Abstract
Purpose
This study investigates the role of bacterial vaginosis (BV) on pregnancy rates during various fertility treatments. BV is known to influence several obstetric outcomes, such as preterm delivery and endometritis. Only few studies investigated the effect of BV in subfertile women, and studies found a negative effect on fecundity especially in the in vitro fertilisation population.
Methods
Observational prospective study, 76 couples attending a fertility clinic in the Netherlands between July 2019 and June 2022, undergoing a total of 133 attempts of intra uterine insemination, in vitro fertilization or intra cytoplasmatic sperm injection. Vaginal samples taken at oocyte retrieval or insemination were analysed on qPCR BV and 16S rRNA gene microbiota analysis of V1–V2 region. Logistic regression with a Generalized Estimated Equations analysis was used to account for multiple observations per couples.
Results
A total of 26% of the 133 samples tested positive for BV. No significant differences were observed in ongoing pregnancy or live birth rates based on BV status (OR 0.50 (0.16–1.59), aOR 0.32 (0.09–1.23)) or microbiome community state type. There was a tendency of more miscarriages based on positive BV status (OR 4.22 (1.10–16.21), aOR 4.28 (0.65–28.11)) or community state type group III and IV. On baseline qPCR positive participants had significantly higher body mass index and smoked more often. Odds ratios were adjusted for smoking status, body mass index, and socioeconomic status.
Conclusion
Bacterial vaginosis does not significantly impact ongoing pregnancy rates but could affect miscarriage rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Between 8 and 12% of couples worldwide are affected by subfertility. There are many causes of subfertility, such as ovulatory dysfunction or male factor, but 15% of couples have no identifiable cause [1, 2]. Bacterial vaginosis (BV) might be a reason (or a cue) for infertility, which is defined as dysbiosis of the vaginal microbiome. It is characterized by the loss of lactic acid-producing bacteria and an increase in the number and diversity of anaerobic bacteria (such as Gardnerella vaginalis). The aetiology and pathogenesis of BV is still unknown, but it is associated with certain lifestyle factors [3]. BV can cause symptoms such as abnormal discharge and fishy odour; however, half of patients with BV are asymptomatic. Various reports measure an incidence of 10–32% of BV among the total population. The incidence of BV is influenced by ethnicity and is shown to be higher in subfertile populations [4, 5]. BV is associated with a higher risk of infections (including sexually transmitted ones), tubal factor infertility, and obstetric complications, such as preterm delivery and endometritis [6, 7].
Some studies have found a negative effect of BV on pregnancy rates among (sub)fertile women, which was primarily studied in an IVF population [6, 8, 9] and only in two studies in a population undergoing intra uterine insemination [10, 11]. Only in recent years, the more sensitive methods like qPCR and microbiota analysis have been used to diagnose BV [12, 13]. Most studies investigate the vaginal microbiome, and some also the endometrial microbiome. There is evidence of a continuum between these two microbiotas [14].
It is suggested that BV influences the endometrium and, thus, the implantation rate. Meta-analysis showed more early pregnancy loss in BV positive women [13, 15]. Furthermore, higher prevalence of unfavourable microbiome was found in multiple studies investigating recurrent implantation failure (RIF) or recurrent miscarriage, except for one study with a larger study size [16, 17].
Therapies for BV include metronidazole and clindamycin, but the recurrence rate within a year remains high [18, 19]. There are also promising data about new treatment options, for example, with probiotics [20]. Up till now, there are no studies with good quality evidence that treatment of BV increases pregnancy rates.
Since evidence on the relation between BV and a subfertile population undergoing intra uterine insemination (IUI) and IVF/ICSI is insufficient, our aim is to investigate the influence of BV on pregnancy rates in this study population. It is hypothesized that BV may have a negative impact on ongoing pregnancy rates in both the IUI and IVF/ICSI population. Additionally, BV could be possibly associated with higher rates of early pregnancy loss.
Materials and methods
This is a prospective single centre cohort study in the Haaglanden Medical Center (HMC) in the Hague, the Netherlands. This fertility clinic is a transport clinic to the IVF laboratory of the Leiden University Medical Center. Microbiological assessment of vaginal swabs was done by external laboratory of Eurofins NMDL and DDL Diagnostic Laboratory in Rijswijk, the Netherlands. Participants (18 years and older) undergoing fertility treatments (intra uterine insemination (IUI), in vitro fertilization (IVF), and intra cytoplasmatic sperm injection (ICSI)) were included between July 2019 and June 2022. Exclusion criteria were inability to understand Dutch or English language, persons with three or more miscarriages, and those on prophylactic antibiotic treatment. There were no couples treated with donor sperm or donor oocytes. Before starting IUI or IVF/ICSI-treatment couples were required to cease smoking.
