Abstract
Since, the organic dyes that harness sunlight are generally considered as the heart of the dye sensitized solar cells (DSSC), the present study was carried out with the aim to design heterocyclic azo dyes that can be potentially used in DSSC application. Hereby, the analysis based on density functional theory (DFT) and time-dependent DFT calculations of the geometries, electronic structures and absorption spectra of the dyes before and after binding to titanium oxide \((\hbox {TiO}_{2})\) were carried out and investigated in detail. The data obtained from these analyses were then used to determine the open-circuit photovoltage \((\hbox {V}_\mathrm{OC})\), and to measure the important parameters such as the light harvesting efficiency (LHE) and the electron injection efficiency associated with the short-circuit photocurrent density \((\hbox {J}_\mathrm{SC})\). Our investigation reveals that all dyes showed absorbance in the visible region (469–521 nm) with high oscillator strength \((f)\) (1.076–1.564) and LHE (0.9176–0.973). Moreover, we found that the dyes after binding to titanium oxide displayed slightly red-shifted absorption (475–527 nm) with improved oscillator strength \((f)\) (1.121–1.664) and LHE (0.921–0.979). In addition, all dyes showed high \(\hbox {V}_\mathrm{OC}\) (1.068–2.232 eV) and high driving force for the electron injection, thus leading to the larger \(\hbox {J}_\mathrm{SC}\). Our findings indicate that the heterocyclic azo dyes investigated in the current study can display better light to power conversion efficiency if used in the DSSC system, where the origin or their better performance can be attributed to the high \(\hbox {J}_\mathrm{SC}\) and \(\hbox {V}_\mathrm{OC}\) values found for these potential dyes. Based on the detailed study and investigation, we believe that the theoretical criteria used in the present study can be employed as an initial screening tool not merely to assess the properties of other organic dyes, but also to potentially design the organic azo dyes for their potential application in the DSSC systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the past two decades, dye-sensitized solar cells (DSSCs) have attracted significant attention from the research community as they emerged as the potential alternatives for the next-generation photovoltaic devices [1]. The sensitizer (material that enhances the sunlight harvesting) is one of the key components in DSSCs and plays a critical role in the power conversion efficiency as well as the device stability [2]. A wide range of photosensitizers, including metal complexes, porphyrins, phthalocyanines and metal-free organic dyes, have been designed and applied to DSSCs in the past two decades [3]. Among several known dye sensitized materials, the ruthenium complexes showed overall conversion efficiency over 11 % [4]. However, besides being the most efficient materials among other known sensitizers, the large scale use of ruthenium based dye sensitized materials is limited due to their significantly high cost, scarce natural reserves of ruthenium and environmental concerns [5]. In order to address these aforementioned challenges associated with the metal based dye sensitized cells, the metal-free organic sensitizers [6–9] are therefore seen as emerging class of materials that display several potential advantages in view of DSSC application. Some of the advantageous features of these metal-free organic dye sensitizers include affordable cost, appreciable durability, inherent environmentally benign nature along with absorption and electrochemical properties which are tunable via suitable molecular tailoring [10].
Since, novel dyes with the donor-conjugated-acceptor \((\hbox {D}{\text {-}}\pi {\text {-}}\hbox {A})\) system can achieve an efficient photovoltaic performance, a significant effort in the domain of DSSC area is to develop metal free organic dyes that can exhibit high light to electric energy conversion efficiency [11]. From literature, we can observe that there are a number of factors that determine the efficiency and potential usefulness of dye for DSSC application [12]. A few of these important factors include (i) wide absorption wavelength in visible to near infrared (IR) region; (ii) easy electron injection from the excited state of the dyes to the conduction band of \(\hbox {TiO}_{2}\) and (iii) good electron transfer from the donor to acceptor [2, 13, 14].
Often trial-and-error approach is used for the synthesis of \(\hbox {D}{\text {-}}\pi {\text {-}}\hbox {A}\) dyes, which is slow process and requires expensive materials. In this prospect, theoretical computation is used for the screening of potential organic dyes and thus reducing the cost to develop efficient dyes. Several new dyes have been computationally designed and screened before their synthesis, thus allowing for a considerable saving of resources and contributing to advances in the DSC field [15].
