Abstract
Purpose
In a preimplantation genetic testing for aneuploidy (PGT-A) cycle, does the blastocyst quality before biopsy, or the day of biopsy, or the embryo hatching status have an impact on either euploidy or the rate of embryo survival after freezing?
Methods
This was a retrospective study including 6130 biopsied blastocysts coming from 1849 PGT-A cycles performed in our center (2016–2022). Embryos were categorized according to the inner cell mass and trophectoderm quality, using Gardner’s scoring (excellent: AA; good: AB, BA, BB; poor: AC, CA, BC, CB, CC); the day of biopsy (5 or 6); and their hatching status (fully hatched blastocysts [FHB] or non-fully hatched blastocysts [nFHB]). The independent relationship between each group and both euploidy and survival rate was assessed.
Results
Excellent-quality embryos were more euploid than both good- and poor-quality embryos (52.69%, 39.69%, and 26.21%; p < 0.001), and day 5–biopsied embryos were more euploid than day 6–biopsied embryos (39.98% and 34.80%; p < 0.001). Survival rates of excellent-quality (92.26%) and good-quality (92.47%) embryos were higher than survival rates in the poor-quality group (84.61%) (p = 0.011 and p = 0.002). Day 5–biopsied embryos survived better than day 6–biopsied embryos (93.71% vs. 83.69%; p < 0.001) and FHB had poorer survival than nFHB (78.61% vs. 93.52%; p < 0.001).
Conclusions
Excellent-quality and day 5–biopsied embryos are more prone to be euploid than good and poor or day 6–biopsied embryos, respectively. Poor-quality, day 6–biopsied embryos, and FHB have significantly lower survival after biopsy and vitrification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preimplantation genetic diagnosis was first designed to select against embryos carrying a mutation responsible for an inherited monogenic disease [1]. Over the years, its use has spread to preimplantation genetic testing for aneuploidy (PGT-A), which stands as the only validated method to increase the chances of a healthy baby born from IVF cycles, by selecting chromosomally normal embryos for transfer [2].
PGT-A was initially performed by removing a polar body [3] or a blastomere from cleavage-stage embryos [4] and analyzing its chromosomes by fluorescence in situ hybridization (FISH). The technique has evolved to its current form, which combines trophectoderm (TE) biopsy with analysis through a comprehensive chromosome screening (CCS) platform and is considered the most reliable method for PGT-A [5, 6]. Compared with blastomere biopsy, the two major benefits of the TE biopsy are that a smaller proportion of the embryo is removed while greater analysis accuracy is achieved because more cells are studied, considerably increasing the number of embryos with a final result.
However, blastocyst biopsy has some drawbacks that must be foreseen carefully to maximize a TE biopsy cycle. Embryos achieve the blastocyst stage anywhere between 110 and 150 h post insemination, and blastulation time can vary even among those coming from the same cohort. This fact makes blastocyst biopsy one of the most time-consuming techniques in the IVF laboratory. The other main concern is that it necessarily implies vitrification as all biopsied embryos must be vitrified while waiting for the CCS analysis to take place. Once the biopsy results are reported, embryos are thawed and replaced in case of normalcy in a deferred cycle [7].
In order to make a PGT-A cycle more efficient, some authors have attempted to search for factors related to TE biopsy that could have an impact on euploidy, survival rate, or the PGT cycle’s general outcome. When looking closely at embryo quality parameters, it has been found that grades 5 and 6 of expansion (according to Gardner’s scoring system [8]) are more prone to be euploid than grade 1 or 2 embryos [9]. The same authors found that when the TE and inner cell mass (ICM) were graded A, the embryos were more likely to be euploid. Other groups have detected differences in the ploidy depending on whether the biopsy is performed on day 5 or day 6 [10, 11], or even on day 7 of development [12]. The relationship between euploidy and the embryo’s hatching status at the time of biopsy and its correlation with the PGT cycle outcomes has also been studied, and no correlation has been found between these two embryonic factors [13].
Although a few publications have addressed the relation between embryonic and biopsy factors regarding PGT-A cycle outcomes [14, 15], scant information is available about the mentioned factors and survival rates after biopsy and vitrification.
The present study aimed to ascertain if, in a PGT-A cycle, morphology-based blastocyst quality at the moment of biopsy, the day of herniation (day 5 or day 6), and the hatching status of the embryo (fully hatched [FHB] or non-fully hatched [nFHB]) are independently related to both embryo euploidy and survival after thawing. Revealing these relationships may help counseling reproductive medicine professionals not only when performing a PGT-A cycle but also in case of a non-PGT-A cycle where embryo morphology and the day of blastulation are the only selection criteria for choosing the best embryo to transfer.
