Abstract
Purpose
To determine the effect of human growth hormone (GH) supplementation during ovarian stimulation in women undergoing IVF/PGT-A cycles, who do not meet the Bologna criteria for poor ovarian response (POR).
Methods
This is a retrospective cohort study of 41 women with suboptimal outcomes in their first cycle of IVF/PGT-A including lower than expected number of MII oocytes, poor blastulation rate, and/or lower than expected number of euploid embryos for their age, who underwent a subsequent IVF/PGT-A cycle with the same fixed dose gonadotropin protocol and adjuvant GH treatment. Daily cotreatment with GH started with first gonadotrophin injection. The IVF cycle outcomes were compared between the control and GH cycle using the Wilcoxon-Signed Rank test.
Results
The total number of biopsied blastocysts (mean ± SD; 2.0 ± 1.6 vs 3.5 ± 3.2, p = 0.009) and euploid embryos (0.8 ± 1.0 vs 2.0 ± 2.8, p = 0.004) were significantly increased in the adjuvant GH cycle compared to the control cycle. The total number of MII oocytes also trended to be higher in the GH cycle (10.2 ± 6.3 vs 12.1 ± 8.3, p = 0.061). The overall blastulation and euploidy rate did not differ between the control and treatment cycle.
Conclusion
Our study uniquely investigated the use of adjuvant GH in IVF/PGT-A cycles in women without POR and without a priori suspicion for poor outcome based on their clinical parameters. Our study presents preliminary evidence that GH supplementation in these women is beneficial and is associated with an increased number of blastocysts for biopsy and greater number of euploid embryos for transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of adjuvant growth hormone (GH) with gonadotropin ovarian stimulation to enhance ovarian response was first proposed by Homburg et al. [1] in 1988. In the last 30 years, there have been numerous studies investigating the use of GH supplementation during ovarian stimulation for IVF in normal responders, women with PCOS, women with poor embryo development, poor responders, and poor prognosis patients (reviewed by [2]).
Growth hormone is produced by the anterior pituitary and is hypothesized to have several modes of action during follicular development. Follicle development is stimulated by both GH and insulin-like growth factor (IGF) [3]. Exogenous GH acts on both GH and IGF receptors on the ovary to increase steroidogenesis and oocyte maturation [4, 5]. These effects could positively translate to IVF by increasing recruitment of preantral and antral follicles to increase the number of mature oocytes obtained in a single cycle. Furthermore, IVF studies have shown that increased follicular GH is correlated with an increase in clinical pregnancy rates [6] and that follicles with higher GH concentration yield oocytes with improved fertilization, embryo development, and implantation [7]. In summary, the theoretical mechanism of adjuvant GH in IVF is to increase the number of mature oocytes available, as well as produce oocytes which, through improved embryo development and implantation, could improve live birth rate.
The routine use of adjuvant GH in IVF in normal responders has previously shown to be of no benefit in ovarian response, embryo quality, or pregnancy rate [8]. The majority of clinical studies on adjuvant GH have focused on women with poor ovarian response (POR)/poor prognosis patients and there have been several meta-analyses to date which have showed significant improved ovarian response and embryo parameters in this population [2, 9, 10]. A recent review on GH use in IVF by Hart et al. [2] included a meta-analysis of the most recent studies in poor responders, which showed that there was a positive benefit on number of oocytes retrieved, number of fertilized oocytes, and clinical pregnancy rate. Despite these findings, this meta-analysis failed to find an increase in number of embryos available for transfer or live birth rate. Moreover, the authors note that the use of GH in “sub-optimal” responders has yet to be investigated. All prior studies investigating GH supplementation have been on fresh IVF cycles with transfer of cleavage stage embryos, so little is known about effect of GH supplementation on blastocyst development or on euploidy rates in IVF/PGT-A cycles.
Due to the abundance of literature on the use of adjuvant GH improving ovarian response, oocyte competence, and embryo development in women with POR/poor prognosis, the physicians in our practice started to routinely offer adjuvant GH treatment to women who did not meet Bologna criteria for POR but had suboptimal outcomes in an initial IVF/PGT-A cycle. The rationale for adjuvant GH in this patient population was that these unexpected suboptimal outcomes such as lower than expected number of MII oocytes, low blastulation rate, and/or fewer than expected euploid embryos may be improved in these “suboptimal responders” much like a POR population.