Technically, the high light to electric energy conversion efficiency can be achieved by using the dyes that absorb light in visible or near IR region [11]. In addition to good spectral response, suppression of charge recombination and dye aggregation are necessary to achieve high \(\hbox {J}_\mathrm{SC}\) [12]. Though, the enhanced conjugation gives an improved spectral response, the same time it increases the chances of charge recombination, which leads to lower IPCEs.
Heterocyclic azo dyes are very good chromophores with strong absorption in entire visible spectrum [16]. From many years, they have been used as colouring agent in textiles and plastics industry. Recently azo dyes bearing thiophene, pyrrole and azoles moieties have been used for optical and electronic applications such as dye sensitized solar cells, optical switching, second harmonic generation and chemosensing [17, 18].
We selected heterocyclic azo dyes due to their small size and absorbance in visible region. Density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations were used to study electronic structure and absorption spectra of dyes before and after binding to \(\hbox {TiO}_{2}\). Moreover, open-circuit photovoltage \((\hbox {V}_\mathrm{OC})\) and key parameters that control the short-circuit current density \((\hbox {J}_\mathrm{SC})\) were also calculated during the course of present study. We highly anticipate that this work would facilitate the future experimental studies in designing and fast screening new efficient organic dyes.
2 Computational details
Well known computational software, Gaussian 09 was used in all the calculations [19]. Structure of free dyes were optimized using B3LYP functional and 6-31+G* basis set, while for the optimization of \(\hbox {dye+TiO}_{2}\) cluster systems B3LYP functional and 6-31+G* basis set for non-metal atoms while LANL2DZ basis set for Ti atom were adopted. Conventional exchange–correlation functional that are considered state-of-the-art for geometry optimization but fail to calculate excitation energy of excited states accurately especially excited states with significant charge-transfer (CT) character [20]. Long-range corrected version of B3LYP (CAM-B3LYP) [21], which has been proved to be appropriate for CT type excitations, was chosen to stimulate the absorption spectra of dyes and \(\hbox {dye+TiO}_{2}\) cluster systems. The LANL2DZ basis set for Ti atom and 6-31+G* basis set for C, H, O, N, S atoms have been selected for the calculation of absorption spectra. Success of this combination of basis sets for determination of transition energies for a series of organic dyes is already reported earlier [22]. The absorption spectra of the dyes were simulated using TD-DFT and solvent effect (acetone) was undertaken using conductor-like polarizable continuum model (CPCM) [23].
3 Results and discussion
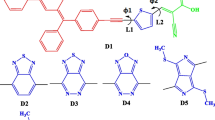
In this study, we presented the results of theoretical calculations with the aim to design efficient sensitizers for dye sensitized solar cells. For this purpose, we designed a variety of Heterocyclic azo dyes, where the structures of these dyes are shown in Fig. 1. In this theoretical study, the DFT and TDDFT calculations were performed and the Key parameters: (i) open-circuit photovoltage \((\hbox {V}_\mathrm{oc})\); (ii) light harvesting efficiency (LHE); (iii) injection driving force \((\Delta G^{inject})\) were calculated accordingly.
3.1 Energy level alignment
The major role of organic dyes in DSSC is to act as the photosensitizer and mainly used to sensitized photoelectrode with large band gap (such as \(\hbox {TiO}_{2})\). Since, the dye is adsorbed on to the surface of photoelectrode and upon illumination the excited electron is injected into the photoelectrode, the energy levels and locations of the HOMOs and LUMOs of the dye sensitizer must need to match the iodine/iodide redox potential and the conduction band edge level of the \(\hbox {TiO}_{2}\) semiconductor [24]. Therefore, the highest few occupied and lowest few unoccupied molecular orbitals are of particular interest, as they involved in the optical transitions that leads to photo-induced electron transfer from the dye to the semiconductor. Based on our calculations, \(\hbox {E}_\mathrm{HOMO}\), \(\hbox {E}_\mathrm{LUMO}\) and energy gap were determined (Table 1). For all dyes that were investigated hereby, the simulated LUMOs lie above the \(\hbox {TiO}_{2}\) conduction band (4.00 eV in vacuum) [25], providing the thermodynamic driving force for favorable electron injection from the excited state of the dye to the \(\hbox {TiO}_{2}\) conduction band. At the same time, the HOMOs of all dyes lie below the iodide redox potential (4.80 eV in vacuum) [26], leading to a fast dye regeneration and avoiding the geminate charge recombination between oxidized dye molecules and photo-injected electrons in the nanocrystalline \(\hbox {TiO}_{2}\) film. High rate of Rapid and efficient electron injection from the excited state of the sensitizer into the conduction band of the \(\hbox {TiO}_{2}\) is desired for high performance of DSSCs.