Material and methods
Patient population and study design
This is a retrospective study conducted in a single fertility clinic (IVIRMA Barcelona). It included 1849 PGT-A cycles (1732 using autologous oocytes and 117 using oocytes from healthy donors) performed in this center from June 2016 to June 2022 in 1591 patients. Population demographics are shown in Table 1, and the distribution of cycles for each PGT-A indication was as follows: 1401 cycles for advanced maternal age (75.79%); 119 cycles for implantation failure (6.42%); 95 cycles for recurrent miscarriage (5.14%); 177 cycles for male factor (9.57%); and 57 cycles for other indications such as previous chromosomopathies (3.08%).
Blastocyst biopsy and a freeze-all policy were common among them and at the time of writing 942 frozen embryo transfers (FETs) were already performed and included in the analysis.
Blastocysts were graded based on their ICM and TE quality according to the Gardner and Schoolcraft scoring system [8]. All biopsied blastocysts were expanding blastocysts initiating hatching or completely hatched before the biopsy. Biopsied blastocysts were stratified into three groups: excellent-quality (blastocysts with both ICM and TE type A); good-quality (blastocysts with either ICM or TE, or both, type B but no type C involved in either the ICM or the TE scoring); and poor-quality blastocysts (when ICM or TE, or both, were type C) (Table 2). Embryos were also clustered depending on the day of the biopsy (day 5 or day 6) and their hatching status (FHB or nFHB). These three features (grouped embryo quality, day of biopsy, and hatching status) were independently analyzed for their relation to both chromosomal euploidy and embryo survival after thawing. Secondarily, global IVF outcomes for these cycles were also calculated.
This study was approved by both the research board and the ethics committee of Clinical Research IVIRMA Valencia, Spain (1909-BCN-083-MF).
Stimulation protocol
Controlled ovarian stimulation was induced following recombinant FSH and/or human menopausal gonadotrophin administration. A flexible GnRH antagonist protocol was used according to ovarian reserve and anti-Mullerian hormone values. Recombinant human chorionic gonadotrophin (hCG) was administered when at least two follicles reached 17 mm in diameter. Patients underwent vaginal oocyte recovery 36 h after hCG administration, always under general sedation.
IVF procedure
Cumulus-oocyte complexes were retrieved and incubated for 2 h before being denudated in a 40 IU hyaluronidase solution (Hyaluronidase; Fertipro, Beernem, Belgium) diluted in culture medium (Gems; Genea Biomedx, Sydney, Australia). All micromanipulation procedures were performed in closed working stations under controlled atmosphere conditions (6% CO2, 5% O2, and 37°C). Two hours after denudation, all metaphase II oocytes underwent intracytoplasmic sperm injection. After microinjection, all oocytes were placed in Embryoslides (Vitrolife, Sweden). Microwells were filled with 20 µL of medium and incubated under a mineral oil layer at 5% CO2 and 5% O2. Embryo development was checked through the time-lapse imaging system, which acquired images at eleven different focal planes every 15 min. Embryos were evaluated at 16–20 h post-insemination for fertilization assessment and at 68–72 h for day 3 evaluation and medium renewal. On day 3, a zona pellucida opening was performed using a non-contact diode laser (Octax Navilase; Vitrolife, Goteborg, Sweden) to allow the TE to herniate while the blastocyst expanded.
Systematic observations for embryo scoring were scheduled at 110–114 h (day 5 in the morning), 118–120 h (day 5 in the afternoon), and 136 h (day 6 in the morning) post insemination. Biopsy call was done when embryologists detected through the time-lapse monitoring system a herniation that permitted the retrieval of TE cells. Biopsy per se was performed on day 5 at 114–116 h and on day 6 at 130–140 h post insemination. Only few cases were performed at 120 h post ICSI (day 5 afternoon), and thus, they were added to the day 5 category. Morphology annotations registered corresponded to the embryo status and quality right before the biopsy. Embryos were occasionally grown until day 7, but this group was excluded from the analysis as the sample size was not comparable to those of the day 5– or day 6–biopsied embryo groups. Biopsy was performed by gently aspirating 4 to 9 TE cells into the biopsy pipette and cutting the TE sample through laser pulses and a flicking pipette movement. Trophectoderm samples were washed three times in phosphate-buffered saline (PBS) solution and placed in microcentrifuge tubes containing 1–2 µL of PBS.
