Abstract
In this work, nano ZnO powders, Bi2O3, Sb2O3, Cr2O3, Co2O3 and a various content of MnO2 were blended thoroughly and pre-calcined at 800℃ and then pressed in to pellets which were sintered at 950℃ to form varistor ceramics. Subsequently, the effects of MnO2 on the microstructure and electrical properties of the ZnO-based varistor were investigated. It was found that the amount of spinel phase (Zn7Sb2O12) and Bi2O3 phase increased with the MnO2 increasing, while the content of pyrochlore (Zn2Bi3Sb3O14) phase decreased. As a result, the growth of ZnO grain was reduced with the average grain size from 9.5 μm down to 5.3 μm, leading to the increase of breakdown field of ZnO-based varistor. Particularly, the ZnO-based varistor with 1.2 mol% MnO2 exhibited the best comprehensive electrical performance with the breakdown field Eb of 901.4 V/mm, the nonlinear coefficient α of 66.7 and the leakage current density JL of 1.1 µA/cm2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the discovery of the nonlinear voltage-current sensitive effect, many materials have been developed in the manufacture of varistor ceramics, such as ZnO [1], TiO2 [2] and SrTiO3 [3]. Particularly for ZnO-based varistor with high breakdown voltage, nonlinear coefficient and greater surge absorption capacity, it has been widely developed and applied in high voltage electrical field, including ZnO-Bi2O3 [4], ZnO-Pr6O11 [5], ZnO-V2O5 [6] and Zn-Cr2O3 [7] based varistor. Specifically, Bi2O3 and other minor additives usually precipitate at the grain boundaries and form an insulate layer around the n-type ZnO grain after sintering, leading to a high breakdown field [8,9,10]. Nevertheless, with the development of science and technology, the high voltage or ultra-high voltage electrical system requires higher varistor properties, i.e., higher breakdown voltage and nonlinear coefficient. As a matter of fact, the properties of varistors are determined on their microstructures, including the grain size, the grain boundary characteristics and the depletion layer [11]. Therefore, it is necessary to control and optimize the grain size, additive homogeneity and selective dopants. In this case, many measures have been taken to improve the electrical properties of ZnO-based varistor, including optimization of composition and sintering process [12]. As one of typical methods, other than above mentioned Bi2O3, Pr6O11, V2O5 and Cr2O3, transition metal oxides are commonly doped into ZnO varistors to improve electrical properties [13, 14]. Since they have multiple valence states, the transition metal cations with low valence states can be oxidized and lost electrons during sintering, enhancing the chemical absorption of oxygen molecules at the grain boundaries and resulting in the formation of additional interface traps [15]. On the other hand, the transition metal cations with high valence states can be reduced and obtain electrons during sintering. For instance, the effects of manganese oxides with different valence on ZnO varistor have been reported in recent years. Han et al. [16]. doped MnO into ZnO powder and found that the sample had a high nonlinear coefficient, attributed to the fact that the secondary ionization Zn ions at the grain boundaries were oxidized after the addition of MnO. Hong et al. [17]. doped 1/3 mol% Mn3O4 into ZnO and found that Mn doping played an important role in the formation of double Schottky barriers in the microstructure of ZnO. Nahm found that the nonlinear coefficient of the ZnO-V2O5-based varistor were improved with the increasing content of Mn3O4 and the varistor doped with 0.5 mol% Mn3O4 possessed the highest nonlinear coefficient of 22.4 [18]. Nahm also found that MnO2 doping inhibited the growth of ZnO varistor. In addition, the electrical properties and stability of ZnO-V2O5-based varistor were enhanced [19].
In this work, different content of MnO2 was doped into ZnO-Bi2O3-Sb2O3-Cr2O3-Co2O3 varistors via high temperature pre-calcining of the mixed oxide powders at 800℃, combined with low temperature sintering route at 950℃ according to our previous work [20]. Meanwhile, the effects of MnO2 on the microstructure and electrical properties of the ZnO-based varistors were discussed.
