Abstract
The main objective of our work is to increase transmittance in the mid infrared region by removing impurities through the pre-heating treatment of zinc sulfide (ZnS) produced by hydrothermal synthesis. The pre-heating treatment proceeded at 450 to 600 °C for 2 h under vacuum atmosphere (10−2 Torr). It was confirmed that the particle size increased as the pre-heating temperature increased. Additionally, all ZnS nano powders had a sphalerite (cubic) structure unaffected by pre-heating treatment. The ZnS nano powders were sintered by hot-press sintering method. As the pre-heating temperature increased, transmittance was improved due to the decreasing of porosity, increase of particle size, and the removal of impurities (carbon and sulfate). However, when the pre-heating treatment temperature was 600 °C, the transmittance slightly decreased due to the formation of a hexagonal phase. The ZnS ceramic with pre-heating treatment at 550 °C showed the highest transmittance (71.6%) and density (99.9%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Zinc sulfide (ZnS), a semiconductor compound, has a wide range of applications such as in light emitting diodes, luminescent devices, photocatalysts, and infrared (IR) windows, based on its unique optical and chemical applicability [1,2,3]. In particular, ZnS has high transmittance in the IR wavelength and moderate hardness compared to chalcogenide glasses which are mainly used for optical application [4, 5]. Out of the optical applications, IR window technologies have been spreading, not only in the more obvious area of military usage, but also in the private sector such as for use in general security, crime monitoring, industrial processes, environmental monitoring, and medical care [5, 6]. However, IR window technology is not efficient enough for practical use, despite the fact that considerable effort has been directed toward IR applications. This is because most commercially available IR windows are very expensive due to their complex production process. Therefore, it is desirable to mass-produce IR windows having high performance at low cost.
It is known that an IR window is normally single phase, high density, and with low defect due to the low scattering of light [7, 8]. For this reason, when ZnS is used for IR windows, the treatment of pressurization and high temperature is necessary. However, ZnS is known to occur at phase transition from a sphalerite (cubic) structure to a wurtzite (hexagonal) structure above 1020 °C [9,10,11]. Also, the temperature of the sublimation point of ZnS is 1180 °C [12]. For this reason, to maintain good optical properties of ZnS ceramics, a pre-heating treatment and a sintering process simultaneously require high density and low defect, capable of suppressing phase transition and decomposition.
Due to such difficulties, ZnS ceramics having good optical properties are prepared by methods such as chemical vapor deposition (CVD) [13]. Most ZnS ceramics fabricated by CVD exhibit high transmittance. However CVD has the disadvantage of being very expensive, complex, and time-consuming [13]. Hot-press (HP) sintering on the other hand is simple, and can make a sintered body of high density [14]. Compared to CVD, HP sintering is suitable for mass production because it is a low cost manufacturing process and generates less pollution [14]. For this reason, research on HP sintering is actively being conducted. [15] However, one problem of HP sintering is that particle sizes are small due to the influence of raw materials, and formation of a single phase is difficult [13]. Therefore, the raw materials (nano powders) used become very important because the shape and characteristics of nano powders are related with the characteristics of ceramics sintered by HP sintering. Since it is important to control the microstructure and to form a single phase, studies on the synthesis of ZnS nano powders are actively conducted [13].
ZnS nano powders for sintering can be synthesized by various methods such as precipitation, spray pyrolysis method, colloidal processing method, and hydrothermal synthesis [2, 15]. Hydrothermal synthesis can control particle sizes, phase homogeneity, morphology, and it has a fast reaction rate. However, it is known that when ZnS nano powders are synthesized by hydrothermal synthesis, impurities adversely affect optical properties [15]. The impurities are removed through the pre-heating treatment, but it is necessary to find a suitable condition of the pre-heating treatment because inappropriate pre-heating treatment is a problem in the formation of other impurities such as zinc oxide as well as the hexagonal phase.
In this study, ZnS nano powders were prepared by hydrothermal synthesis, and a pre-heating treatment was performed to remove impurities. The pre-heating treated ZnS nano powders were HP sintered to produce ZnS ceramics with high density and transmittance. We also tried to confirm the change of optical characteristics according to the temperature of the pre-heating treatment.