Sample size calculation was performed before starting the study. To detect a difference of 15% in pregnancy rate, assuming a 30% prevalence of BV 372 fertility treatments should be included to reach a power of 90% (alpha 0.05, percentages based on the results of the study of Haahr) [6].
Patient recruitment, sample, and data collection
Persons were asked to join the study at their initial fertility assessment (IFA). In the Netherlands, persons need to have a referral (e.g. from a general practitioner) for an IFA. When participants gave informed consent, the first vaginal swab (e-swab, Copan Italia SpA, Breschia, Italy) and pH measurement (pH-Fix 4.0-7.0, ref 92137, Macherey-Nagel, Düren, Germany) were taken from the posterior fornix after inserting a speculum. The measurements were not performed if the woman was menstruating or post-coital. Participants and fertility doctors were blinded for the outcome of the swab. If participants had subsequent IUI or IVF/ICSI treatment, the sampling was performed again, prior to the ovum pick up or insemination, up to a maximum of 4 procedures, to obtain an accurate BV status for each individual IUI and IVF/ICSI attempt. During ovum pick up, antibiotics are not routinely administered, but only given for certain indications (for example with endometriomas). In case of a frozen embryo cycle, the sampling was performed at the last ultrasound before transfer. After an ongoing pregnancy, no new swabs were collected. The vaginal samples were frozen within 24 h in the HMC laboratory and later transported to the external laboratories for molecular analysis. If participants had complaints of BV which required treatment, they were tested separately from our study. When tested positive for BV, they were treated according to the standard protocol.
Blood samples for hCG measurement were collected two weeks after insemination, ovum pick up, or embryo transfer. Follow-up ended at cessation of treatment, end of study (August 2022) or live birth (follow-up until June 2023). Information about patient characteristics, fertility treatment, and pregnancy outcomes were extracted from the electronic patient files. This information was managed using Castor EDC, a cloud-based clinical data management service.
BV qPCR
Extracted DNA from all swabs was tested with a CE-IVD marked multiplex quantitative PCR assay (AmpliSens® Florocenosis/Bacterial vaginosis-FRT PCR kit, InterLabService, Moscow, Russia) according to the manufacturer’s instructions. Based on the presence of Lactobacillus species, Gardnerella vaginalis, Atopobium vaginae (recently reclassified as Fannyhessea vaginae) [21], and total amount of bacteria, swabs were categorised as BV positive (amount of G. vaginalis and/or A. vaginae is almost equal or exceeds the amount of Lactobacillus spp.), BV negative (G. vaginalis and/or A. vaginae are absent or its amount is substantially less than the Lactobacillus spp. amount), unspecified dysbiosis (amount of Lactobacillus spp. is reduced relative to the total amount of bacteria, whereas G. vaginalis and/or A. vaginae are absent or its amount is substantially less than total amount of bacteria), or suspected dysbiosis (amount of G. vaginalis and/or A. vaginae is similar to the amount of Lactobacillus spp. but does not exceed the limit value) using the software tool provided by the kit manufacturer. Unspecified dysbiosis and suspected dysbiosis were regarded as qPCR BV positive.
Microbiota analysis
Microbiota analysis was performed on the extracted DNA of swabs of participants who started directly with IUI or IVF. Microbiota analysis was done on their IFA swab, and on their subsequent swabs during IUI or IVF/ICSI treatment until pregnant or until switch from IUI to IVF/ICSI. A fragment of ~ 421bp of the V1–V2 region of the 16S rRNA gene was amplified using the primers described by Ravel et al. (2011) and Walker et al. (2015) with Illumina overhang adaptor sequences added [22, 23]. Results were classified in one of five vaginal microbiome community state types (CST), as described by Ravel et al. (2011). CST I is dominated by L. crispatus and respectively CST II by L. gasseri, CST III by L. iners, CST IV by non-lactobacilli, and CST V by L. jensenii [22]. For more details on the nucleic acid extraction and microbiota analysis, see Supplementary File 1.
Outcomes
Primary endpoint of the study was ongoing pregnancy rate at 12 weeks gestation. Secondary endpoints were miscarriages (including biochemical pregnancy and clinical pregnancy with or without foetal heartbeat at 7 weeks gestation), live birth, ectopic pregnancy, and preterm delivery (< 37 weeks).