2 Experimental
2.1 Preparation procedure of varistors
All the raw materials including nano ZnO (99.99 wt%, 1314-13-2), Bi2O3(99.99 wt%,1304-76-3), Sb2O3 (99.99 wt%, 1309-64-4), Cr2O3 (99.99 wt%, 1333-82-0), Co2O3 (99.99 wt%, 1308 − 389), MnO2 (99.99 wt%, 1313-13-9), were purchased from Chengdu Chron Chemicals Co., Ltd., China and directly used without further purification. In this work, nano ZnO powders are used as the matrix of varistors and the designed mole ratio of compositions of the samples, including a fixed content of Bi2O3, Sb2O3, Cr2O3, Co2O3 and a various content of MnO2, is listed in Table 1. The fabrication route of samples, including mixing of the given powders, pre-calcination, granulation, tableting and sintering, is shown in Fig. 1.
2.2 Characterization of the microstructure and electrical properties
A DX-1000 diffractometer operated at 40 kV/25 mA with Cu Kα radiation is used to identify the phase composition of the variator samples. The Aztec Live ULTIM detector (EDS) is used to perform semi-quantitative analysis of the elements, and the valence states of some elements are verified by AXIS Ultra DLD photoelectron spectrometer (XPS). Meanwhile, a JSM-7500 F scanning electron microscope (SEM) is applied to characterize the morphologies of the samples. The average grain size (d) is calculated by the linear intercept through the equation accordingly [21]:
Where L is the length of a random straight line on the micrograph, M refers to the magnification of the micrograph, and N is the number of grain boundaries intercepted by the lines.
The true density (pt) of sample is measured by the Archimedes method via ET-320 solid densimeter and the relative density (pr) is calculated based on the following equations:
where m0 refers to the mass of the dry specimen in air, m1 is the mass of the same specimen in deionized water, \(\:{\rho\:}_{0}\) refers to the theoretical density of the designed composition, which can be calculated as follows.
where Mi is the molar mass of each component, mi is the molar percentage of each component (i = 1,2…), and ρi is the theoretical density of each component.
The electrical properties, including breakdown fields (Eb), nonlinear coefficient (α) and leakage current density (JL), are gauged at room temperature by the MY-3 kV meter and calculated by the following equation:
Where U1mA is the voltage detected at a current of 1 mA, h is the thickness of the sample.
In which U0.1mA is the voltage detected at a current of 0.1 mA.
Where IL is the leakage current measured at 0.75 U1mA, S is the area of the silver electrode.
3 Results and discussion
3.1 Phase composition of ZnO-based varistors
The XRD patterns of ZnO-based varistors doped with different content of MnO2 are demonstrated in Fig. 2. It can be seen that the main ZnO phase and the secondary phases including Zn7Sb2O12 spinel phase, Bi2O3 phase and a small amount of Zn2Bi3Sb3O14 pyrochlore phase coexist. Nevertheless, it can be found that the spinel phase and Bi2O3 phase increases slowly while pyrochlore phase decreases gradually with the doping content of MnO2 increasing.
According to the binary phase diagram of ZnO-Bi2O3, Bi2O3 would melt and dissolve into ZnO matrix, or react with Sb2O3 and ZnO to form Zn2Sb3Bi3O14 when the temperature is higher than 750℃ [22]. However, the pyrochlore phase would continue to react with ZnO to form Zn7Sb2O12 accompanied by liquid Bi2O3 with the temperature increasing to 950℃ based on the following chemical equation [23]:
Generally, the liquid phase (Bi2O3) would increase the solid dissolution of the other additives in the matrix, accelerate the mass transfer and promote ceramic densification during the sintering process. Specifically, the increase solid dissolution of additives with unequal valence to Zn2+ would improve the charge effects. At the same time, the secondary phases, including Bi2O3, the pyrochlore phase (Zn2Sb3Bi3O14) and spinel phase (Zn7Sb2O12) would precipitate on the grain boundary to form an insulate layer, would reduce the grain size of ZnO grain, as well as enhance the barrier height ф in the ZnO-Bi2O3 based varistor [24], leading to a high breakdown field [25,26,27].