From June 2016 until August 2019, samples were sent for chromosomal analysis to one genetic laboratory (Igenomix S.L., Valencia, Spain) and from August 2019 until June 2022 samples were sent to a different genetic laboratory (Juno Genetics, Oxford, UK). Igenomix used whole-genome amplification while Juno used a targeted amplification prior to next-generation sequencing (NGS). Differences in methodology could represent a limitation for this study but resolution, non-informative rates (0.77% in global), and cut-off points for euploidy/aneuploidy of both laboratories have been investigated and found to be comparable. The distribution of the non-informative and mosaic embryos among the categories of the three variables of study is represented in Supplementary Figs. 1 and 2 respectively.
Before August 2020, mosaic embryos were categorized as euploid or aneuploid depending on the percentage of euploid-aneuploid discordance among the retrieved TE cells. Since the publication of some reassuring studies about healthy babies born after mosaic embryo transfers [16], mosaic embryos were reported as such and they were considered transferable or non-transferable considering the Preimplantation Genetic Diagnosis International Society (PGDIS) guidelines [17] and Grati’s scoring system for prioritizing mosaic embryos [18]. On account of the policy change on reporting mosaic embryos, in this study, the completely euploid and the low-risk mosaic embryos were clustered together in the “transferable embryos” group while the completely aneuploid and the high-risk mosaic embryos were grouped as “non-transferable embryos.” After the biopsy, embryos were immediately vitrified according to the manufacturer’s protocol (Kitazato vitrification/thawing kit cryotop method; Kitazato, Shizuoka, Japan).
Results were uploaded to our patient management system within the following 2 weeks. Once the chromosomal analysis results were reported, FET cycles were scheduled when at least one transferable embryo (euploid or low-risk mosaic) was available. Following either a programmed or natural hormone replacement therapy [19], embryos were thawed. Immediate embryo survival was assessed under the microscope to avoid interrupting embryo culture conditions once the embryo is let in the incubator. The laboratory policy stated that for an embryo to be considered for embryo transfer, intact cells had to be over 50% in both ICM and TE. If the percentage of cell loss was close to 50%, a second look 1 h after warming was performed to confirm embryo transfer. Embryo morphology was also evaluated at that moment according to the blastocyst quality prior to vitrification, the day of vitrification, and its hatching status. Embryos were transferred to the patient 3 to 5 hours after thawing. Embryo re-expansion was not taken into account as a transfer decision-maker as there does not seem to be a consensus on the predictive value that this embryonic feature has over the implantation potential of thawed embryos [20].
Study outcomes
A database including patient and embryo parameters from both fresh PGT-A and FET cycles was built. The primary outcomes of this study were the transferable embryo rate and survival rate. The transferable embryo rate was calculated as the percentage of the ensemble of euploid and low-risk mosaic embryos, while the survival rate was the proportion of intact embryos after thawing. The secondary outcomes were the clinical pregnancy, miscarriage, ongoing pregnancy, and implantation rates. The clinical pregnancy rate was described as the proportion of FET cycles where at least one embryo was transferred that showed a rise in beta hCG level to over 50 IU/mL 12 days after the transfer and a gestational sac was visualized on ultrasound at 5 gestational weeks. The miscarriage rate was defined as the percentage of the latter that resulted in a miscarriage before 22 weeks of gestation. The ongoing pregnancy rate was calculated as the residual clinical pregnancies that did not end in a miscarriage. The implantation rate was calculated as the quotient of gestational sacs over transferred embryos.
Data and statistical analysis
A chi-square test was used to compare categorical variables and the Student t-test was used to compare continuous variables when normality could be accepted; otherwise, the Mann–Whitney U test was used. Comparison among categories (excellent, good, poor; day 5, day 6; FHB, nFHB) of the three features analyzed in reference to the two study variables “transferable embryo” and “survival rate” were analyzed using binary logistic regression models. Univariate models were proposed and then the step function from the stats package was used to find the multivariate model with the lowest AIC (Akaike information criterion) using the three independent variables. A multivariate logistic regression analysis using maternal age as one of the confounders was also performed. In all models, the applicability conditions were assessed and a significance level of 5% was used. All calculations were performed using R 4.0.3 (R Core Team, 2020, Vienna, Austria).
Results
About the study: clinical and laboratory performance
The details of the cycles’ flow and the overall clinical outcomes are presented in Fig. 1. From the initial 1849 PGT-A cycles, 1017 (55%) ended up having transferable embryos for transfer. At the time of writing, 942 FET cycles had been performed with at least one embryo being thawed. In 92.78% of them (n = 874), at least one embryo survived the thawing process, so the replacement was finally performed. After the replacement of at least one transferable embryo, 68.87% (n = 602) of the transfers resulted in a positive clinical pregnancy. Seventy-seven of these cases ended in a miscarriage (12.79%) between 5 and 22 weeks of gestation and, considering that four cases were lost to follow-up, a final 59.61% (n = 521) of the cycles were counted as having ongoing pregnancies. The mean number of embryos transferred was 1.05 ± 0.22, and the overall implantation rate of the PGT-A program was 53.41%. Additionally, the correlation between maternal age and the percentage of transferable/non-transferable embryos has been assessed and is represented in Supplementary Fig. 3.