3.2 UV–Vis spectra of dyes
To gain an insight into the excited states giving rise to the intense absorption spectra of sensitizers, TD-DFT calculations were performed at the CAM-B3LYP/6-31+G* level using C-PCM in acetone. In the TD-DFT calculations of absorption spectra, the ten lowest singlet-singlet transitions were taken into account.
The computed maximum absorption wavelengths \((\uplambda _\mathrm{max})\), absorption energy, absorption energy, oscillator strengths \((f)\) and nature of the transitions are summarized in Table 2. Absorption spectra are given in Figs. 2 and 3. All dyes showed absorption in the visible region (469–521 nm). Absorbance in visible region is required for high efficiency. Oscillator strengths were also high (1.076–1.564). Light harvesting efficiency (LHE) is another factor which determines the efficiency of DSSC. LHE should be high as possible to maximize the photocurrent response. The LHE can be expressed as [27]:
Values of LHE are given in Table 2. LHE values were high (0.921–0.979).
3.3 Adsorption of dyes on \(\hbox {TiO}_{2}\) surface
Nature of excited states of semiconductor/dye interface controls the electron injection rate from dye to semiconductor. Hence, the computational modeling of semiconductor/dye interface is considered to be a very useful tool to optimized DSSCs. In this regard, simulations of the electronic and optical properties of \(\hbox {TiO}_{2}\) nanoparticles and the dye excited states were performed.
3.3.1 Adopted model for \(\hbox {TiO}_{2}\) film
Semiconductor is one of the main components in the DSSCs and nano structured \(\hbox {TiO}_{2}\) has frequently been used in the most efficient DSSC systems. \(\hbox {TiO}_{2}\) has three major crystalline structures; rutile, anatase and brookite. However, the electrodes in photovoltaic cells are often based on anatase and the (101) surface of anatase is thermodynamically most stable surface [28, 29]. Finally, \(\hbox {Ti}_{5}\hbox {O}_{20}\hbox {H}_{22}\) (101) model from crystal structures is adopted as surface of \(\hbox {TiO}_{2}\) film in the current work. This model is small enough that the large basis set (6-31+G*) can be adopted in calculation. From several adsorption configurations, bidentate chelating adsorption mode is used and it is energetically favorable [28].
3.3.2 Electronic and optical properties of the dye@\(\hbox {TiO}_{2}\) system
It is found that electronic and optical properties of dyes showed significant changes when absorbed to \(\hbox {TiO}_{2}\), might be due to interaction between the dyes and the semiconductor. Therefore, we also simulated the UV/Vis absorption spectra of the dyes after binding to \(\hbox {Ti}_{5}\hbox {O}_{20}\hbox {H}_{22 }\) cluster at the same level of theory for the free dyes except that the LANL2DZ basis set was used for Ti atom. The results from these investigations are listed in Table 3. We found that after binding to \(\hbox {TiO}_{2}\), dyes showed red-shift in the maximum absorption wavelengths. Red-shift of absorption spectra of dye after binding \(\hbox {TiO}_{2 }\) can be explain on the basis of interactions between electron acceptor group of dye (–COOH) and the 3d orbitals of the Ti atom, resulting an overall decrease in the LUMO energies compared with the isolated dyes. Oscillating strength \((f)\) was improved due to the interaction between the dyes and the semiconductor comparing with the free dyes. HOMOs and LUMOs also demonstrate that there is significant charge transfer from the donor part to the acceptor \(\hbox {Ti}_{5}\hbox {O}_{20}\hbox {H}_{22 }\) (Fig. 4). In addition, after binding to the semiconductor, the LHE of the dyes showed an increase.

