Abstract
The electrical resistance of gas sensors, based on polycrystalline metal-oxide semiconductors, obeys a power-law response with the pressure of different gases (R ~ pγ). The exponent γ can be derived resorting to the mass action law and its value depends on chemical reactions that take place at the surface of the grains. To explain the gas sensitivity, we revisit two conceptual models, regularly used in the literature: the ionosorption and the vacancy models. We show that they predict different values for the exponent γ. Also, the consequences of considering the bulk oxygen vacancies as deep levels are analyzed. Comparison of γ values obtained from both conceptual models with those found in experiments can indicate what mechanisms are possible to occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solid-State gas sensors can transduce a gas concentration into an electric signal. Polycrystalline metal-oxide semiconductors (MOXs) are the most common inorganic materials employed in chemical sensing of inflammable and toxic gases, breath analysis in medical diagnosis, and quality control in the chemicals, food, and cosmetics industries [1,2,3,4]. In this type of sensor material, the signal detection comes from the change of resistivity under target gas exposure. The details of the basic mechanisms that cause such a response are still controversial, but there are two widely accepted models to explain the sensor behavior.
The first model, known as the ionorsorption model, suggests that oxygen ionosorbs at the surface of the grains, trapping electrons from the bulk of the grains [5, 6]. This leads to a change in electronic density near the surface, resulting in the formation of an electron depletion region and band bending. Therefore, the film conductivity decreases. For large enough grains, depletion regions do not overlap, creating a quasi-neutral region at the center of the grains. In small grains, depletion regions tend to overlap, resulting in practically flat bands for grains smaller than 10 nm. The second model (referred hereafter as the vacancy model) postulates that changes in oxygen vacancy density at the surface and their ionization, are the determining factor in the chemiresistive behavior [7, 8]. Reduction or oxidation of the surface by ambient oxygen (Mars–van Krevelen mechanism) controls the surface conductivity and therefore the overall sensing behavior [9, 10]. Within these conceptual models, the change in the sensor response in presence of oxygen and reducing gases is regularly described by a set of surface reactions.
It is regularly observed that the electrical resistance (R), or conductance (G ), of the MOX sensor films obeys a power-law response with gas pressure (\(G\propto p^{-\gamma}\)) [11]. Then, the exponent γ is defined as the slope of response against the gas concentration in logarithmic scale as follows:
Equation (1) is regularly expressed as:
where eVs is the band bending. Equation (2) clearly indicates that the sensor sensitivity presents two contributions. One comes from the first factor of the RHS in Eq. (2), G−1dG/Vs, known as the transducer function, which relates the conductivity behavior to the barrier height, eVs. The second factor, pdVs/dp, known as the receptor function, accounts for changes in the barrier height due to the charge transfer process between the adsorbed gas molecules and the semiconductor.
Various metal oxides have been reported as potential gas sensing materials, but tin dioxide (SnO2) is the most used material in practical applications [12]. Tin dioxide is an n-type semiconductor since oxygen vacancies are the dominant defects in the bulk and they behave as donors. Within the ionosorption model, it is regularly considered that adsorbed oxygen on the SnO2 surface can ionosorb (dependent on the working temperature) non-dissociatively as O2− or O22− or dissociatively as O− or O2−. In this model, the negative charge is localized at the surface of the grains, creating a charge depletion region below the surface. This, in turn, forms a potential barrier that prevents electrons from crossing the grain boundary increasing the resistance of the sensor [5,6,7]. Gases, such as CO, react with the adsorbed oxygen reducing its density and the band bending, and, consequently, the electrical resistance of the sensing layer decreases. Conversely, oxidizing gases, such as NO2, can adsorb and trap more electrons increasing the electric resistance. According to the vacancy model, CO removes oxygen from the surface of the lattice producing CO2 and oxygen vacancies that, after ionization, release electrons to the conduction band, and the electrical conductivity increases.
In this work we compute the theoretical values of the power-law exponent γ. These values are derived by applying the mass action law to the surface reactions proposed for both frameworks. The aim is to explain the sensor response under dry air and in the presence of a simple reducing gas [11, 13]. Furthermore, we analyze the consequences of considering the bulk oxygen vacancies as deep levels. Specifically, we are interested in how proposed surface mechanisms determine the parameter γ and the compatibility with experiments. To avoid dealing with band bending and charge transfer through a potential barrier, which controls the electrical conductivity in large grains, we will restrict to grains small enough so that bands can be considered practically flat within the grains.