Embryological outcomes of the cycles are shown in Table 3, which summarizes the census for total and mature (metaphase II) oocytes, fertilized oocytes, biopsied embryos, informative embryos, transferable and non-transferable embryos, thawed embryos, and surviving embryos. Regarding the distribution of embryos among the different categories of each studied feature (embryo quality, day of biopsy, and the hatching status of the embryo), it is notable that the majority of them were classified as being of good quality (63.73%) compared with both excellent (14.32%) and poor (21.95%). Additionally, they were mostly biopsied on day 5 rather than on day 6 of embryo development (73.40% vs. 26.60%) and they were not completely hatched at the time of biopsy and freezing (89.59% vs. 10.41%) (Fig. 2).
Embryo quality, day of biopsy, hatching status, and ploidy
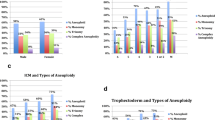
After adjusting by age, the transferable embryo rate was significantly higher in embryos classified as being of excellent-quality (n = 459; 52.69%) compared with the rates in both good-quality (n = 1539; 39.69%) (p < 0.001; OR = 0.574; IC 95%, 0.49–0.67) and poor-quality (n = 350; 26.21%) (p < 0.001; OR = 0.324; IC 95%, 0.26–0.39) embryos. Moreover, statistically significant differences were also found between the euploidy rate of good-quality compared with poor-quality embryos (p < 0.001; OR = 1.858; IC 95% = 1.61–2.13) (Fig. 3).
Regarding the day embryos were biopsied and its relationship to chromosomal normalcy, embryos that were suitable for biopsy on day 5 appeared to be significantly more euploid than embryos that were biopsied on day 6 (n = 1785, 39.98% vs. n = 563, 34.80%, respectively) (p < 0.001; OR = 0.816; IC 95%, 0.71–0.92) independently of their quality at the moment of the biopsy (p = 0.002; OR = 0.806; IC 95%, 0.70–0.92) (Fig. 4A).
In regard to the hatching status of the embryo and its ploidy category, the data showed that hatched embryos were significantly more euploid than non-fully hatched embryos (n = 314, 49.60% vs. n = 2034, 37.32%, respectively) (p < 0.001; OR = 1.621; IC 95%, 1.36–1.93) again, independently of the embryo quality (p < 0.001; OR = 1.761; IC 95%, 1.45–2.12) (Fig. 4B).
Embryo quality, day of biopsy, hatching status, and survival after freezing
The data showed that the survival rate was also dependent on embryo quality, as both excellent- and good-quality embryos had significantly better chances of surviving than did poor-quality embryos (n = 310, 92.26% and n = 774, 92.47% respectively, vs. n = 121, 84.61%) (p = 0.009; OR = 0.444; IC 95%, 0.24–0.81 and p = 0.002; OR = 2.328; IC 95% = 1.37–3.93) (Fig. 5).
Concerning the day of the biopsy, statistically significant differences were found between survival rates of embryos biopsied on day 5 compared with embryos biopsied on day 6 (n = 969, 93.71% vs. n = 236, 83.69%) (p < 0.001; OR = 0.341; IC 95%, 0.22–0.51) regardless of the embryo quality group to which they belonged (p = 0.002; OR = 0.490; IC 95%, 0.31–0.76) (Fig. 6A).
Finally, as for the hatching status of the embryo and its relationship to the probability of surviving after freezing, non-fully hatched embryos were found to survive better than fully hatched embryos when thawed (n = 1069, 93.52% vs. n = 136, 78.61%) (p < 0.001; OR = 0.247; IC 95%, 0.16–0.38) independently of the embryo quality they presented at the moment of the biopsy (p < 0.001; OR = 0.300; IC 95%, 0.18–0.48) (Fig. 6B).
Discussion
This monocentric study investigated the prevalence and significance of embryo quality, blastocyst development rate, and hatching status in relation to euploidy and survival rates after embryo biopsy. A better understanding of the factors that could influence these two end points seems to be of crucial interest when counseling patients after a TE biopsy cycle.
According to our data, excellent- and good-quality embryos, day 5–biopsied embryos and FHB are each independently more likely to be euploid than are poor-quality embryos, day 6 embryos, or nFHB. Regarding embryo survival after biopsy and freezing, excellent- and good-quality embryos, day 5–biopsied embryos, and nFHB have better chances of surviving than poor-quality, day 6, and FHB, respectively, when there is an independent relationship among these three factors.