Compliance with ethical standards
This study was approved by the Medical Ethics Committee of Leiden Den Haag Delft, reference Z21.031. All participants gave their informed consent to involve in this study.
Statistical analysis
IBM SPSS statistics for Macintosh, version 27, was used for all analysis. Continuous parametric variables were analysed using an unpaired t test. Continuous non-parametric variables are analysed using the Mann-Whitney U test. Categorical variables are examined using the Chi2 or Fischer’s exact test. To account for the repeated measurements per case, a general estimating equations analysis (GEE regression model) is used, with an exchangeable correlation matrix.
Results
Description of included participants
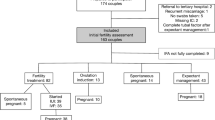
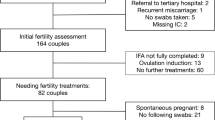
Figure 1 shows a flow diagram of included couples. Some couples started IUI or IVF/ICSI treatment but had no subsequent swabs done and therefore were excluded from the analysis. In total, 76 couples could be included, of which 68 couples underwent fertility treatment with subsequent vaginal swabs. Couples conceiving spontaneously before fertility treatment and couples starting fertility treatments after expectant management or ovulation induction were included for analysis. Microbiome CST was analysed in the subgroup of direct indication for fertility treatment, with eight couples conceiving spontaneously before fertility treatment started.
Table 1 describes baseline characteristics of the couples based on BV qPCR results at IFA. One participant had no start qPCR result, but two subsequent negative qPCR results, and was regarded as BV negative at IFA.
The maximum age of participants was 42 years. Almost no discharge complaints were reported; only one participant was treated for discharge complaints. BV positive participants at baseline have a significant higher BMI and a higher percentage of smokers. The measured pH was significantly higher in the BV qPCR positive group.
Separate analysis comparing ongoing pregnant versus non pregnant participants or participants experiencing a miscarriage versus no miscarriage did not show significant differences for BMI or smoking (data not shown).
Pregnancy outcomes
Pregnancy results are shown in Table 2, based on BV status at the time of attempt. In total, 133 attempts were included, of which 26% of samples tested positive on BV. One of those attempts had a total fertilization failure (IVF-procedure), so the participant could not receive a fresh ET. Ongoing pregnancy and live birth rates were lower in BV qPCR positive attempts, however not significant (20% vs 11%, OR 0.50). Table 2 shows a higher percentage of miscarriages in qPCR BV positive attempts. Analysed on number of miscarriages in pregnant participants, this also was significant (25% vs 60%, OR 4.22). To adjust for confounders, correction was applied for BMI, smoking and SES. The adjusted odds ratios were in line with the non-adjusted odds. Cause of subfertility and age had no significant influence and were therefore excluded from the model.
Separate analysis of the IVF/ICSI attempts are in line with data above; however, data did show an on average stronger effect of BV positivity than in IUI attempts (data not shown).
Four participants had a preterm delivery, three of whom delivered between 32 and 37 weeks gestation. One participant delivered at 24 weeks gestation after a weeklong episode of cramps and vaginal blood loss, which required admission to the hospital. The neonate died 7 days postpartum due to its prematurity. This participant tested negative for BV qPCR with every IUI attempt. However, the microbiota analysis of this patient at the start of treatment showed microbiome CST IV and later CST III. One of four preterm delivered participants tested BV positive and was in preterm labor around 32 weeks, although delivered eventually at 36 weeks. One participant had a termination of pregnancy around 20 weeks’ gestation because of a serious birth defect (Noonan’s syndrome, BV qPCR negative, CST III). One pregnancy of unknown location was reported.
Table 3 shows pregnancy results based on microbiome community state type (CST) at the moment of IUI/IVF/ICSI attempt. In total, 95 attempts were analysed on microbiome CST. CST II and CST V together were detected in only 5 attempts and were not included in the statistical analysis. CST I (L. crispatus) has a tendency of higher chance of an ongoing pregnancy compared to CST III and CST IV (32% in CST I vs 13% in CST III vs 22% in CST IV). Live birth rate in CST III was even lower because of one termination of pregnancy. CST III (L. iners) and CST IV have a tendency of higher chance of miscarriage (5% in CST I vs 13% in CST III and 22% CST IV). Numbers were too small for a clinically significant effect.