2 Modeling ionosorption using the mass action law
In this section, and within the ionosorption model, the dependence of conductivity on the partial pressure of the target gases is derived, resorting to the mass action law applied to the surface chemistry regularly used in the literature to describe the receptor function. To keep the models as simple as possible, a one-dimensional approach will be adopted since results are essentially the same. It is generally accepted that oxygen can adsorb on MOX surfaces via a series of consecutive reduction/dissociation steps, non-dissociatively as O2− or O22− and dissociatively as O− or O2− [5, 6, 11]. The superoxide (O2−) species was observed, in SnO2, after oxygen adsorption at T < 150 °C by temperature programmed desorption studies [14]. Oxygen atomic species like O−, extensively used within the ionosorption framework, has not been observed by direct spectroscopic studies [6]. Also, Sopiha et al. [15], based on density-functional theory calculations showed that none of the O− adsorption configurations are stable, and they found that the only stable species is O2−. Accordingly, experimental measurements of the sensor power law response using dry air predicts a γ exponent ¼, which is compatible with O2− ionosorption. In the commonly employed ionosorption theory applied in most papers, the neutral adsorbed oxygen plays no role in gas sensing. Only the ionized species are considered to influence the electrical conductivity. Thus, the oxygen adsorption–desorption process for O2− is alternately presented in the literature as [5, 11]:
A priori, both equations are chemically equivalent and, as we will show below, predict the same value for the exponent, γ=1/4. Indeed, by applying the mass action law to Eq. (3), assuming a low coverage, we obtain:
where σS is the surface charge density, due to O2−, which is practically constant for a small grain. Thus, the electron density n, and consequently the conductance G, is proportional to pO2−1/4. For Eq. (4), the mass action law leads to:
and, the electron density (conductivity), is also found proportional to pO2−1/4.
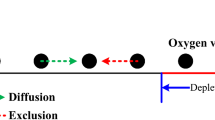
On the other hand, it is important to note that, according to the Wolkenstein theory of chemisorption (WTC), oxygen desorption occurs mainly through neutral oxygen [16]. Figure 1(a) shows the band scheme before oxygen adsorption for a very small grain. Note that the Fermi level lies close to the conduction band and then the conductivity is high. Sensing properties are attributed to intergranular charge after oxygen adsorption that directly affects the electrical conduction. Depending on the relative energy position of its LUMO (lowest unoccupied molecular orbital), EA, with respect to the Fermi level, EF, adsorbed oxygen at a semiconductor surface will be ionized or neutral (Fig. 1(b)). Electrons that ionize adsorbed oxygen come from the semiconductor bulk, producing an electron-depleted surface region, known as the space-charge layer. In the case of very small grains, the depletion regions are fully overlapped, and the bands are practically flat. Thus, the grains present only depleted regions with a very low electron concentration, as illustrated in Fig. 1(b). These electrons at the conduction band are responsible for the electrical conductivity of the film. We can rewrite Eqs. (3, 4) for dissociative chemisorption within the WTC as
and the subsequent ionosorption for doubly ionized oxygen
(a) 1D band scheme for an n-type small grain of width d before oxygen adsorption. EC, EV, EF, and Ed denote the energy of the conduction band minimum, the valence band maximum, the Fermi level, and the donor level. (b) 1D band scheme for an n-type small grain of width d after oxygen adsorption. EA denotes the oxygen acceptor surface site energy (indicated in grey)
The ratio between the density of singly charged and neutral oxygen can be determined with the Fermi–Dirac statistics, i.e.,
Applying the mass action law to Eq. (7, 8) and using Eq. (9), we can write
Noting that EC-EF is equal to (EC-EA) + (EA-EF), see Fig. 1, and that the surface charge is practically constant, this can be rewritten as:
Finally, remembering that
it can be derived that:
This result can be also derived by directly applying the mass action law to Eq. (7, 8)
Combining Eq. (14, 15) we obtain
For a sufficiently small grain, which is fully depleted, most conduction electrons transfer to the surface and then the amount of negative charge at the surface is practically constant. Equation (16) leads to n \(\propto\) pO2−1/4 such as Eq. (13). This analysis is much more direct than that of Eqs. (9–13). However, we included the detailed analysis presented above to emphasize the role of neutral oxygen in determining the response function.