In this study, more than three-quarters of the patients were performing a PGT-A cycle due to their advanced maternal age. This percentage is consistent with previous publications where advanced maternal age indication is present in the 77% of the PGT-A patients [21]. It has already been published that the history of a patient in terms of previous implantation failure, miscarriages, or the lack of euploid embryos in past PGT-A cycles have no relationship with the euploidy rate of the present cycle [22]. This is the rationale behind clustering all patients regardless of their PGT-A indication. By doing so, the total number of cases analyzed is increased and a more practical approach reflecting the reality of an IVF center is presented. Furthermore, the aim of this study was not to relate maternal age with the three embryonic parameters studied. Nonetheless, results have been adjusted by age to confirm that maternal age was not acting as a confounder.
The global advanced maternal age of the population could explain why the usable blastocyst rate was unusually low (39.49%), the global euploidy rate was 38.60%, and that almost half of the patients (45%) ended up having no euploid embryos available to transfer. Our PGT-A program’s global results are comparable to those of other works with a similar maternal mean age [23, 24]. Maternal age is the strongest predictor of PGT-A cycle outcome [25], and it not only affects the percentage of euploid embryos and thus the chances for a patient to have at least one transferable embryo [26], but it also impacts embryo quality and development rate [14, 27, 28] and therefore the chances of survival after vitrification [29].
Besides maternal age, prior studies have investigated the correlation between chromosomal abnormalities and embryo quality [30]. The data shown here agree with other data showing that better embryo quality is related to better chances of an embryo of being euploid [7, 14, 31] without disregarding the contribution that poor-quality embryos can make to the total number of embryos available for transfer [32]. Concerning the biopsy time, the present results correlate with those of previous publications [10, 11, 31, 33, 34] in concluding that day 5–biopsied embryos are more prone to be euploid than day 6–biopsied embryos. Some authors have previously stated that delayed blastulation is related to higher chances of aneuploidy and that this delay increases with every additional chromosomal abnormality [35]. It has already been reported that the time to blastocyst formation may depend on culture conditions [36, 37] but, since a considerable number of studies (performed in different culture conditions) agree with our findings on this point, it seems that reaching the blastocyst stage between 110 and 120 h after insemination may be a sign of good prognosis.
The fact that fully hatched blastocysts were more euploid than non-fully hatched ones, independent of the day-of-biopsy variable, does not appear to align with the results of Rodriguez-Purata and colleagues [13], who found no differences in euploidy between these two groups. The disparity with our results could be caused by the smaller size of their sample and the younger maternal age of their participants.
Compared with non-PGT cycles, and as opposed to other TE biopsy analysis [26, 38], in this study, we performed assisted hatching on day 3 of embryo development to facilitate embryo herniation by day 5. Embryos could escape the zona pellucida once they started expanding before it was expected from their degree of expansion. Therefore, the fact that some (compared with others) were already hatched may correspond to an earlier expansion not related to the moment when blastocysts would naturally abandon the zona pellucida. Under these circumstances, the hatching status of the embryo must be considered a sign of the degree of expansion rather than a biological event that merits being related to euploidy. Therefore, the results may demonstrate that earlier-expanded blastocysts appear to be more euploid than late-expanded ones, in concordance with Minasi’s observations [9].
Concerning survival after freezing in relation to the studied features, the data support previous investigations in concluding that embryo quality before vitrification is the clue to predicting the chances of a certain embryo’s survival [39, 40]. The results emphasize the importance of genetic and cytoplasmic events occurring during development that intervene in both the development into a good-quality embryo and the ability to survive a freezing protocol. The effect of euploidy alone on survival rate has not been addressed in this study and it has already been settled for future investigations.
When offering PGT to patients, a high embryo survival rate is crucial, and there is still little information available on the impact of performing an embryo biopsy before vitrification. Besides, there does not seem to be a consensus on the fact that time to blastulation could be related neither to survival rates [41, 42] nor to live birth rates after thawing embryos [43, 44]. Our data show a better survival rate for day 5–biopsied embryos compared with day 6 ones, independent of embryo quality and hatching status. This may indicate that (1) embryos that reach the blastocyst stage earlier are more competent in protecting themselves against cryopreservation insult, or (2) embryos should be cryopreserved when they naturally reach the blastocyst stage. Even if the use of time-lapse incubators has allowed embryologists to adjust as much as possible the time of biopsy to the time of herniation, the day 6–biopsy group results should be viewed with caution because the group might have been artificially enriched by embryos that reached herniation late on day 5 and during the night, when performing biopsies was not possible. Understanding the differences in survival rate between day 5 and day 6 of development is especially relevant when discussing the implementation of new PGT approaches such as niPGT (non-invasive PGT). It seems that niPGT’s effectiveness depends on the embryo being cultured until day 6 [45], and some authors have agreed that day 6 embryos can survive as well as day 5 ones [46, 47]. However, their data do not correlate with our findings as, in their studies, embryos were vitrified when they had reached the blastocyst stage naturally; no studies have examined the differences in survival rate between day 5–blastulated embryos that were vitrified on day 5 compared with day 6–vitrified embryos that had already blastulated on day 5.