Description of microbiome results
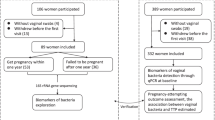
A total of 161 samples were tested with BV qPCR and microbiota analysis (including swabs at IFA and at insemination or IVF/ICSI procedure). The microbiome was analysed in a subgroup of the study, as seen in Fig. 1. These 161 samples belonged to 73 different people, so a median of 2 samples per person. At species level, all five CST classes are present. CST group II and V had only few samples as these Lactobacilli types are less common. One participant could not be classified with the classification system of Ravel (with dominating L. johnsonii and L. ultunensis). Of these, 161 samples, 40 (25%) were tested positive for BV qPCR (Supplemental Fig. 1). Shannon Diversity Index was significantly higher in the BV qPCR positive samples (2.62 vs 0.95, p < 0.001). Group IV, the type of microbiome described as the most abnormal in literature, contained 37 samples (23% of total number of samples). Thirty-three of the 37 CST IV samples tested positive for BV qPCR (89%, p < 0.001). Supplementary file 2 shows a more detailed description about different bacteria found.
Out of the in total 73 participants, 15 persons had no subsequent attempts. The microbiota of all the swabbed IUI/IVF/ICSI attempts of in total 58 persons is shown in Fig. 2. The samples are ordered per class, with the highest percentage of Lactobacilli shown first. Shannon Diversity Index did not significantly differ between samples whether a pregnancy was established or not (p = 0.07).
When zooming in on the 29 microbiome samples on which a pregnancy was directly established (Fig. 3), CST I, III, and IV are present in both groups. The only CST I sample with a subsequent miscarriage contained 61% of L. crispatus. The samples with ongoing pregnancy contained on average 74% Lactobacilli, and samples with subsequent miscarriage contained on average 59% Lactobacilli (p = 0.33). Shannon Diversity Index did not significantly defer between samples with subsequent ongoing pregnancies or miscarriages (p = 0.16).
Discussion
This is one of the first studies to date researching the effect of bacterial vaginosis (diagnosed by qPCR) in a broader population than solely IVF patients. This study shows an increase in miscarriages due to qPCR BV positive status or microbiome CST III and IV during IUI or IVF/ICSI. A slight decrease in ongoing pregnancies due to qPCR BV positivity was observed, however not significant. The CST I (L. crispatus) group showed a small (not significant) increase in ongoing pregnancies compared to other community state types.
Previous studies had a very specific IVF population [6, 8, 9], with probably a higher significant influence of BV status on pregnancy outcomes then this relative ‘good prognosis’ study group treated with also IUI. Haahr et al. described a difference in IVF pregnancy results of 35% based on qPCR.[6] Koedooder et al. reported a 5.9% IVF pregnancy rate when using the specific unfavourable microbiome algorithm, whereas they observed a 54% pregnancy rate in their most favourable group with less than 60% L. crispatus [8]. This could suggest that BV only has a significant impact in a certain (poor prognosis) population. There was a difference on smoking status and BMI between groups in our study. Smoking was measured as smoking at moment of sampling. Other studies do not mention the definition of smoking or classify smoking as ‘has ever smoked’. Probably BMI and currently smoking (and thereby the immune system) play a role in BV status [3]. Participants had a relatively high socioeconomic status (SES), which could result in bias. However, a slightly lower SES in participants with BV was shown. This could indicate a relationship between lifestyle and dietary intake on the microbiome [24]. Most other studies did not record the SES thoroughly, so possibly the effect of BV lies more in being a proxy for a healthy lifestyle.
A consensus should be reached on how to define and measure an abnormal microbiome or BV status to minimize heterogeneity in further studies. The predictive model of Koedooder et al. (2019) described a negative impact on pregnancy outcomes when there was a high percentage of Proteobacteria and L. jensenii [8]. However, in this study, there were almost no samples with these percentages of Proteobacteria or L. jensenii (detailed in supplementary file 2). This study showed that CST III and CST IV have similar pregnancy results. The similarity of CST III (L. iners) and CST IV is previously described [6]. Since treatment of CST III is not possible yet, qPCR testing could be sufficient in future clinical settings. In the absence of evidence that treatment of BV leads to better pregnancy outcomes, testing of asymptomatic subfertile individuals for BV (qPCR or microbiome sequencing) should be discouraged at this moment.