After MnO2 is doped, it would also transform into liquid phase during the pre-calcining and sintering process owing to the low melting point as 535℃. In this situation, liquid MnO2 can promote the liquid phase sintering and accelerate the mass transfer, combined with the active effect of nano ZnO, reaction temperature of Eq. (8) would decrease. Simultaneously, the spinel phase turned to be difficult to react with the Bi2O3 phase to form pyrochlore reversibly during the cooling process after dissolved Cr, Mn and other elements [28]. As a result, the spinel phase and Bi2O3 phase increase with the rising doping of MnO2, by contrast, the pyrochlore phase decreases.
3.2 Morphologies and microstructures of ZnO-based varistors
The SEM micrographs of ZnO varistor doped with different content of MnO2 are illustrated in Fig. 3. As can be seen, the secondary phase particles distributed at the ZnO grains increase, while the grain size of ZnO grains decreases with the doping content of MnO2 increasing from 0 to 1.2 mol%. The average size of ZnO grains with different content of MnO2 calculated from the linear intercept method [21] is shown in Fig. 4. As illustrated, the average size of ZnO grains decreased from 9.5 μm to 5.3 μm with the MnO2 content increasing from 0 to 1.2 mol%. Combined with the XRD analysis in Fig. 2, it can be speculated that the increased secondary phase particles around the main phase ZnO grains, i.e., Zn7Sb2O12 spinel particles, hinder the further growth of the grains. As a result, the smaller ZnO grains are observed in the SEM images with the increase of MnO2 doping in the sample.
Figure 5 displays that the average relative density of all the ZnO varistors doped with different doping content of MnO2 is higher than 99%, consistent with fewer pores being observed in Fig. 4, indicating that all the samples transformed into dense ceramics successfully. Under the synthetic effect of MnO2, Bi2O3 with low melting point, liquid phase sintering plays an important role.
Figure 6 demonstrates EDS mapping of Mn in ZnO varistors doped with 0.9 mol% Mn and Fig. 7 reveals the elemental composition in the grain and on the grain boundary, respectively. Combined the result listed in Table 2, it can be seen that Mn distributes inside the ZnO grains and at the grain boundaries since MnO2 would melt and dissolve into the ZnO grains and Bi-rich liquid phase during the sintering process. The Bi-rich liquid phase containing Mn element will segregate at the grain boundaries of the ZnO grain or in the secondary phase particles during the cooling process, which is the reason why Mn element is detected both inside the ZnO grain and at the grain boundaries [29, 30].
Figure 8 presents The XPS spectrum of Mn element of ZnO varistor doped with 0.9 mol% MnO2, where the peak of Mn 2P3/2 can be fitted by Mn2+ peak at 638.7 eV and Mn3+ peak at 641.1 eV [31], proving that the valence of Mn4+ can be reduced as Mn2+ during the sintering process of ZnO varistor. According to the chemical reaction formula (9) and (10), MnO2 would decompose and release oxygen at different temperature, leading to the decrease of valence of Mn ions [32].
3.3 The electrical properties of ZnO-based varistors
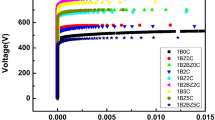
Figure 9 shows the average breakdown field of ZnO varistor doped with different content of MnO2. Obviously, it can be found the average breakdown field of ZnO varistor increases from 349.9 V/mm to 901.4 V/mm with the content of MnO2 increasing from 0 to 1.2 mol%. In theory, the breakdown field of varistor depends on the following two factors [33]: the breakdown voltage of the single grain Ugb and the number of grains per unit thickness N.
From the results of XRD and SEM, the number of secondary phase particles increases, which inhibits the growth of ZnO grains, leading to the decrease of ZnO grain size. Therefore, the breakdown field of varistor increases.
The nonlinear coefficients and leakage current densities of ZnO varistors with different content of MnO2 are shown in Fig. 10. The nonlinear coefficients become higher with the increasing of the content of MnO2. By contrast, the changes of leakage current density are opposite to that of content of MnO2.