3.4 Performance of DSSCs based on dyes
The overall efficiency of the DSSC can be calculated from the integral of short-circuit photocurrent density \((\hbox {J}_\mathrm{SC})\), open circuit potential \((\hbox {V}_\mathrm{OC})\), the fill factor (ff) and the intensity of light \((\hbox {I}_\mathrm{S})\),which is expressed by following equation:
3.4.1 Factors influencing \(\hbox {J}_\mathrm{sc}\)
The \(\hbox {J}_\mathrm{sc}\) in DSSCs is determined by the following equation:
In Eq. (3), LHE\((\uplambda )\) is the light harvesting efficiency at a given wavelength, \(\Phi _\mathrm{inject. }\) is the electron injection efficiency, and \(\upeta _\mathrm{collect. }\) is the charge collection efficiency. For the same DSSCs with only different dyes, just as for the organic dyes under study, it is reasonable to assume that the \(\upeta _\mathrm{collect.} \) is a constant. As a result, the enhancement of \(\hbox {J}_{SC}\) should focus on improving the LHE and \(\Phi _\mathrm{inject.}\)
As discussed above, if the charge collection efficiency \((\Phi _\mathrm{inject.})\) could be considered as a constant for the same cell architecture, LHE and \(\Phi _\mathrm{inject. }\) would be the two main factors influencing \(\hbox {J}_\mathrm{SC}\). According to Eq. (3), in order to obtain a high \(\hbox {J}_\mathrm{SC}\), the efficient organic dyes used in DSSCs should have a large LHE. The LHE of dyes was found high in the present study.
Another way to enhance \(\hbox {J}_\mathrm{SC}\) is to improve \(\Phi _\mathrm{inject.}\), which is related to the driving force (\(\Delta G^{inject})\) of the electron injection from the photoinduced excited states of organic dyes to the TiO\(_{2 }\) surface. In general, larger \(\Delta G^{inject}\) leads to larger \(\Phi _\mathrm{inject.}\).
Computation of electron injection rate is very useful to study photovoltaic data. Following equation is used to calculate free energy change (in eV) for the electron injection:
In Eq. (4), \(E_{OX}^{dye*}\) is the oxidation potential of the dye in the excited state, \(E_{CB}^{TiO_2 }\) is the reduction potential of the conduction band of the \(\hbox {TiO}_{2}\) \((E_{CB}^{TiO_2 } =4.0\hbox {eV})\).
In Eq. (5), \(E_{OX}^{dye}\) is the redox potential of the ground state. \(E_{OX}^{dye}\) can be estimated as negative \(\hbox {E}_\mathrm{HOMO}\) [30]. Moreover, in this equation \(\lambda _{\max }^{ICT}\) is the energy of the intra-molecular charge transfer (ICT). By using this scheme, we calculated \(\Delta G^{inject}\),as well as \(E_{OX}^{dye}\) and \(E_{OX}^{dye*}\) for dyes and the results are listed in Table 4. We found that all the calculated \(\Delta G^{inject}\) were negative, which means that the dye excited state lies above the \(\hbox {TiO}_{2}\) conduction band edge, favoring the injection of the electron from the excited state dye to the \(\hbox {TiO}_{2}\) conduction band edge. Dyes would show higher \(\hbox {J}_\mathrm{SC}\) due higher LHE and \(\Delta G^{inject}(\Phi _\mathrm{inject.})\).
3.4.2 Open-circuit photovoltage \((\hbox {V}_\mathrm{OC}\))
The \(\hbox {V}_\mathrm{OC}\) is obtained only by experiment, the relationship among these quantities and the electronic structure of dye are still unknown. The analytical relationship between \(\hbox {V}_\mathrm{OC}\) and \(\hbox {E}_\mathrm{LUMO}\) may exist. According to the sensitized mechanism (electron injected from the excited dyes to the semiconductor conduction band) and single electron and single state approximation, the energy relationship can be shown as follows [31]:
In Eq. (6), \(\hbox {E}_{CB}\) is the energy of the semiconductor’s conduction band edge. It is known that the higher the \(\hbox {E}_\mathrm{LUMO}\), the larger the \(\hbox {V}_\mathrm{OC}\). Eq. (6) provides only an ideal value for Voc. However, the real Voc of a DSSC is generally smaller than this theoretical limit, and one of the reasons is a backward reaction between electrons and redox electrolyte [32, 33]. Once the photogenerated electrons are not rapidly transferred to conducting substrate, the facile recombination of electrons and oxidized ionic species of the electrolyte results in a downward photovoltage. Relatively high values of LHE, \(\Delta G^{inject}\) and \(\hbox {V}_\mathrm{OC }\) indicate that all dyes would show high efficiency if used in the dye sensitized solar cell.