A strength of this study is to analyse several consecutive attempts of IUI or IVF/ICSI instead of only one attempt, which could show an effect if it takes longer to become pregnant with a certain condition. Another strength is that the follow-up period was long enough to report live birth rates. Thirdly, this study compares qPCR BV and (partly) the microbiota analysis. This was also suggested by Skafte-Holm, to make it easier to compare studies in the future [13]. Sample outcomes of BV qPCR were in concordance with 16S microbiota analyses. This suggests that qPCR analysis is probably sufficient for a classification in certain patients. Low rates of Lactobacilli are correlated with lower ongoing pregnancy rates, and this is exactly what the BV qPCR tests. The incidence of bacterial vaginosis is high in this multi-ethnic subfertile group, which aligns with other literature. The pathogens and vaginal CST classes identified in earlier research were also seen in this study. However, one participant’s vaginal microbiome was dominated by L. johnsonii/L. ultunensis, which could not be classified into the known CST classes.
A limitation of this study is not reaching the sample size needed for the power calculation. The inclusion rate was much lower than expected, mainly due to a temporary closure of the fertility department during the COVID pandemic, and individuals being less willing to participate in scientific studies. Another limitation is that microbiota analysis was performed in a selected group of samples. Microbiota data of all samples might have resulted in stronger evidence of the outcome results, as seen in other literature [8]. Furthermore, there was no data on the microbiome of participants’ partners’ semen. The male partner’s microbiome could also play an interacting role on influencing the vaginal microbiome and pregnancy outcomes.
Further research, especially a randomized trial, should be performed to see if treatment or lifestyle interventions, based on BV qPCR status as a marker, improves pregnancy outcomes. Factors such as smoking, BMI, SES, and ethnicity should be reported to investigate if there is a causal effect of BV on pregnancy results. Further research about BV in persons with RIF or recurrent miscarriage is necessary, and the same kind of randomized trial should be performed in this patient group as well. This study suggests that an unfavourable microbiome or BV could predict for a miscarriage in a population with (yet) no RIF or recurrent miscarriages. In the future, it could be a possibility to wait for a third or last IVF treatment until a more favourable microbiome is reached, to lower the chances of having a miscarriage. It is not clear in current literature if BV is the causal reason for unfavourable pregnancy outcomes or a marker for a certain lifestyle. Testing BV positive could be used as an additional reason to encourage couples to adopt a healthier lifestyle to increase the success of having a healthy baby with fertility treatment.
Conclusions
This study suggests an increased risk in miscarriages in BV qPCR positive IUI/IVF/ICSI attempts (OR 4.22 (1.10–16.22), aOR 4.28 (0.65–28.11)) and in the microbiome CST III and CST IV group (in a subcohort of the study). BV qPCR positive IUI/IVF/ICSI attempts had a slight lower ongoing pregnancy rate, however not significant (OR 0.50 (0.16–1.59), aOR 0.32 (0.09–1.23)).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76.
vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10.
Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(suppl_1):S29–S35.
Babu G, Singaravelu BG, Srikumar R, Reddy SV, Kokan A. Comparative study on the vaginal flora and incidence of asymptomatic vaginosis among healthy women and in women with infertility problems of reproductive age. J Clin Diagn Res [Internet]. 2017;11:DC18–22. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=28969122&retmode=ref&cmd=prlinks.
Borgdorff H, van der Veer C, van Houdt R, Alberts CJ, de Vries HJ, Bruisten SM, et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. Fredricks DN, editor. PLoS One. 2017;12(7):e0181135. https://doi.org/10.1371/journal.pone.0181135.
Haahr T, Jensen JS, Thomsen L, Duus L, Rygaard K, Humaidan P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum Reprod [Internet]. 2016;31:795–803. https://doi.org/10.1093/humrep/dew026.
Ravel J, Moreno I, Simón C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am J Obstet Gynecol [Internet]. 2021;224:251–7. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=33091407&retmode=ref&cmd=prlinks.
Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, de Jonge JD, et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod [Internet]. 2019;34:1042–54. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=31119299&retmode=ref&cmd=prlinks.
Moreno I, Garcia-Grau I, Perez-Villaroya D, Gonzalez-Monfort M, Bahceci M, Barrionuevo MJ, et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome [Internet]. 2022;10:1–17. https://doi.org/10.1186/s40168-021-01184-w.