2 Experimental
2.1 ZnS nano powders synthesis and the pre-heating treatment
ZnSO4 ∙ 7H2O (High Purity Chemicals, 99.9%) and Na2S ∙ 9H2O (Sigma-Aldrich, 99.99%) were used as starting materials for hydrothermal synthesis of the ZnS nano powders. ZnSO4 ∙ 7H2O and Na2S ∙ 9H2O were dissolved separately in 160.9 ml distilled water while being stirred at 85 °C for 30 min using a hotplate stirrer. The Na2S solution was poured into the ZnSO4 solution and mixed by stirring at 85 °C for 1 h. This mixture was transferred to a 500 ml teflon-lined stainless steel autoclave, which was synthesized at 180 °C for 70 h in a forced convection oven. After the reaction was completed, the mixture was removed from the autoclave and cooled to room temperature in air. The synthesized white precipitate was then washed using distilled water and filter paper to remove impurities, and dried at 100 °C for 3 h in an oven. The chemical reaction of the hydrothermal synthesis process described is as follows [16]:
The dried ZnS nano powders were then pre-heating treated using an alumina crucible to remove impurities. The pre-heating treatment conditions were performed under vacuum (about 10−2 Torr) at 450, 500, 550, and 600 °C for 2 h. The pre-heating treated ZnS nano powders were analyzed to confirm the change of characteristics according to the pre-heating treatment temperature by scanning electron microscopy (SEM, JEOL JSM-7610F), X-ray diffraction (XRD, RIGAKU DMAX 2500), and Fourier transform infrared spectroscopy (FT-IR, JASCO FT/IR 4100).
2.2 HP sintering of ZnS nano powders
The pre-heating treated ZnS nano powders were sintered by HP under vacuum (about 10−2 Torr). Additionally, a 12.5 mm silicon carbide mold was used for sintering ZnS nano powders. The HP sintering was performed at 950 °C for 2 h under 30 MPa, and the heating rate was 10 °C/min. To prevent breakage of the ZnS ceramics, the pressure was removed immediately after the holding time. The ZnS ceramics were investigated to confirm structural characteristics, density, and optical properties by SEM, XRD, and FT–IR.
3 Results and discussion
3.1 ZnS nano powders
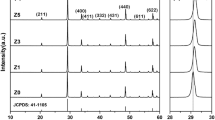
Figure 1 shows the XRD patterns of the ZnS nano powders prepared through hydrothermal synthesis and the pre-heating treatment. In order to use ZnS ceramics as an optical material, it is necessary to secure a cubic single phase [11]. The observed peaks show diffraction peaks at 2Ɵ values of 28.6°, 33.1°, 47.6°, 56.4°, and 59.0°, corresponding to the diffraction planes (111), (200), (220), (311), and (222), respectively. All of the peaks were perfectly indexed to the cubic phase of ZnS. In order to clarify the effect of the pre-heating treatment temperature on the ZnS nano powders, the microstructure of the pre-heating treated ZnS nano powders was confirmed by microstructure images. Figure 2 shows the microstructure images of the non pre-heating treated ZnS nano powders and pre-heating treated ZnS nano powders. Microstructure images show that the particles were found to be mainly plate shaped with significant differences in particles size. It can be seen that it is mainly in the cubic phase when we see the plate form [17]. As shown in Fig. 2(a), the particle size of the ZnS nano powders prepared through hydrothermal synthesis is 50 ~ 100 nm. Figure 2(b–e) shows the growth of particle size with the increase of pre-heating temperature. It can be seen that the nano-sized and micron-sized particles are mixed during the pre-heating treatment up to 500 °C. When the pre-heating temperature was 600 °C, the particle size of the ZnS nano powders increased to 1 μm.