It is worth noting that the oxygen desorption occurs, as proposed in Eqs. (3, 4), through oxygen adions, while in the WTC it occurs only through neutral oxygen. Even though both mechanisms yield the same value for exponent γ, they are fundamentally different. Models based on Eqs. (3) or (4) assume that oxygen at the surface is always ionized. Thus, oxygen desorption rate is related to the surface density of oxygen ions. In contrast, the WTC states that only a fraction of the adsorbed oxygen is ionosorbed, which is determined by the difference between the acceptor level and the Fermi level, and desorption rate directly depends on the surface density of neutral oxygen. Also, it is important to note that Eq. (4) is stoichiometrically correct since half a mole of molecular oxygen adsorbs as a mole of atomic oxygen. However, by applying the mass action law, the adsorption rate would be proportional to the square root of the gas pressure when it should be linearly proportional, as physically expected. On the other hand, according to the RHS of Eq. (6), desorption would be a first order reaction, but two oxygen atoms are needed to form O2(g), which implies a second order reaction.
Other forms of adsorption lead to different values of the exponent γ. Indeed, if oxygen adsorbs non-dissociatively and singly charged, as O2−, γ = 1, and if oxygen adsorbs dissociatively and singly charged, as O−, γ = 1/2. These results can be easily obtained applying the mass action law to equations of the form of Eqs. (3) or (4), or by using the WTC as shown in Ref. 17.
It is important to note that the mass action law states that the rate of a reaction is proportional to the product of the concentrations of each reactant. This is valid for elementary reactions, in which no reaction intermediates are present. In this sense, although questionable, the reactions presented here have been regularly treated as elementary in the gas sensor literature [5,6,7, 11]. This assumes that possible intermediate oxygen species do not play a relevant role in determining the general dependencies we want to find out.
3 The ionosorption framework in the presence of reducing gases
Within the ionosorption framework, models based on Eqs. (3, 4) propose that the key mechanism in sensing a reducing gas is its reaction with ionosorbed oxygen. In contrast, models based on the WTC propose that reducing gases remove neutral oxygen from the surface, which in turn reduces the amount of ionized oxygen. Oxygen can adsorb on SnO2 surface in molecular and atomic forms and subsequently can ionosorb by trapping electrons from the conduction band. It has been proposed that simple reducing gases, such as H2 or CO, react with adsorbed oxygen ions, which leads to the release of H2O or CO2. As a result, electrons return to the conduction band, and the conductivity increases. Thus, the surface concentration of oxygen adopts a new value determined by a steady-state situation in which the oxygen adsorption equals its elimination from the surface that now includes the oxidation of the reducing gas. Following Ref. 11, the chemical processes that occur at the surface for doubly ionized oxygen are described as
where A denotes the reducing gas. We will refer to this as Model 1. Under steady state, the mass action law leads to:
where k1, k-1, and k2 are the reaction rate constants for oxygen adsorption, for oxygen desorption, and for the reaction between the reducing gas and oxygen at the surface, respectively.
The reducing gas is effective when the second term in the RHS is dominant. In this case, it is found that:
Model 1 predicts that the conductivity would increase as pA1/4 and decrease with the oxygen pressure as pO2−1/4, and the simultaneous change in the pressure of oxygen and the reducing gas, keeping the ratio pA/pO2 constant, would not affect the conductivity.
Alternatively, in Ref. [5], the oxygen chemisorption, already presented in Eq. (4), is written as:
then, the mass action law leads to
We will refer to this as Model 2. So, just as we did in dealing with Eq. (19), by assuming that the second term in the RHS is dominant, it is found that:
giving rise to a different dependency of the conductivity with the reducing gas pressure than that obtained in Eq. (20).