Amato V, Papaleo E, Pasciuta R, Viganò P, Ferrarese R, Clementi N, et al. Differential composition of vaginal microbiome, but not of seminal microbiome, is associated with successful intrauterine insemination in couples with idiopathic infertility: a prospective observational study. Open Forum Infect Dis [Internet]. 2020 [cited 2022 Oct 14];7. Available from: /pmc/articles/PMC6942492/
Vieira-Baptista P, Silva-Soares S, Lyra J, Falcão V, Póvoa AM, Calejo L, et al. Wet mount microscopy of the vaginal milieu does not predict the outcome of fertility treatments: a cross-sectional study. J Low Genit Tract Dis. 2022;26(2):176–80.
van den Munckhof EHA, van Sitter RL, Boers KE, Lamont RF, Te Witt R, le Cessie S, et al. Comparison of Amsel criteria, Nugent score, culture and two CE-IVD marked quantitative real-time PCRs with microbiota analysis for the diagnosis of bacterial vaginosis. Eur J Clin Microbiol Infect Dis. 2019;38:959–966. https://doi.org/10.1007/s10096-019-03538-7.
Skafte-holm A, Humaidan P, Bernabeu A, Lledo B, Jensen JS, Haahr T. The association between vaginal dysbiosis and reproductive outcomes in sub-fertile women undergoing IVF-treatment: a systematic PRISMA review and meta-analysis. Pathogens. 2021;10(3):295.
Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun [Internet]. 2017;8:811–75. Available from: https://www.nature.com/articles/s41467-017-00901-0.
van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod [Internet]. 2013;28:1809–15. https://doi.org/10.1093/humrep/det096.
Jiao X, Zhang L, Du D, Wang L, Song Q, Liu S. Alteration of vaginal microbiota in patients with recurrent miscarriage. J Obstet Gynaecol [Internet]. 2022;42:248–55. https://doi.org/10.1080/01443615.2021.1904851.
Saxtorph MH, Hallager T, Persson G, Petersen KB, Eriksen JO, Larsen LG, et al. Assessing endometrial receptivity after recurrent implantation failure: a prospective controlled cohort study. Reprod Biomed Online [Internet]. 2020;41:998–1006. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=32978074&retmode=ref&cmd=prlinks.
Haahr T, Elbaek HO, Laursen RJ, Alsbjerg B, Jensen JS, Humaidan P. Treatment of abnormal vaginal microbiota before frozen embryo transfer: case-report and minireview to discuss the longitudinal treatment efficacy of oral clindamycin. Front Physiol [Internet]. 2017;8:415. https://doi.org/10.3389/fphys.2017.00415/full.
Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis [Internet]. 2006;193:1478–86. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=16652274&retmode=ref&cmd=prlinks.
Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, et al. Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. N Engl J Med [Internet]. 2020;382:1906–15. https://doi.org/10.1056/NEJMoa1915254.
Nouioui I, Carro L, García-López M, Meier-Kolthoff JP, Woyke T, Kyrpides NC, et al. Genome-based taxonomic classification of the phylum actinobacteria. Front Microbiol. 2018;22(9):2007.
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108(suppl. 1):4680–7.
Walker AW, Martin JC, Scott P, Parkhill J, Flint HJ, Scott KP. 16S rRNA gene-based profiling of the human infant gut microbiota is strongly influenced by sample processing and PCR primer choice. Microbiome. 2015;22(3):26.
Bowyer RCE, Jackson MA, le Roy CI, Ni Lochlainn M, Spector TD, Dowd JB, et al. Socioeconomic status and the gut microbiome: a TwinsUK cohort study. Microorganisms. 2019;7(1):17.
Acknowledgements
A special thanks to the HMC fertility doctors and their team for including participants and retrieving samples.
Funding
This study was funded by a grant from the Research Fund of the Haaglanden Medical Center (2021) and Stichting Researchfonds Bronovo.
Author information
Authors and Affiliations
Contributions
MMvdT: protocol/project development, data collection and management, data analysis, manuscript writing. EHAvdM: protocol/ project development, microbiome data analysis, manuscript editing. MvdZ: including participants and sample collection, data analysis, manuscript editing. AM: protocol/project development, microbiome data analysis, manuscript editing. JMMvL: manuscript editing, critical discussion. SlC: statistical protocol development, statistical data analysis, manuscript editing KEB: protocol/project development, data analysis, manuscript editing.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Medical Ethics Committee of Leiden Den Haag Delft, reference Z21.031.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van den Tweel, M.M., van den Munckhof, E.H., van der Zanden, M. et al. Bacterial vaginosis in a subfertile population undergoing fertility treatments: a prospective cohort study. J Assist Reprod Genet 41, 441–450 (2024). https://doi.org/10.1007/s10815-023-03000-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-03000-w