FT–IR analysis was performed to confirm whether the impurities were removed by the pre-heating treatment. Figure 3 shows the FT–IR spectra of the ZnS nano powders before and after the pre-heating treatment. The ZnS nano powders contain several absorption peaks between 500 and 4000 cm−1. The broad bands at 3420 cm−1 and 1630 cm−1 represent the presence of water [17, 18]. The bands are attributed to the O-H stretching vibration and H-O-H bending mode, respectively [18]. The absorption band in the range of 400–900 cm−1 is characteristic of Zn-O vibration [19]. The absorption bands at 1200 and 1400 cm−1 indicate the S-O vibration band [20]. Additional absorption bands observed at 2926, 2856, 2363 cm−1 are due to Zn-S microstructure formation of the ZnS nano powders [21]. The characteristic bands of water, Zn-O, and S-O of the ZnS nano powders after the pre-heating treatment are reduced. It can be confirmed that the temperature of the pre-heating treatment affects the impurities in the ZnS nano powders [22]. However, the impurities in the ZnS nano powders are not completely removed. Since the purity of the ZnS nano powders is closely related to the optical properties, a systematic study on ZnS nano powder refinement is needed in future work.
3.2 ZnS ceramics
The ZnS ceramics were sintered by HP sintering to investigate the influence of microstructure and impurity changes on the characteristics due to the pre-heating treatment of ZnS nano powders.
The ZnS ceramics with different pre-heating treatment exhibit different colorations in Fig. 4. Coloration in transparent ZnS ceramics is often mentioned in literature, and is a major obstacle to achieving excellent optical properties [22]. The gray color of ZnS ceramic [Fig. 4(a)] obtained by HP sintered ZnS nano powders without pre-heating treatment is due to carbon contamination [22]. On the other hand, when the pre-heating temperature of the ZnS nano powders increases [Fig. 4(b–e)], carbon contamination is removed and becomes transparent. It is known that carbon reacts with Na2SO4 to generate CO2, and disappears, as shown in the following reaction formula [23]:
The reacted Na2SO4 is the product during hydrothermal synthesis by the following reaction formula:
As a result of this reaction, the conversion of Na2SO4 to Na2S becomes active, and carbon contamination is reduced as the pre-heating treatment temperature increases.
In Fig. 5, the cross section of ZnS ceramics was analyzed by SEM to confirm the density and microstructure change of ZnS ceramics according to the pre-heating treatment temperature. As shown in Fig. 5(a), the ZnS ceramic obtained by HP sintered ZnS nano powders without pre-heating treatment shows that many residual pores existed. The particles are homogeneous with an average of about 1 μm. The ZnS ceramic pre-heating treatment at 450 °C [Fig. 5(b)] shows an inhomogeneous microstructure in which nano-sized and micron-sized particles are mixed. The ZnS ceramic pre-heating treatment at 500 °C [Fig. 5(c)] exhibits an increase of the micron-sized particles and the absence of pores. In the case of the ceramics obtained from ZnS nano powders pre-heating treated above 550 °C [Fig. 5(d) and (e)], it was confirmed that small particles disappeared and large particles existed. It is observed that the particle growth of the ZnS nano powders affects the coarsening of the ZnS ceramics after HP sintering as the pre-heating treatment temperature increases [24]. Density analysis was performed to determine how the microstructure changes affect the density variation, and the results are shown in Table 1. The theoretical density of ZnS is 4.09 g/cm−3 [25], and the density is measured using Archimedes’ method. The density of ZnS ceramic without pre-heating treatment is 98.4%, which was poor due to many pores. The densities of 98.4, 98.9, 99.4, 99.9, and 99.9% were observed in the ZnS ceramics obtained by pre-heating treatment at 450, 500, 550, and 600 °C, respectively. When the fracture surface of the ZnS ceramics is observed, the presence of large particles appears to be more densified than the presence of small particles. As mentioned previously, the change of the ZnS ceramics structure is expected to help in the reduction of light scattering.
The XRD patterns to confirm the structural change in the ZnS ceramics according to the pre-heating treatment of the ZnS nano powders are shown in Fig. 6. The observed peaks show the diffraction peaks at 2Ɵ values of 28.6°, 33.1°, 47.6°, 56.4°, and 59.0°, corresponding to the diffraction planes (111), (200), (220), (311), and (222), respectively. Figure 6(b) is an enlargement to confirm the presence of a hexagonal phase after HP sintering. In the ceramics sintered after the pre-heating treatment of the ZnS nano powders, the diffraction peak at 26.8° shows a hexagonal phase corresponding to the diffraction planes (100). The ZnS ceramics with the pre-heating treatment indicate the presence of the cubic as the major phase and the hexagonal as the minor phase. In the ZnS ceramic, the hexagonal peaks formed because of the hexagonal phase being stable at high temperature. When the pre-heating treatment temperature increased, the hexagonal peaks increased because of the difference particle size, impurity, and density by pre-heating treatment.