To be consistent with the WTC, we should consider that oxygen desorption occurs through neutral oxygen, Eq. (7). Also, the reaction of a reducing gas with oxygen takes place mainly with neutral oxygen. The reaction with a charged oxygen is not favorable since it involves the energy needed to return an electron to the conduction band and then this is negligible. Thus,
We will refer to this as Model 3. Now, we can write:
and then, the mass action law, by assuming that the second term in the RHS is dominant, leads to
Since the ratio between the density of charged and neutral oxygen can be determined with the Fermi–Dirac statistics, Eq. (9), we obtain
Thus, Model 3 predicts that the conductivity would increase as pA1/2 and decrease with the oxygen pressure as pO2−1/2, and the simultaneous change in the pressure of oxygen and the reducing gas, keeping the ratio pA/pO2 constant, would not affect the conductivity [17]. It is important to emphasize that the conductivity in presence of a reducing gas not only depends on the partial pressure of the reducing gas but also on the oxygen partial pressure.
Note that the exponents predicted by the three models Eqs. (20, 23, and 27) are different. We can make a similar analysis for dissociative and non-dissociative singly ionized oxygen adsorption. Results are summarized in Table 1.
Within the ionosorption model, it is regularly considered that an exponent γ = 1/2, corresponds to singly charged ionosorbed oxygen, and γ = 1/4 to doubly charged ionosorbed oxygen. Interestingly, we have shown that, within the WTC, for doubly charged ionosorbed oxygen, under the presence of a reducing gas, leads to γ = 1/2.
It is important to mention that systematic studies carried out by Shimanoe and co-workers [18, 19] show that the conductivity of SnO2, with a small crystallite size, depends on CO pressure as pCO1/2. According to this finding, Model 1 indicates that oxygen adsorbs singly charged, while Models 2 and 3 indicate that oxygen adsorbs doubly charged. Recently, we carried out experiments with a SnO2 film made of very small grains and using H2 as the reducing gas and we found that the conductivity depends on (pA/pO2)γ, with γ = 1/2. This suggests that under the ionosorption model, the WTC, in which oxygen is adsorbed/desorbed neutral and always ionosorbs doubly charged (Model 3), correctly describes the sensor response [17].
4 Oxygen-vacancy framework
So far, we have described the ionosorption framework which assumes that the basic mechanism involved in sensing a reducing gas is its reaction with adsorbed oxygen [20, 21]. For an n-type semiconductor, electrons are transferred from the conduction band to the adsorbed oxygen, and reducing gases, such as CO, react with adsorbed oxygen releasing electrons back to the conduction band. However, some researchers criticize the evidence for ionosorption asserting that there is not enough spectroscopic evidence that support the existence of charge adions and consequently this is just a phenomenological model [6, 7]. Indeed, oxygen vacancies at the surface could be the determining species in the sensing process [6, 7, 22,23,24,25,26]. Basically, reduction and oxidation of the surface by gaseous oxygen through a Mars-van Krevelen mechanism would control the film conductivity and then the sensing behavior. Regularly, it is proposed that CO would remove oxygen from the surface of the lattice to give CO2 leaving an oxygen vacancy at the surface. Since oxygen vacancies behave electrically as donors, they would contribute to increasing the film conductivity [26].
The possible mechanisms have been summarized in an excellent review (Ref. 7), where reactions according to the ionosorption and oxygen-vacancy models are described. In that paper, the oxygen adsorption–desorption process for the oxygen-vacancy equilibrium is described as
where O0x accounts for neutral lattice oxygen. Equation (28) describes the adsorption process in the case of oxygen deficient sites (V+) on the metal oxide surface. Oxygen molecules from the ambient adsorb at oxygen vacant sites by consuming one free electron per atom; this reduces the conductance for n-type materials as well as it reduces the number of active sites for further adsorption. For doubly ionized vacancies, V 2+,
According to Eq. (29), if oxygen pressure decreases the density of vacancies increases. Thus, electrons are released from the surface to the bulk making the film more conductive. Note that, within the ionosorption model, the conductivity is reduced by increasing the difference between the conduction band and the Fermi level, which leads to the formation of intergranular barriers in large enough grains. Whereas, in the vacancy model the surface cannot be negatively charged to generate an appreciable band bending, as vacancies behave as donors.