Regarding embryo hatching status, our results concur with those of others who have proposed that the zona pellucida might provide physiological protection against both mechanical and chemical events that occur during vitrification [39, 48]. As some groups have already postulated, assisted hatching right before the biopsy could be an alternative method to avoid having a hatched blastocyst at biopsy and vitrification [38]. Zona drilling on day 3 was supposed to be an adequate strategy to facilitate TE biopsy, particularly as failure to hatch has previously been described as a limitation for implantation [49] but, given the present results, this strategy should be re-examined.
To our knowledge, this study includes the largest number of biopsied embryos cultured in a timelapse incubator among monocentric studies that have attempted to correlate embryonic factors with both euploidy and survival after thawing giving a complete overview of the embryonic parameters that can affect the success of a PGT-A cycle. In our opinion, it provides a better understanding of the impacts that embryo quality, developmental speed, and embryo hatching may have on both euploidy and survival rates in blastocyst PGT cycles. Additionally to maternal age, this may clarify the counseling given to patients undergoing a TE biopsy PGT-A about their chances of having a transferable embryo capable of surviving after vitrification.
Data Availability
The data supporting the findings of this study are available from the corresponding author, MF, upon request.
References
Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from biopsied human preimplantational embryos sexed by Y-specific DNA amplification. Nat. 1990;344:768–70.
Sermon K. Novel technologies emerging for preimplantation genetic diagnosis and preimplantation genetic testing for aneuploidy. Expert Rev Mol Diagn. 2017;17(1):71–82.
Verlinsky Y, Cieslak J, Freidine M, Ivakhenko V, Wolf G, Kovalinskaya L, White M, Lifchez A, Kaplan B, Moise J, Valle J, Ginsberg A, Strom C, Kuliev A. Diagnosing and preventing inherited disease: pregnancies following pre-conception diagnosis of common aneuploidies by fluorescence in-situ hybridization. Hum Reprod. 1995;10:923–7.
Munne S, Lee A, Rosenwaks Z, Grifo J, Cohen J. Diagnosis of major chromosome aneuploidies in human preimplantation embryos. Hum Reprod. 1993;8:2185–91.
McArthur SJ, Leigh D, Marshall JT, De Boer KA, Jansen RPS. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril. 2005;84:1628–36.
Scott RT, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100:624–30.
Minasi MG, Greco E. Current aspects of blastocyst culture, biopsy, and vitrification. CCE Curr Trends Clin Embryol. 2014;I:27–33.
Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards Reproductive Certainty: Infertility and Genetics beyond 1999. Carnforth: Parthenon Press; 1999. p. 378–88.
Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarcelli F, Spinella F, Fiorentino F, Varrichio MT, Greco E. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31:2245–54.
Kaing A, Kroener LL, Tassin R, Li M, Liu L, Buyalos R, et al. Earlier day of blastocyst development is predictive of embryonic euploidy across all ages: essential data for physician decision-making and counselling patients. J Assist Reprod Genet. 2018;35:119–25.
Taylor TH, Patrick JL, Gitlin SA, Wilson JM, Crain JL, Griffin DK. Comparison of aneuploidy, pregnancy and live birth rates between day 5 and day 6 blastocysts. Reprod Biomed Online. 2014;29:305–10.
Hammond ER, Cree LM, Morbeck DE. Should extended blastocyst culture include Day 7? Hum Reprod. 2018;33:991–7.
Rodriguez-Purata J, Gingold J, Lee J, Whitehouse M, Slifkin R, Briton-Jones C, et al. Hatching status before embryo transfer is not correlated with implantation rate in chromosomally screened blastocysts. Hum Reprod. 2016;31:2458–70.
Li N, Guan Y, Ren B, Zhang Y, Du Y, Kong H, Zhang Y, Lou H. Effect of blastocyst morphology and developmental rate on euploidy and live birth rates in preimplantation genetic testing for aneuploidy cycles with single-embryo transfer. Front Endocrinol. 2022;858042:13.