In order to investigate the influence of microstructure change and hexagonal phase on the infrared transmittance, we analyzed the transmittance in the mid IR region. Figure 7 shows the transmittance at 3 ~ 8 μm at room temperature on the polished 1 mm thickness of the ZnS ceramics. The overall transmittance of the ZnS ceramics was improved when the ZnS nano powders were pre-heated. The ZnS ceramic without the pre-heating treatment exhibits a significantly low transmittance due to the impurities, small particle size, and many residual pores. Generally, pores act as a scattering source to reduce transmittance by generating light scattering [15]. The transmittance of the ZnS ceramics was improved when the pre-heating treatment temperature of the ZnS nano powders increased to 550 °C. It is assumed that the transmittance increased because of decreased light scattering source due to the decrease of pores, decrease of small particles, and effect of particle coarsening [26, 27]. Also, as the pre-heating temperature increased from 550 to 600 °C, transmittance decreased slightly due to the formation of the hexagonal phase. It is judged that transmittance decreased due to the increase of light scattering by anisotropy of the hexagonal phase and interface between the cubic and hexagonal phase. In addition, as the pre-heating treatment temperature of the ZnS nano powders increased, the decrease of the absorption peak observed at about 7 μm was confirmed. The absorption peaks at around 7 μm indicate Na2SO4, as shown in Eq. (4) [13]. As shown in Eq. (3), as the pre-heating treatment temperature increases, the conversion of Na2SO4 to Na2S is activated. From this result, the absorption peaks at around 7 μm decreased as the pre-heating treatment temperature increased. Transmittance at the mid IR region is summarized in Table. 2 in order to accurately check transmittance in the mid IR region. Transmittance of the ZnS ceramics in the mid IR region increased to about six times or more according to the presence or absence of the pre-heating treatment of the ZnS nano powders. It is judged that transmittance rapidly increases due to the reduction of pores. Also, when the pre-heating temperatures increase, it is judged that transmittance increases due to the coarsening of particles. From the result, it has been confirmed that a process of suitable pre-heating treatment is required to minimize porosity and hexagonal phase during the manufacturing of optical ZnS ceramics.
4 Conclusions
In this study, we applied the pre-heating treatment for the effective synthesis and impurity removal of ZnS ceramics, and analyzed the structure and optical characteristics according to pre-heating treatment temperature. First, ZnS nano powders with a cubic single phase were synthesized by hydrothermal synthesis. The ZnS nano powders were pre-heated to remove impurities, and sintered using hot-press (HP) sintering. The result of the pre-heating treatment of the ZnS nano powders are as follows: decrease of Na2SO4 and C affecting transmittance, densification and coarsening of microstructure, and reduction of pores. With this effect, elaboration of the transparent ZnS ceramics in the mid IR region was successfully achieved. However, it was shown that transmittance decreased slightly due to the hexagonal phase when the pre-heating treatment temperature increased. Therefore, pre-heating treatment of a suitable temperature is required. The highest transmittance was obtained from the ZnS ceramics with pre-heating treatment of ZnS nano powders at 550 °C, and the average transmittance was 71.6%. As a result, it has been confirmed that pre-heating treatment after hydrothermal synthesis is a suitable method for removing impurities and improving optical characteristics of the ZnS ceramics sintered by HP sintering. The ZnS ceramics produced by this method is expected to be applied to many applications as an optical material.
