all procedural ablation steps [25]. The repetitive need to assess catheter position, tissue contact, and movement using fluoroscopic imaging results in a significant increase in FT and might explain the unfavorable outcomes noted with the use of EAM in teaching cases reported in our study. This concept was highlighted in a recent study demonstrating the importance of using multiple modalities to assess tissue catheter contact. The combination of manual catheter feedback, catheter tip force sensors, and EAM did not eliminate the need for 2-D fluoroscopic imaging [26]. Integrating EAM and other imaging modalities is technically challenging for trainees and requires development of procedural skills. This was supported by a significant improvement in FT and complication rates throughout the training year.
Routine additional training techniques are warranted to optimize fellows’ training and procedural outcomes in academic teaching settings. Medical training has been continuously evolving, and the use of simulators to enhance technical skills in the cardiac catheterization laboratory has resulted in improved procedural skills and patient safety [27, 28]. Prospective data assessing the clinical usefulness of simulators in the cardiovascular training of novice electrophysiology fellows demonstrated better procedural performance and decreased fluoroscopy times [28, 29].
5 Limitations
This study was conducted in a single center, which limits the generalizability of our results to other training centers. Nonetheless, to our knowledge, this is the largest study comparing RFCA utilizing EAM or conventional mapping techniques in procedures performed exclusively by trainees. Our conclusions do not apply to experienced operators as demonstrated by previous well-designed prospective studies that show improved outcomes with the use of EAM technology. Our study does not include radiation dose, as it was not possible to retrieve data to calculate dose-area product for a substantial percentage of our patients. In addition, the use of intracardiac ultrasound or catheter-tissue contact force technology was not utilized or controlled for in our dataset. Such technologies may be critical for ultimately improving RFCA outcomes and reducing exposure to IR [18, 30]. Data compilation was performed retrospectively, and the absence of randomization could have permitted unknown confounders to influence our results. We could not control for the degree of participation trainees had in each case. Given retrospective nature of the study, we could not control for the degree of participation trainees had in each case, nor for individual case difficulty, as when operators anticipated a more challenging procedure, the tendency was to perform the procedure with EAM assistance leading to a longer procedure and an increase in FT. Finally, these results cannot be applied to RFCA of AF since all cases were performed using EAM and no control group was available for comparison.
6 Conclusion
The use of EAM systems during teaching cases did not significantly reduce fluoroscopy time or improve acute outcomes when compared with conventional mapping. Prospective randomized studies are needed to determine the true effects of EAM during teaching cases. Alternative teaching strategies that enhance confidence and safety during catheter manipulation and allow for reduced fluoroscopy use should be employed. The use of intracardiac ultrasound and force-sensing catheters should help to reduce exposure to ionizing radiation by providing real-time visualization and information that would normally be sought by fluoroscopic imaging. Our findings support the notion that further research and development of specific training techniques are necessary to reduce physician and patient exposure to IR during RFCA.
Abbreviations
- AF:
-
Atrial fibrillation
- AFL:
-
Atrial flutter
- ANOVA:
-
Analysis of variance
- AOR:
-
Adjusted odds ratios
- AVNRT:
-
Atrial tachycardia atrioventricular nodal reentry tachycardia
- AT:
-
Atrial tachycardia
- RFCA:
-
Radio frequency catheter ablation
- IR:
-
Ionized radiation
- WPW:
-
Wolff-Parkinson-White syndrome
- VT:
-
Ventricular tachycardia
References
Packer, D. L. (2005). Three-dimensional mapping in interventional electrophysiology: techniques and technology. Journal of Cardiovascular Electrophysiology, 16(10), 1110–1116.
Bhakta, D., & Miller, J. M. (2008). Principles of electroanatomic mapping. Indian Pacing and Electrophysiology Journal, 8(1), 32–50.
Wittkampf, F. H., Wever, E. F., Derksen, R., Wilde, A. A., Ramanna, H., Hauer, R. N., et al. (1999). LocaLisa: new technique for real-time 3-dimensional localization of regular intracardiac electrodes. Circulation, 99(10), 1312–1317.
Sporton, S. C., Earley, M. J., Nathan, A. W., & Schilling, R. J. (2004). Electroanatomic versus fluoroscopic mapping for catheter ablation procedures: a prospective randomized study. Journal of Cardiovascular Electrophysiology, 15(3), 310–315.
Della Bella, P., Fassini, G., Cireddu, M., Riva, S., Carbucicchio, C., Giraldi, F., et al. (2009). Image integration-guided catheter ablation of atrial fibrillation: a prospective randomized study. Journal of Cardiovascular Electrophysiology, 20(3), 258–265.
Casella, M., Pelargonio, G., Dello Russo, A., Riva, S., Bartoletti, S., Santangeli, P., et al. (2011). “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavX mapping system: personal experience and review of the literature. Journal of Interventional Cardiac Electrophysiology, 31(2), 109–118.
Earley, M. J., Showkathali, R., Alzetani, M., Kistler, P. M., Gupta, D., Abrams, D. J., et al. (2006). Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. European Heart Journal, 27(10), 1223–1229.
Shurrab, M., Laish-Farkash, A., Lashevsky, I., Morriello, F., Singh, S. M., Schilling, R. J., et al. (2013). Three-dimensional localization versus fluoroscopically only guided ablations: a meta-analysis. Scandinavian Cardiovascular Journal, 47(4), 200–209.
Morady, F. (1999). Radio-frequency ablation as treatment for cardiac arrhythmias. New England Journal of Medicine, 340(7), 534–544.