Figure 11 illustrates the E-J curves and lnJ-E0.5 curves of ZnO varistors doped with different content of MnO2. According to the following formula [4], the calculated grain boundary barrier height φB, barrier width ω, donor concentration Nd and interface state density Ns of the corresponding sample are listed in Table 3.
As can be seen from Table 3, with the increasing content of MnO2, the grain boundary barrier height φB, the donor concentration Nd and the interface state density Ns increase, but the barrier width ω decreases.
In the process of formation of ceramic, the MnO2 added to the green pallet migrates with the growth of ZnO grains. When the sintering temperature is low, MnO2 diffuses and distributes on the surface of the powder aggregated. With the sintering temperature increasing, the ZnO grains begin to contact, leading to the formation of the “grain-grain boundary-grain”. Therefore, MnO2 would distribute at the grain boundary. At this time, the sintering temperature provides energy for MnO2, which would decompose and diffuse from the grain boundary with higher concentration to the interior of the grain with lower concentration, and react with ZnO, represented by the Kroger-Vink defect symbol as follows:
The reaction can not only provide more electronegative oxygen to increase the interface state density Ns, but also produce positive centers and additional carriers, which would diffuse to the ZnO grain boundary due to its low solubility in ZnO grains in the cooling process of ceramic. After the diffusion of \(\:{\text{Mn}}_{\text{Zn}}^{\bullet\:}\) to the grain boundary, in addition to further increasing Ns, the resistivity of ZnO grains would be effectively suppressed and the impedance difference between grain boundaries be reduced.
In practice, the interface state density NS has the following relationships with the grain boundary barrier height φB and the nonlinear coefficient α [34, 35]:
Therefore, with the interface state density NS increases, the grain boundary barrier height φB increases, enhancing the nonlinear coefficient.
In sum, MnO2 would decompose according to the Eqs. (9), (10) and (17) during the transformation of green pallet to ceramic. Based on the Eq. (20), the O2 generated by the decomposition of MnO2, would absorb electrons in the grain boundary layer and convert them into O2−.
After the electrons in the grain boundary layer are captured, the interface state density NS and the grain boundary barrier height φB increase, thus the nonlinear coefficient α of ZnO varistor is improved significantly.
4 Conclusion
After sintering from the pre-calcined mixture of nanosized ZnO and the corresponding additives, the content of Zn7Sb2O12 phase and Bi2O3 phase in the varistor increases, while the content of pyrochlore Zn2Bi3Sb3O14 phase decreases with the increased content of MnO2. The Zn7Sb2O12 phase particles can inhibit the growth of ZnO grains, making the average size of ZnO grains reduce from 9.5 μm down to 5.3 μm. The ZnO varistor with 1.2 mol% MnO2 obtains the best comprehensive electrical performance. The breakdown field Eb is 901.4 V/mm, the nonlinear coefficient α is 66.7, and the leakage current density JL is 1.1 µA/cm2. In practice, the MnO2 doped in the varistor can release oxygen by decomposition. The interface state density NS in ZnO varistors can be improved by two ways: obtaining electrons from oxygen and releasing oxygen by reaction of MnO2 and ZnO, making the grain boundary barrier height φB increase. As a result, the nonlinear coefficient (α) of ZnO varistor rises up.
Data availability
Data sharing not applicable to this article as all raw date were generated by the authors.