4 Conclusions
In this study, open-circuit photovoltage \((\hbox {V}_\mathrm{OC})\), factors influencing the short-circuit current density \((\hbox {J}_\mathrm{SC})\) including LHE and electron injection efficiency were calculated using DFT and TDDFT methods and discussed comprehensively. All the dyes showed absorbance in visible region (469–521 nm) with high oscillator strength \((f)\) (1.076–1.564) and LHE (0.9176–0.973). After binding to titanium oxide, all dyes showed slightly red-shifted absorption (475–527 nm) with improved oscillator strength \((f)\) (1.121–1.664) and light harvesting efficiency (LHE) (0.921–0.979). All the dyes showed high driving force for electron injection, thus leading to the larger \(\hbox {J}_\mathrm{SC}\). These dyes also showed larger \(\hbox {V}_\mathrm{OC}\) (1.068-2.232 eV). These results indicate that heterocyclic azo dyes will show better light to power conversion efficiency in DSSCs due high \(\hbox {J}_\mathrm{SC}\) and \(\hbox {V}_\mathrm{OC}\). These dyes will display high efficiency in DSSCs.
References
O’Regan, B., Gratzel, M.: A low-cost, high-efficiency solar cell based on dye-sensitized colloidal \(\text{ TiO }_{2}\) films. Nature 353, 737–740 (1991)
Abdullah, M.I., Janjua, M.R.S.A., Nazar, M.F., Mahmood, A.: Quantum chemical designing of efficient TC4-based sensitizers by modification of auxiliary donor and \(\uppi \)-spacer. Bull. Chem. Soc. Jpn. 86, 1272–1281 (2013)
Boschloo, G., Hagfeldt, A.: Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 42, 1819–1826 (2009)
Nazeeruddin, M.K., Angelis, F.D., Fantacci, S., Selloni, A., Viscardi, G., Liska, P., Ito, S., Takeru, B., Gratzel, M.: Combined experimental and DFT–TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 127, 16835–16847 (2005)
Abdullah, M.I., Janjua, M.R.S.A., Mahmood, A., Ali, S., Ali, M.: Quantum chemical designing of efficient sensitizers for dye sensitized solar cells. Bull. Korean Chem. Soc. 34, 2093–2098 (2013)
Mishra, A., Fischer, M.K.R., Bäuerle, P.: Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. Engl. 48, 2474–2499 (2009)
Zhang, G.L., Bala, H., Cheng, Y.M., Shi, D., Lv, X.J., Yu, Q.J., Wang, P.: High efficiency and stable dye-sensitized solar cells with an organic chromophore featuring a binary \(\uppi \)-conjugated spacer. Chem. Commun. 16, 2198–2200 (2009)
Choi, H., Choi, H., Paek, S., Song, K., Kang, M.-S., Ko, J.: Novel organic sensitizers with a quinoline unit for efficient dye-sensitized solar cells. Bull. Korean Chem. Soc. 31, 125–132 (2010)
Li, Y.-T., Chen, C.-L., Hsu, Y.-Y., Hsu, H.-C., Chi, Y., Chen, B.-S., Liu, W.-H., Lai, C.-H., Lin, T.-Y., Chou, P.-T.: Donor–acceptor organic sensitizers assembled with isoxazole or its derivative 3-oxopropanenitrile. Tetrahedron 66, 4223–4229 (2010)
Irfan, A., Al-Sehemi, A.: Quantum chemical study in the direction to design efficient donor-bridge-acceptor triphenylamine sensitizers with improved electron injection. J. Mol. Model. 18, 4893–4900 (2012)
Al-Eid, M., Limb, S.H., Park, K.-W., Fitzpatrick, B., Han, C.-H., Kwak, K., Hong, K.: Graeme Cooke Facile synthesis of metal-free organic dyes featuring a thienylethynyl spacer for dye sensitized solar cells. Dyes Pigments 104, 197–203 (2014)
Lee, W., Choi, J., Namgoong, J.W., Kim, S.H., Sun, K.C., Jeong, S.H., Yoo, K., Ko, M.J., Kim, J.P.: The effect of five-membered heterocyclic bridges and ethoxyphenyl substitution on the performance of phenoxazine-based dye-sensitized solar cells. Dyes Pigments 104, 185–193 (2014)
Preat, J., Jacquemin, D., Perpete, E.A.: Towards new efficient dye-sensitised solar cells. Energy Environ. Sci. 3, 891–904 (2010)