Scheinman, M. M. (1995). NASPE survey on catheter ablation. Pacing and Clinical Electrophysiology, 18(8), 1474–1478.
Katritsis, D., Efstathopoulos, E., Betsou, S., Korovesis, S., Faulkner, K., Panayiotakis, G., et al. (2000). Radiation exposure of patients and coronary arteries in the stent era: a prospective study. Catheterization and Cardiovascular Interventions, 51(3), 259–264.
Rehani, M. M. (2007). Training of interventional cardiologists in radiation protection—the IAEA’s initiatives. International Journal of Cardiology, 114(2), 256–260.
Bernardi, G., Padovani, R., Trianni, A., Morocutti, G., Spedicato, L., Zanuttini, D., et al. (2008). The effect of fellows’ training in invasive cardiology on radiological exposure of patients. Radiation Protection Dosimetry, 128(1), 72–76.
Eckardt, L., & Breithardt, G. (2009). Catheter ablation of ventricular tachycardia. From indication to three-dimensional mapping technology. Herz, 34(3), 187–196.
Coggins, D. L., Lee, R. J., Sweeney, J., Chein, W. W., Van Hare, G., Epstein, L., et al. (1994). Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. Journal of the American College of Cardiology, 23(6), 1333–1341.
Waldo, A. L., Henthorn, R. W., Plumb, V. J., & MacLean, W. A. (1984). Demonstration of the mechanism of transient entrainment and interruption of ventricular tachycardia with rapid atrial pacing. Journal of the American College of Cardiology, 3(2 Pt 1), 422–430.
Gepstein, L., Hayam, G., & Ben-Haim, S. A. (1997). A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation, 95(6), 1611–1622.
Razminia, M., Manankil, M. F., Eryazici, P. L., Arrieta-Garcia, C., Wang, T., D’Silva, O. J., et al. (2012). Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. Journal of Cardiovascular Electrophysiology, 23(10), 1078–1086.
Gellis, L. A., Ceresnak, S. R., Gates, G. J., Nappo, L., & Pass, R. H. (2013). Reducing patient radiation dosage during pediatric SVT ablations using an “ALARA” radiation reduction protocol in the modern fluoroscopic era. Pacing and Clinical Electrophysiology, 36(6), 688–694.
Bulava, A., Hanis, J., & Eisenberger, M. (2015). Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing and Clinical Electrophysiology, 38(7), 797–806.
Stellbrink, C., Siebels, J., Hebe, J., Koschyk, D., Haltern, G., Ziegert, K., et al. (1994). Potential of intracardiac ultrasonography as an adjunct for mapping and ablation. American Heart Journal, 127(4 Pt 2), 1095–1101.
Ullah, W., Hunter, R. J., Baker, V., Dhinoja, M. B., Sporton, S., Earley, M. J., et al. (2014). Target indices for clinical ablation in atrial fibrillation: insights from contact force, electrogram, and biophysical parameter analysis. Circulation. Arrhythmia and Electrophysiology, 7(1), 63–68.
Fisher, J. D., & Krumerman, A. K. (2011). Tamponade detection: did you look at the heart borders (redux)? Pacing and Clinical Electrophysiology, 34(1), 8.
Ferguson, J. D., Helms, A., Mangrum, J. M., Mahapatra, S., Mason, P., Bilchick, K., et al. (2009). Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circulation. Arrhythmia and Electrophysiology, 2(6), 611–619.
Gallagher, A. G., & Cates, C. U. (2004). Virtual reality training for the operating room and cardiac catheterisation laboratory. Lancet, 364(9444), 1538–1540.
Di Biase, L., Paoletti Perini, A., Mohanty, P., Goldenberg, A. S., Grifoni, G., Santangeli, P., et al. (2014). Visual, tactile, and contact force feedback: which one is more important for catheter ablation? Results from an in vitro experimental study. Heart Rhythm, 11(3), 506–513.
Nestel, D., Groom, J., Eikeland-Husebo, S., & O’Donnell, J. M. (2011). Simulation for learning and teaching procedural skills: the state of the science. Simulation in Healthcare, 6(Suppl), S10–13.
De Ponti, R., Marazzi, R., Doni, L. A., Tamborini, C., Ghiringhelli, S., & Salerno-Uriarte, J. A. (2012). Simulator training reduces radiation exposure and improves trainees’ performance in placing electrophysiologic catheters during patient-based procedures. Heart Rhythm, 9(8), 1280–1285.
De Ponti, R., Marazzi, R., Ghiringhelli, S., Salerno-Uriarte, J. A., Calkins, H., & Cheng, A. (2011). Superiority of simulator-based training compared with conventional training methodologies in the performance of transseptal catheterization. Journal of the American College of Cardiology, 58(4), 359–363.
Stabile, G., Solimene, F., Calo, L., Anselmino, M., Castro, A., Pratola, C., et al. (2014). Catheter-tissue contact force for pulmonary veins isolation: a pilot multicentre study on effect on procedure and fluoroscopy time. Europace, 16(3), 335–340.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Andrew Krumerman is consultant for Biosense Inc., Biotronik Inc., and Speak2mdbyphone.com.
Additional information
Jorge Romero and Florentino Lupercio contributed equally to this work.
Rights and permissions
About this article
Cite this article
Romero, J., Lupercio, F., Goodman-Meza, D. et al. Electroanatomic mapping systems (CARTO/EnSite NavX) vs. conventional mapping for ablation procedures in a training program. J Interv Card Electrophysiol 45, 71–80 (2016). https://doi.org/10.1007/s10840-015-0073-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-015-0073-6