The objective of this study was to investigate the use of adjuvant GH in subsequent IVF/PGT-A cycles in women who were not poor responder patients, but who had suboptimal outcomes in an initial IVF/PGT-A cycle.
Material and methods
Study period and participants
All IVF/PGT-A cycles were reviewed from January 2014 to September 2019 to identify women who met the following inclusion and exclusion criteria.
Inclusion criteria are as follows: Women who did not meet the Bologna criteria for POR, defined as follows, at least two of the following three criteria had to be present to establish the definition: (1) advanced maternal age (> 40 years) or any other risk factor for POR, (2) a previous POR (≤ 3 oocytes with a conventional stimulation protocol), and (3) an abnormal ovarian reserve test, i.e., antral follicle count (AFC) less than 5–7 follicles or anti-Müllerian hormone (AMH) below 0.5–1.1 ng/ml [11]. In addition, women had to have one or more of the following suboptimal outcomes in an initial IVF/PGT-A cycle (control cycle with no GH): (1) lower than expected number of MII oocytes based upon age/AFC/AMH as informed by prior literature [12,13,14]; (2) poor blastulation rate as predicted by age, e.g., < 40.5% for women < 40 years old and < 22.2% ≥ 40 years old [15]; (3) lower than expected number of euploid embryos as predicted by age using approximate % euploidy rates stratified by age from a study performed by Franasiak et al. [16]. Women meeting only one suboptimal inclusion criteria were eligible for inclusion. Included patients had at least one subsequent IVF/PGT-A using the identical stimulation protocol as their control cycle with the addition of GH (GH cycle). Exclusion criteria are as follows: women with POR per Bologna criteria, women whose initial and subsequent IVF cycles used different stimulation protocols, women whose initial and subsequent IVF cycles occurred greater than 1 year apart, and women with incomplete data.
The option to use adjuvant GH was offered as part of routine clinical care to women who met the above inclusion criteria and participation was voluntary after appropriate counseling by the treating physician and self-funded by the patients.
A total of 41 women met the inclusion criteria and underwent 41 control and 41 subsequent treatment IVF/PGT-A with adjuvant GH cycles during the study period. For retrospective data collection and analysis, IRB approval was granted through the UT Austin Institutional Review Board.
Treatment protocols
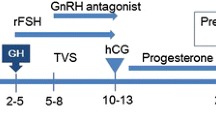
All the IVF protocols used were fixed dose gonadotropin protocols. Each woman used the same stimulation protocol with the same total dose of gonadotropin in the control and treatment cycle. The only difference in the treatment cycle compared with control cycle was the additional GH initiated with start of gonadotropin start. All IVF protocols started with a minimum of 2 weeks of oral contraceptive pills. The demi-halt protocol is equivalent to a stop-protocol [17] in which early cessation of GnRH agonist is used in combination with high dose gonadotrophins (300 FSH and 150 HMG). The delayed start protocol is a clomid flare protocol preceded by 7 days of GnRH antagonist [18]. During the adjuvant GH treatment cycle, 1.45 mg subcutaneous injection daily of GH (equivalent 4.35 IU Omnitrope@) was initiated on day 1 of ovarian stimulation and continued throughout the stimulation phase up to a total of 12 days. From prior studies, a minimum of 4 IU daily has been shown beneficial for poor responders [19]; therefore, we used this dose to minimize the cost to the patient. In practice, each patient was dispensed a total of three vials (total does 17.4 mg/52.2 IU) of Omnitrope and instructed to use a 1/4 vial of Omnitrope to achieve 4.35 IU daily dosing.