In Ref. [24], Zemel proposes that surface defect sites are neutral and that the surface becomes negatively charged when vacancies are occupied by oxygen drawn from the gas phase. Thus, the electrostatics arising from healing an oxygen vacancy at the surface would be formally identical to an oxygen ionosorption. In ionic crystals, a Schottky defect forms when oppositely charged ions leave their lattice sites creating oppositely charged vacancies. Thus, even though Zemel does not mention it, it can be understood that tin vacancies are the source for the negative charge required at the surface to build a potential barrier. Another alternative would be to include intrinsic surface states as proposed in Ref. [26]. Anyway, in what follows we would not include any other species than those proposed in Ref. [7], i.e. those present in Eq. (29).
By applying the mass action law to Eq. (29),
We will assume a low surface coverage of oxygen vacancies. Also, due to electroneutrality, the charge due to electrons must be equal to the total charge due to donors, in the bulk and at the surface. Assuming that surface donors are dominant, for doubly charged vacancies, 2n.d = [V2+], where d is the grain size. Thus, as proposed by Zemel, the electron density is found proportional to pO2−1/6. Whereas if single charged vacancies are considered, Eq. (28), the electron density would be proportional to pO2−1/4. Thus, experimental results indicate that oxygen vacancies at the surface should be singly ionized [17].
Within the oxygen vacancy framework, a simple reducing gas A (carbon monoxide, for example) would act as:
assuming doubly ionized vacancies [7]. Similarly, in Ref. [27], this process is presented in two steps, one for the vacancy formation and the other one for the vacancy charging/discharging. Under steady state, the mass action law leads to:
The reducing gas is effective when the second term in the RHS is dominant. In this case, and considering a low coverage of vacancies, it can be found that:
We can make a similar analysis for singly ionized oxygen vacancies. In this case, the electron density becomes proportional to (pA/pO2)1/4. These results are summarized in Table 2. Thus, in any case, the oxygen vacancy model does not lead to the experimental results, i.e. (pA/pO2)1/2 [17].
5 Considering bulk oxygen vacancies as deep levels
Defects at metal oxide semiconductor have been a controversial issue for decades. Since years ago, researchers in the field of sensors have usually assumed that the dominant donor levels in bulk MOXs are shallow and due to oxygen vacancies [28,29,30]. However, these assumptions have been questioned [31, 32]. In Ref. [33], Kilic and Zunger conclude that the formation energy of surface vacancies, specifically calculated for In2O3, is much lower than that of their bulk counterparts, and that surface vacancies create states which lie considerably higher in energy than the respective states of the oxygen vacancy in the bulk. Moreover, bulk donor levels of oxygen vacancies are very deep. This idea has been incorporated in the field of gas sensors by Kozhushner and coworkers [34].
If oxygen vacancies behave as shallow donors, then, most of them would be regularly ionized. Conversely, regularly deep donors are mostly nonionized [34]. If vacancies behave as single donors, the density of ionized donors would be:
and the density of electrons is giving in Eq. (12):
For a band structure as shown in Fig. 2, in which the bulk donor level is below the Fermi level several kT’s, the density of singly ionized donors, see Eq. (34), becomes:
1D band scheme for a small n-type grain of width d for deep bulk donors. EC, EV, EF, and Ed denote the energy of the conduction band minimum, the valence band maximum, the donor level, and the Fermi level, respectively. EA and Eds are possible acceptor and donor levels at the surface indicated in grey
If the surface is not charged, without ionized surface acceptors of donors, then the density of electrons must be equal to the density of ionized bulk donors to guarantee electroneutrality. With Eqs. (35, 36) and considering that EF-Ed = (EC-Ed)-(EC-EF):
Thus, the bulk conductivity would be determined not only by the density of bulk oxygen vacancies, but it would also be strongly affected by their level energy in the gap, Ed. We can now incorporate the influence of states at the surface within the ionosorption or the vacancy frameworks considering bulk oxygen vacancies as deep levels.
5.1 The ionosorption framework with bulk oxygen vacancies as deep levels
Let’s check first the consequences of applying the mass action law within the ionosorption model by including the presence of acceptor levels at EA. If oxygen adsorbs doubly charge, following the surface reaction described by Eq. (17) presented in Sect. 3, Model 1, we can write:
By applying the mass action law, assuming a low coverage:
Charge at the bulk is due to ionized oxygen vacancies, which now depends on the Fermi level position as shown in Eq. (36). As the Fermi level drops, n decreases as Nd+ increases, becoming the dominant charge at the bulk. For a 1D model and singly charged bulk vacancies, the electroneutrality reduces to σs \(\propto\) dNd+ where d is the grain size. In Fig. 2 we see that EF-Ed = (EC-Ed)-(EC-EF). Thus, having Eqs. (36, 39) in mind, electroneutrality leads to:
Interestingly, if bulk vacancies are considered doubly charged, the electron density becomes proportional to pO2−1/8. A similar analysis can be done using the WTC (referred as Model 3 in Sect. 3). Obtained exponents are summarized in Table 3.