Tiegs AW, Sun L, Patounakis G, Scott RT Jr. Worth the wait? Day 7 blastocysts have lower euploidy rates but similar sustained implantation rates as day 5 and day 6 blastocysts. Hum Reprod. 2019;34:1632–9.
Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373:2089–90.
Cram DS, Leigh D, Handyside A, Rechitsky L, Xu K, Harton G, et al. PGDIS Position Statement on the Transfer of Mosaic Embryos. Reprod Biomed Online. 2019;39(Suppl 1):e1–4.
Grati FR, Gallazzi G, Branca L, Maggi F, Simoni G, Yaron Y. An evidence-based scoring system for prioritizing mosaic aneuploid embryos following preimplantation genetic screening. Reprod Biomed Online. 2018;36:442–9.
Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simón C. The endometrial receptivity array for diagnosis and personalised embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818–24.
Giunco H, Connerney M, Boylan C, Koelper N, Mersereau J, Berger DS. Embryo re-expansion does not affect clinical pregnancy rates in frozen embryo transfer cycles: a retrospective study. J Assist Reprod Genet. 2021;38:2933–9.
Patrizio P, Shoham G, Shoham Z, Leong M, Barad DH, Gleicher N. Worldwide live births following the transfer of chromosomally “Abnormal” embryos after PGT/A: results of a worldwide web-based survey. J Assist Reprod Genet. 2019;36:1599–607.
Cimadomo D, Capalbo A, Dovere L, Tacconi L, Soscia D, Giancani A, et al. Leave the past behind: women’s reproductive history shows no association with blastocysts’ euploidy and limited association with live birth rates after euploid embryo transfers. Hum Reprod. 2021;36:929–40.
Sacchi L, Albani E, Cesana A, Smeraldi A, Parini V, Fabiani M, et al. Preimplantation genetic testing for aneuploidy improves clinical, gestational, and neonatal outcomes in advanced maternal age patients without compromising cumulative live-birth rate. J Assist Reprod Genet. 2019;36(12):2493–504.
Sanders KD, Silvestri G, Gordon T, Griffin DK. Analysis of IVF live birth outcomes with and without preimplantation genetic testing for aneuploidy (PGT-A): UK Human Fertilisation and Embryology Authority data collection 2016–2018. J Assist Reprod Genet. 2021;38(12):3277–85.
La Marca A, Capuzzo M, Imbrogno MG, Donno V, Spedicato GA, Sacchi S, Minasi MG, Spinella F, Greco P, Fiorentino F, Greco E. The complex relationship between female age and embryo euploidy. Minerva Obstet Gynecol. 2020;73:103–10.
Awadalla MS, Vestal NL, McGinnis LK, Ahmady A, Paulson RJ. Effect of age and morphology on sustained implantation rate after euploid blastocyst transfer. Reprod Biomed Online. 2021;43:395–403.
Irani M, O’Neill C, Palermo GD, Xu K, Zhang C, Qin X, et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110:95-102.e1.
Reig A, Franasiak J, Scott RT, Seli E. The impact of age beyond ploidy: outcome data from 8175 euploid single embryo transfers. J Assist Reprod Genet. 2020;37:595–602.
Cimadomo D, Capalbo A, Levi-Setti PE, Soscia D, Orlando G, Albani E, et al. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Hum Reprod. 2018;33:1992–2001.
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, Katz-Jaffe MG, Wells D. The relationship between blastocyst morphology, chromosomal abnormality and embryo gender. Fertil Steril. 2011;95:520–4.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–81.
Cimadomo D, Soscia D, Vaiarelli A, Maggiulli R, Capalbo A, Ubaldi FM, Rienzi L. Looking past the appearance: a comprehensive description of the clinical contribution of poor-quality blastocysts to increase live birth rates during cycles with aneuploidy testing. Hum Reprod. 2019;34:1206–14.
Lane S, Reed L, Schoolcraft W, Katz-Jaffe M. Euploid day 7 blastocysts of infertility patients with only slow embryo development have reduced implantation potential. Reprod Med Online. 2022;44:858–65.
McDaniel K, Awadalla M, McGinnis L, Ahmady A. Transfer the best and biopsy the rest? Blastocyst euploidy rates differ by morphology and day of biopsy. Arc Gyn Obstet. 2021;303:249–58.
Vega M, Breborowicz A, Moshier EL, McGovern PG, Keltz MD. Blastulation rates decline in a linear fashion from euploid to aneuploid embryos with single versus multiple chromosomal errors. Fertil Steril. 2014;102:394–8.