References
T.A. Nogueira, C. de Salles, E.T.W. Neto, F.F. Pinheiro, Short duration current impulse waveform effects on the degradation and energy absorption capability of zinc oxide varistors, Electr. Power Syst. Res. 220, 109336 (2023)
X. Liao, Y. Pu, D. Zhu, Synergistic effect of co-doping of nano-sized ZnO and Nb2O5 on the enhanced nonlinear coefficient of TiO2 varistor with low breakdown voltage. J. Alloys Compd. 886 (2021)
K. Tsuji, W.-T. Chen, H. Guo, X.-M. Chen, T.-K. Lee, W.-H. Lee, C.A. Randall, Valence and electronic trap states of manganese in SrTiO3-based colossal permittivity barrier layer capacitors. RSC Adv. 6(94), 92127–92133 (2016)
L. Zhang, W. Liu, J. Gao, F. Kong, Y. Li, S. Li, Effects of the Er2O3 doping on the microstructure and electrical properties of ZnO-Bi2O3 based varistor ceramics. Ceram. Int. 47(22), 32349–32356 (2021)
W. Cao, X. Xie, Y. Wang, M. Chen, Y. Qiao, P. Wang, Y. Zhang, J. Liu, Effect of Pr6O11 doping on the microstructure and electrical properties of ZnO varistors. Ceram. Int. 45(18), 24777–24783 (2019). Part A)
T.K. Roy, High nonlinearity in 0.1 mol% In2O3 added ZnO–V2O5 based varistors prepared at different sintering temperatures. Ceram. Int. 47(24), 35152–35159 (2021)
T. Tian, L. Zheng, S. Bernik, Z. Man, X. Shi, X. Ruan, G. Li, Influence of Cr2O3 doping on the electrical characteristics of novel ZnO-Cr2O3-based varistor ceramics. Mater. Res. Bull. 159, 112111 (2023)
M. Matsuoka, Nonohmic properties of zinc oxide, ceramics, Jpn. J. Appl. Phys. 10(6), 736– (1971)
T.K. Gupta, Application of zinc-oxide, varistors, J. Am. Ceram. Soc. 73(7), 1817–1840 (1990)
D.R. Clarke, Varistor ceramics. J. Am. Ceram. Soc. 82(3), 485–502 (1999)
C.W. Nan, D.R. Clarke, Effect of variations in grain size and grain boundary barrier heights on the current-voltage characteristics of ZnO varistors. J. Am. Ceram. Soc. 79(12), 3185–3192 (1996)
D. Xu, L.Y. Shi, Z.H. Wu, Q.D. Zhong, X.X. Wu, Microstructure and electrical properties of ZnO-Bi2O3-based varistor ceramics by different sintering processes. J. Eur. Ceram. Soc. 29(9), 1789–1794 (2009)
H.-I. Hsiang, H.-R. Tsai, C. Pithan, Effects of Sr(Co, Nb, Ta)O3 addition on the defect structures and electrical properties of ZnO-based varistors. J. Mater. Chem. C 10(25), 9644–9654 (2022)
N. Daneu, N. Novak Gramc, A. Rečnik, M. Maček Kržmanc, S. Bernik, Shock-sintering of low-voltage ZnO-based varistor ceramics with Bi4Ti3O12 additions. J. Eur. Ceram. Soc. 33(2), 335–344 (2013)
J.J. Cheng, J.M. Wu, Effect of powder characteristics on electrical properties of (Ba,Bi,Nb)-added TiO2 ceramics. Jpn J. Appl. Phys. 35(9a), 4704–4710 (1996)
J.P. Han, A.M.R. Senos, P.Q. Mantas, Deep donors in polycrystalline Mn-doped ZnO. Mater. Chem. Phys. 75(1–3), 117–120 (2002)
Y.W. Hong, J.H. Kim, The electrical properties of Mn3O4-doped ZnO. Ceram. Int. 30(7), 1301–1306 (2004)
C.W. Nahm, The effect of sintering temperature on varistor properties of (Pr, Co, Cr, Y, Al)-doped ZnO ceramics. Mater. Lett. 62(29), 4440–4442 (2008)
C.-W. Nahm, Influence of Mn doping on microstructure and DC-accelerated aging behaviors of ZnO-V2O5-based varistors. Mater. Sci. Eng. B 150(1), 32–37 (2008)
J. Li, S. Yang, Y. Pu, D. Zhu, Effects of pre-calcination and sintering temperature on the microstructure and electrical properties of ZnO-based varistor ceramics. Mater. Sci. Semicond. Process. 123 (2021)