Tai, C.-K., Chen, Y.-J., Chang, H.-W., Yeh, P.-L., Wang, B.-C.: DFT and TD-DFT investigations of metal-free dye sensitizers for solar cells: effects of electron donors and \(\uppi \)-conjugated linker. Comput. Theor. Chem. 971, 42–50 (2011)
Abbotto, A., Barolo, C., Bellotto, L., De Angelis, F., Gratzel, M., Manfredi, N., Marinzi, C., Fantacci, S., Yum, J., Nazeeruddin, M.K.: Heteroaromatic conjugated bipyridine based ruthenium sensitizer for efficient dye-sensitized solar cells. Chem. Commun. 42:5318–5320 (2008)
Towns, A.D.: Developments in azo disperse dyes derived from heterocyclic diazo components. Dyes Pigments 42, 3–25 (1999)
Yesodha, S.K., Pillai, C.K.S., Tsutsumi, N.: Stable polymeric materials for nonlinear optics: a review based on azobenzene systems. Prog. Polym. Sci. 29, 45–74 (2004)
Matharu, A., Jeeva, S., Huddleston, P.R., Ramanujam, P.S.: Synthesis and optical storage properties of a thiophene-based holographic recording medium. J. Mater. Chem. 17, 4477–4482 (2007)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, P.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, N.J., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A.1. Gaussian Inc., Wallingford (2009)
Dreuw, A., Head-Gordon, M.: Single-reference ab initio methods for the calculation of excited states of large molecules. Chem. Rev. 105, 4009–4037 (2005)
Yanai, T., Tew, D.P., Handy, N.C.: A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004)
Zhang, J., Li, H.-B., Wu, Y., Geng, Y., Duan, Y.-A., Liao, Y., Su, Z.-M.: Chem. J. Chin. Univ. 32, 1343–1348 (2011)
Barone, V., Cossi, M.: Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998)
Qin, P., Yang, X.C., Chen, R.K., Sun, L.C., Marinado, T., Edvinsson, T., Boschloo, G., Hagfeldt, G.: Influence of \(\uppi \)-conjugation units in organic dyes for dye-sensitized solar cells. J. Phys. Chem. C 111, 1853–1860 (2007)
Gratzel, M.: Photoelectrochemical cells. Nature 414, 338–344 (2001)
Zhang, G.L., Bai, Y., Li, R.Z., Shi, D., Wenger, S., Zakeeruddin, S.M., Gratzel, M., Wang, P.: Employ a bisthienothiophene linker to construct an organic chromophorefor efficient and stable dye-sensitized solar cells. Energy Environ. Sci. 2, 92–95 (2009)
Qin, C., Clark, A.E.: DFT characterization of the optical and redox properties of natural pigments relevant to dye-sensitized solar cells. Chem. Phys. Lett. 438, 26–30 (2007)
Hagfeldt, A., Graetzel, M.: Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 95, 49–68 (1995)
Persson, P., Bergström, R., Ojamäe, L., Lunell, S.: Quantum-chemical studies of metal oxides for photoelectrochemical applications. Adv. Quant. Chem. 41, 203–263 (2002)
Pearson, R.G.: Absolute electronegativity and hardness: application to inorganic chemistry. Inorg. Chem. 27, 734–740 (1988)
Zhang, C.R., Liu, Z.J., Chen, Y.H., Chen, H.S., Wu, Y.Z., Yuan, L.H.: DFT and TDDFT study on organic dye sensitizers D5, DST and DSS for solar cells. J. Mol. Struct. 899, 86–93 (2009)
Jung, H.S., Lee, J.K.: Dye sensitized solar cells for economically viable photovoltaicsystems. J. Phys. Chem. Lett. 4, 1682 (2013)
Choi, H., Kamat, P.V.: Know thy nano neighbor. Plasmonic versus electron charging effects of metal nanoparticles in dye-sensitized solar cells. ACS Nano 6, 4418 (2012)
Acknowledgments
The authors would like to sincerely appreciate the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no RGP-VPP-255.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmood, A., Khan, S.UD. & Rana, U.A. Theoretical designing of novel heterocyclic azo dyes for dye sensitized solar cells. J Comput Electron 13, 1033–1041 (2014). https://doi.org/10.1007/s10825-014-0628-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-014-0628-2