The effect of reducing gases, following the surface chemistry adopted in Model 1, can be described as:
When the action of the reducing gas is dominant, under steady state, the mass action law leads to:
Now, by applying the electroneutrality condition:
Remembering that EF-Ed = (EC-Ed)-(EC-EF), we can write:
A similar analysis can be done using the WTC. Results are summarized in Table 4.
5.2 Oxygen-vacancy framework with bulk oxygen vacancies as deep levels
Let’s analyze next the consequences of having shallow surface donors due to surface vacancies, as indicated in Fig. 2 as Eds. This was described in Eq. (29):
By applying the mass action law, as done before:
If the positive charge generated by surface vacancies is dominant respect to bulk vacancies, electroneutrality implies 2d.n = [V2+] and then with Eq. (46) we can write:
If vacancies are singly charged, the electron density is proportional to p−1/4.
Finally, a simple reducing gas as CO would act as proposed in Eq. (41):
Under steady state, the mass action law leads to:
The reducing gas is effective when in the RHS the second term is dominant. In this case, and considering a low coverage of vacancies, it can be found that:
This result is equal to that of Eq. (33) because the same surface reactions are dominant, as described in Eqs. (32) and (49). For singly charged vacancies the exponent for the conductivity dependence on oxygen and the reducing gas pressures becomes ¼.
6 Conclusions
The electrical conductivity of a gas sensor film, based on a polycrystalline metal-oxide semiconductor such as SnO2, has been found to present a power-law dependence on the pressure of different gases. This dependence has been regularly derived resorting to the mass action law applied to possible reactions at the surface together with semiconductor Physics. In this work, we calculated the power-law exponents predicted by widely accepted frameworks, regularly used in the literature, to explain the gas sensitivity: the ionosorption and the vacancy frameworks. The consequences of considering the bulk oxygen vacancies as deep levels are also analyzed.
We found that none of the predicted exponents derived within the vacancy framework is compatible with the experimental results. The predicted exponents by considering bulk oxygen vacancies as deep donor levels agree with those found in experiments for an atmosphere with oxygen and a reducing gas (if adsorbed oxygen and bulk oxygen vacancies can be singly ionized). However, the predicted dependence on the oxygen pressure, when this is the only gas present, does not match experiments. Within the ionosorption framework, resorting to the regular reactions proposed in the literature, a similar incompatibility with experiments arises. Conversely, all experimental findings are reproduced by applying the WTC when oxygen vacancies are doubly ionized shallow levels and oxygen ionosorbs doubly ionized.