Deng J, Qianying Z, Cinnioglu C, Kayali R, Lathi R, Behr B. The impact of culture conditions on blastocyst formation and aneuploidy rates: a comparison between single-step and sequential media in a large academic practice. J Assist Reprod Genet. 2020;37:161–9.
Sfontouris I, Martins W, Nastri C, Viana I, Navarro P, Raine-Fenning N, van der Poel S, Rienzi L, Racowsky C. Blastocyst culture using single versus sequential media in clinical IVF: a systematic review and meta-analysis of randomized controlled tria’ls. J Assist Reprod Genet. 2016;33:1261–72.
Maggiulli R, Giancani A, Cimadomo D, Ubaldi F, Rienzi L. Human blastocyst biopsy and vitrification. J Vis Exp. 2019;26(149).
Oliva M, Briton-Jones C, Gounko D, Lee J, Copperman A, Sekhon L. Factors associated with vitrification-warming survival in 6167 euploid blastocysts. J Assist Reprod Gen. 2021;38:2671–8.
Shear MA, Vaughan DA, Modest AM, Seidler EA, Leung AQ, Hacker MR, Sakkas D, Penzias AS. Blasts from the past: is morphology useful in PGT-A tested and untested frozen embryo transfers? Reprod Biomed Online. 2020;41:981–9.
Muthukumar K, Kamath MS, Mangalaraj AM, Aleyamma TK, Chandy A, George K. Comparison of clinical outcomes following vitrified warmed day 5/6 blastocyst transfers using solid surface methodology with fresh blastocyst transfers. J Hum Reprod Sci. 2013;6:59–64.
Sunkara SK, Siozos A, Bolton VG, Khalaf Y, Braude PR, El-Toukhy T. The influence of delayed blastocyst formation on the outcome of frozen-thawed blastocyst transfer: a systematic review and meta-analysis. Hum Reprod. 2010;25:1906–15.
Ferreux L, Bourdon M, Sallem A, Santulli P, Barraud-Lange V, Le Foll N, Maignien C, Chapron C, de Ziegler D, Wolf J-P, Pocate-Cheriet K. Live birth rate following frozen–thawed blastocyst transfer is higher with blastocysts expanded on day 5 than on day 6. Hum Reprod. 2018;33:390–8.
Liebermann J, Tucker M. Comparison of vitrification and conventional cryopreservation of day 5 and day 6 blastocysts during clinical application. Fertil Steril. 2006;86:20–6.
Navarro L, García-Pascual C, Rubio C, Simon C. Non–invasive preimplantation genetic testing for aneuploidies: an update. Reprod Biomed Online. 2022;44:817–28.
Kaye L, Will EA, Bartolucci A, Nulsen J, Benadiva C, Engmann L. Pregnancy rates for single embryo transfer (SET) of day 5 and day 6 blastocysts after cryopreservation by vitrification and slow freeze. J Assist Reprod Genet. 2017;34:913–9.
Viñals Gonzalez X, Odia R, Naja R, Serhal P, Saab W, Seshadri S, Ben-Nagi J. Euploid blastocysts implant irrespective of their morphology after NGS-(PGT-A) testing in advanced maternal age patients. J Assist Reprod Genet. 2019;36:1623–9.
Rubino P, Tapia L, de Assin Ruiz, Alonso R, Mazmanian K, Guan L, Dearden L, Thiel A, Moon C, Kolb B, Norian JM, Nelson J, Wilcox J, Tan T. Trophectoderm biopsy protocols can affect clinical outcomes: time to focus on the blastocyst biopsy technique. Fertil Steril. 2020;113:981–9.
Practice Committees of the ASRM and SART. Role of assisted hatching in in vitro fertilization: a guideline. Fertil Steril. 2014;102:348–51.
Author information
Authors and Affiliations
Contributions
MF conceived the study, analyzed the data, and wrote the manuscript. AC and AB collected the data. ME was involved in conceiving the study and revising the manuscript. All authors approved the content and contributed to drafting and revising the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by both the research board and the ethics committee of Clinical Research IVIRMA Valencia, Spain (1909-BCN-083-MF).
Employment
The authors have neither a present, nor a past, or an anticipated employment that may gain or lose financially or non-financially through the publication of this manuscript.
Data attestation statement
The subjects included in this study have not concomitantly been involved in other randomized trials. Data regarding any of the subjects in the study has not been previously published unless specified. Data will be made available to the editors of the journal for review or query upon request.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Florensa, M., Cladellas, A., Ballesteros, A. et al. Preimplantation genetic testing for aneuploidy: predictive embryonic factors. J Assist Reprod Genet 41, 1329–1339 (2024). https://doi.org/10.1007/s10815-024-03061-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-024-03061-5