References
M. Bredol, J. Merikhi, J. Mater. Sci. 33(2), 471–476 (1998)
Y. Li, Y. Wu, J. Am. Ceram. Soc. 98(10), 2972–2975 (2015)
J.S. Hu, L.L. Ren, Y.G. Guo, H.P. Liang, A.M. Cao, L.J. Wan, C.L. Bai, Angew. Chem. Int. Ed. 117(8), 1295–1299 (2005)
I. Masafumi, S. Masahiko, in Window and Dome Technologies and Materials XV, ed. by B.J. Zelinski (SPIE, Bellingham, 2017), p. 101790L–1
A. Rogalski, K. Chrzanowski, Opto-electron. Rev. 10, 111 (2002)
C.B. Willingham, J.M. Wahl, P.K. Hogan, L.C. Kupferberg, T.Y. Wong, A.M. De, Proc. of. SPIE 5078, 179 (2003)
O. Merdrignac-Conance, N. Hakmeh, G. Durand, X.-H. Zhang, Proc. of SPIE 9822, 1 (2016)
C. Chlique, G. Delaizir, O. Merdrignac-Conance, C. Roucau, M. Dolle, P. Rozier, V. Bouquet, X.H. Zhang, Opt. Mater. 33(5), 706–712 (2011)
Y. Chen, L. Zhang, J. Zhang, P. Liu, T. Zhou, H. Zhang, D. Gong, D. Tang, D. Shen, Opt. Mater. 50, 36–39 (2015)
T.T.Q. Hoa, L.V. Vu, T.D. Canh, N.N. Long, J. Phys. Conf. Ser. 187, 012081 (2009)
Y.-D. Kim, K. Sonezaki, H. Maeda, A. Kato, J. Mater. Sci. 33, 5101 (1997)
N. Uzar, M.C. Arican, Bull. Mater. Sci. 34(2), 287–292 (2011)
Z. Shizen, M.A. Hongli, R. Jean, M.-C. Odile, A. Jean-Luc, L. Jacques, Z. Xianghua, J. Optoelectron. Adv. M. 1, 667 (2007)
Y. Li, L. Zhang, K. Kisslinger, Y. Wu, Opt. Mater. Express 4, 1140 (2014)
X. Fang, T. Zhai, U.K. Gautam, L. Li, L. Wu, Y. Bando, D. Golberg, Prog. Mater. Sci. 56(2), 175–287 (2011)
D. Denzier, M. Olschewski, K. Sattler, J. Appl. Phys. 84(5), 2841–2845 (1998)
I.N. Bhattacharya, P.K. Gochhayat, P.S. Mukherjee, S. Paul, P.K. Mitra, Mater. Chem. Phys. 88(1), 32–40 (2004)
P. Aubry, A. Bensalah, P. Gredin, G. Patriarche, D. Vivien, M. Mortier, Opt. Mater. 31(5), 750–753 (2009)
A. Kajbafvala, S. Zanganeh, E. Kajbafvala, H.R. Zargar, M.R. Bayati, S.K. Sadrnezhaad, J. Alloys Compd. 497(1-2), 325–329 (2010)
R. Kugel, H. Taube, J. Phys. Chem. 79(20), 2130–2135 (1975)
S. Ummartyotin, N. Bunnak, J. Juntaro, M. Sain, H. Manuspiya, Solid State Sci. 14(3), 299–304 (2012)
C. Chlique, O. Merdrignac-Conanec, N. Hakmeh, X. Zhang, J. L. Adam, J. Am. Ceram. Soc. 96, 3070 (2013)
M. Nassar, Energy Sources 25(8), 837–844 (2003)
M.N. Rahaman, Ceramic Processing and Sintering, 2nd Edn (Taylor and Francis, Boca Raton, 2003)
B. Mokili, M. Froment, D. Lincot, J. Phys. lV. 15, 261 (1995)
T. Ueno, M. Hasegawa, M. Yoshimura, H. Okada, T. Nishioka, K. Teraoka, A. Fujii, S. Nakayama, SEI Theh. Rev. 69, 48 (2009)
C. S. Park, S. Y. Yeo, T. H. Kwon, W. I. Park, J. S. Yun, Y. H. Jeong, Y. W. Hong, J. H. Cho, J. H. Paik, J. Korean Inst. Electr. Electron. Mater. Eng. 30, 722 (2017)
Acknowledgements
This work was supported by the Materials and Components Technology Development Program of MOTIE/KEIT [No. 10067243, The development of TeO2 based optical glass and sintered ZnS for mid infrared applications in smart devices].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeo, SY., Kwon, TH., Park, CS. et al. Sintering and optical properties of transparent ZnS ceramics by pre-heating treatment temperature. J Electroceram 41, 1–8 (2018). https://doi.org/10.1007/s10832-018-0137-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-018-0137-y