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
References
D.R. Miller, S.A. Akbar, Sens. Actuators B: Chem. 204, 250–272 (2014)
I.D. Kim, A. Rothschild, H.L. Tuller, Acta Mater. 61, 974–1000 (2013)
W.T. Moon, Y.K. Jun, H.S. Kim, W.S. Kim, S.H. Hong, J. Electroceram. 23, 196–199 (2009)
S. Mahajan, S. Jagtap, Appl. Mater. Today. 18, 100483 (2020)
N. Barsan, U. Weimar, J. Electroceram. 7, 143–167 (2001)
A. Gurlo, Chem. Phys. Chem. 7, 2041–2052 (2006)
A. Gurlo, R. Riedel, Angew. Chem. Int. Ed. 46, 3826–3848 (2007)
S. Kucharski, P. Ferrer, F. Venturini, G. Held, A.S. Walton, C. Byrne, J.A. Covington, S.K. Ayyala, A.Q.M. Beale, C. Blackman, Chem. Sci. 13, 6089–6097 (2022)
P. Mars, D.W. van Krevelen, Chem. Eng. Sci. 3, 41–59 (1954)
C. Doornkamp, V. Ponec, J. Mol. Catal. A: Chem. 162, 19–32 (2000)
N. Yamazoe, K. Shimanoe, Sens. Actuators B: Chem. 128, 566–573 (2008)
G. Eranna, B.C. Joshi, D.P. Runthala, R.P. Gupta, Crit. Rev. Solid State Mater. Sci. 29, 111–188 (2004)
N. Yamazoe, K. Suematsu, K. Shimanoe, Sens. Actuators B: Chem. 176, 443–452 (2013)
N. Yamazoe, J. Fuchigami, M. Kishikawa, T. Seiyama, Surf. Sci. 86, 335–344 (1979)
K.V. Sopiha, O.I. Maliyi, C. Persson, P. Wu Appl. Mater. Interfaces 13, 33664–33676 (2021)
T. Wolkenstein, Electronic Processes on Semiconductor Surfaces during Chemisorption (Consultants Bureau, New York, 1991)
P.M. Desimone, F. Schipani, R. Procaccini, D.A. Mirabella, C.M. Aldao, Sens. Actuators B: Chem. 370, 132387 (2022)
N. Yamazoe, K. Suematsu, K. Shimanoe, Sens. Actuators B: Chem. 163, 128–135 (2012)
N. Ma, K. Suematsu, M. Yuasa, T. Kida, K. Shimanoe, A.C.S. Appl, Mater. Interfaces 7, 5863–5869 (2015)
P. Shankar, J.B.B. Rayappan, Sci. Lett. J. 2015 4, 126 (2014)
M. Habgood, N. Harrison, Sur. Sci. 602(5), 1072–1079 (2008)
C. Blackman, ACS Sensors 6, 3509–3516 (2021)
M. Eslamian, A. Salehi, E. Nadimi, Surf. Sci. 708, 121817 (2021)
J.N. Zemel, Thin Solid Films 163, 189–202 (1988)
S. Kucharski, C. Blackman, Chemosensors 9, 270 (2021)
J. Ding, T.J. McAvoy, R.E. Cavicchi, S. Semancik, Sens. Actuators B: Chem. 77, 597–613 (2001)
L. Zhao, X. Gong, W. Tao, T. Wang, P. Sun, F. Liu, X. Liang, F. Liu, Y. Wang, G. Lu, ACS Sensors 7, 1095–1104 (2022)
S. Samson, C.G. Fonstad, J. Appl. Phys. 44, 4618–4621 (1973)
J. Maier, W. Göpel, J. Solid State Chem. 72, 293–302 (1988)
K.G. Godinho, A. Walsh, G.W. Watson, J. Phys. Chem. C 113, 439–448 (2009)
A.K. Singh, A. Janotti, M. Scheffler, C.G. Van de Walle, Phys. Rev. Lett. 101, 055502 (2008)
S. Lany, A. Zakutayev, T.O. Mason, J.F. Wager, K.R. Poeppelmeier, J.D. Perkins, Phys. Rev. Lett. 108, 016802 (2012)
C. Kilic, A. Zunger, Phys. Rev. Lett. 88, 095501 (2002)
M.A. Kozhushner, L.I. Trakhtenberg, V.L. Bodneva, T.V. Belisheva, A.C. Landerville, I.I. Oleynik, J. Phys. Chem. C 118, 11440–11444 (2014)
Acknowledgements
This work was partially supported by the National Council for Scientific and Technical Research (CONICET) of Argentina, and the National University of Mar del Plata (Argentina). C.M.A. acknowledges invaluable discussions with Professor Chris Blackman.
Funding
This work was partially supported by the National Council for Scientific and Technical Research (CONICET) of Argentina and the National University of Mar del Plata (Argentina).
Author information
Authors and Affiliations
Contributions
Conceptualization, D.A. Mirabella, P.M. Desimone, and C.M. Aldao; investigation, D.A. Mirabella, P.M. Desimone, and C.M. Aldao, writing original draft preparation D.A. Mirabella and C.M. Aldao; writing review and editing, D.A. Mirabella, P.M. Desimone, and C.M. Aldao; funding acquisition, C.M. Aldao. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflic of interest
The authors declare they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirabella, D.A., Desimone, P.M. & Aldao, C.M. On the mass action law and the power law response in tin dioxide gas sensors. J Electroceram (2024). https://doi.org/10.1007/s10832-024-00351-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10832-024-00351-3