J.C. Wurst, J.A. Nelson, Lineal intercept technique for measuring grain size in two-phase polycrystalline ceramics. J. Am. Ceram. Soc. 55(2), 109–109 (1972)
J. Ott, A. Lorenz, M. Harrer, E.A. Preissner, C. Hesse, A. Feltz, A.H. Whitehead, M. Schreiber, The influence of Bi2O3 and Sb2O3 on the Electrical properties of ZnO-Based varistors. J. Electroceram. 6(2), 135–146 (2001)
E.R. Leite, M.A.L. Nobre, E. Longo, J.A. Varela, Microstructural development of ZnO varistor during reactive liquid phase sintering. J. Mater. Sci. 31(20), 5391–5398 (1996)
W.W. Lin, Z.J. Xu, Z.H. Wang, J.H. Yang, C.X. Zhu, R.Q. Chu, Influence of Bi3Zn2Sb3O14 pre-synthesis phase on electrical properties of the ZnO-Bi2O3 based varistor ceramics. J. Alloy Compd. 834, 6 (2020)
M. Matsuoka, Nonohmic properties of Zinc Oxide ceramics. Jpn. J. Appl. Phys. 10(6), 736–746 (1971)
T.K. Gupta, Application od zinc-oxide varistors. J. Am. Ceram. Soc. 73(7), 1817–1840 (1990)
D.R. Clarke, V. Ceramics, J. Am. Ceram. Soc. 82(3), 8 (1999)
Y.S. Lee, T.Y. Tseng, Effects of spinel phase formation in the calcination process on the characteristics of ZnO-glass varistors. J. Mater. Sci. - Mater. Electron. 8(2), 115–123 (1997)
S.G. Cho, H. Lee, H.S. Kim, Effect of chromium on the phase evolution and microstructure of ZnO doped with bismuth and antimony. J. Mater. Sci. 32(16), 4283–4287 (1997)
H.R. Bai, S.H. Li, Y.H. Zhao, Z.J. Xu, R.Q. Chu, J.G. Hao, C. Chen, H.Y. Li, Y.Y. Gong, G.R. Li, Influence of Cr2O3 on highly nonlinear properties and low leakage current of ZnO-Bi2O3 varistor ceramics, Ceram. Int. 42(9), 10547–10550 (2016)
H. Zhang, R. Xiong, S. Peng, D. Xu, J. Ke, Highly active manganese oxide from Electrolytic Manganese Anode Slime for efficient removal of Antibiotics Induced by Dissociation of Peroxymonosulfate. Nanomaterials. 13(10), 1600 (2023)
I. Barbariol, S. Roitti, An electroconductometric investigation of Mn3O4 and the Mn3O4 -Mn2O3 transformation. J. Mater. Sci. Lett. 3(2), 181–183 (1984)
C.-W. Nahm, Low-temperature sintering effect on varistor properties of ZnO–V2O5–MnO2–Nb2O5–Bi2O3 ceramics. Ceram. Int. 39(2), 2117–2121 (2013)
G.D. Mahan, Fluctuations in Schottky barrier heights. J. Appl. Phys. 55(4), 980–983 (1984)
H.B. Jiang, X. Ren, X.B. Lao, A.T. Kong, M.L. Zhong, Y. Sun, Y.C. Wu, Z. Yao, L.Y. Shi, Effect of NiO doping on grain growth and electrical properties of ZnO-based varistors. J. Eur. Ceram. Soc. 42(9), 3898–3904 (2022)
Funding
This research is supported by the Fundamental Research Funds for the Central Universities of People’s Republic of China.
Author information
Authors and Affiliations
Contributions
Xiaolong Huang: Software; Validation; Investigation; Writing - Review & Editing. Jiaqi Li: Conceptualization; Methodology; Software; Validation; Writing - Original Draft. Guangxu Pan: Software; Validation. Dachuan Zhu: Methodology; Resources; Writing - Review & Editing; Supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Statement regarding Research Involving Human participants and/or animals
Not applicable.
Competing interests
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Li, J., Pan, G. et al. The effects of MnO2 on the microstructure and electrical properties based on ZnO-Bi2O3-Sb2O3-Cr2O3-Co2O3 varistors. J Electroceram (2024). https://doi.org/10.1007/s10832-024-00360-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10832-024-00360-2