Follicular growth was monitored throughout ovarian stimulation as per standard clinic protocol and 10,000 IU hCG trigger was given when at least two 18-mm follicles were seen on ultrasound. Oocyte retrieval was performed 35.5 h after hCG trigger. ICSI was performed on all MII oocytes in one IVF laboratory. The following day, fertilization check was performed and only 2PN embryos were kept for extended embryo culture. These embryos were group-cultured in mini-desk top tri-gas incubators in 5% O2.The embryos were hatched on day 3 and laser trophectoderm biopsy performed on day 5 and day 6 on all embryos graded 3BB (Gardner) or better. The trophoblast cells were sent to one commercial reference laboratory for NexGen sequencing during this study period. Blastulation rate was the percentage of 2PN embryos that developed into biopsied blastocysts per cycle. Euploid rate was the percentage of biopsied blastocysts which were found to be euploid per cycle.
The primary outcomes were number of biopsied embryos and number of euploid embryos. Secondary outcomes were number of metaphase II (MII), fertilization rate, blastulation rate, and euploidy rate.
Statistical analysis
The primary and secondary outcomes were compared between the control cycle and subsequent GH treatment cycles and as our data was non-parametric and paired, a Wilcoxon-Signed Rank test was used for statistical analysis as each woman underwent both a control and subsequent GH treatment cycles. A post hoc power analysis showed that our study was adequately powered for the primary outcomes but underpowered to show a difference in secondary outcomes. The statistical analysis was performed using STATA 15.
Results
Forty-one women underwent a total of forty-one subsequent adjuvant GH treatment cycles. Table 1 shows the demographic data of the women in this study. No adverse side effects were reported by any of the women with using GH, and there were no cases of ovarian hyperstimulation syndrome (OHSS). Although, the ovarian stimulation protocols differed among patients, for each individual patient, the identical stimulation protocol was used in the initial and subsequent GH treatment cycle. Table 2 shows the cycle outcome of the control and subsequent adjuvant GH treatment cycles. The adjuvant GH IVF-PGT/A cycles trended towards a greater number of MII oocytes retrieved; however, this did not reach statistical significance. The fertilization rate and blastulation rate was not significantly different across cycles. The number of biopsied blastocysts and euploid embryos was significantly higher in the GH cycle; however, the blastulation and euploid rate was not significantly different between the control and GH treatment cycles.
We then stratified our control and treatment cycles by patient age and by AMH level to investigate whether GH treatment was of greater benefit to older women or women with lower ovarian reserve parameters (AMH < 1ng/ml). In our subgroup analysis, the significant increase in number of biopsied embryos and number of euploid embryos with adjuvant GH was still observed in women under 37 and those with AMH > 1. However, women with AMH < 1 had only a significant increase in number of biopsied blastocysts (Tables 3 and 4).
Discussion
To the best of our knowledge, this is the first published study investigating the primary outcome of the impact of growth hormone use in women without the diagnosis of poor ovarian reserve but suboptimal response. This demonstrates promise for these “suboptimal responders” (women without the classic Bologna criteria who have either responded less favorably to stimulation, retrieving fewer mature oocytes and/or obtaining fewer blastocyst and/or fewer euploid embryos available for transfer). While previous research has focused on women with POR and poor prognosis patients, these data demonstrate the clinical benefit of GH supplementation in women without this diagnosis.
Ours is also the first study to our knowledge to investigate GH in PGT-A cycles. This not only allows for the study of GH on embryo ploidy, but also allows for separation of clinical outcomes based on the availability of euploid embryos. Given that prior GH studies were conducted in the setting of untested embryos, the underlying reason for failure of live birth and whether GH could reasonably be expected to contribute to improvement were not able to be investigated. A prior study investigating the factors that influence embryo euploidy showed that the strongest predictors are age, serum AMH, and number of oocytes retrieved [20]. Given that age and AMH are immutable in a patient, the number of oocytes retrieved in a cycle is the one parameter that could potentially be improved with an intervention like GH to obtain more euploid embryos. Indeed, our study did demonstrate a trend towards an increase in MII oocytes in the GH cycle, without change in gonadotropin dose or protocol.
Our study uniquely reports the number of biopsied embryos and euploid embryos was significantly increased in the subsequent GH cycle. However, the euploid rate (percentage of embryos biopsied that were euploid) was not significantly different across cycles. One plausible explanation for this is that a women’s euploidy rate is inherently predetermined based on her age, genetics, and other factors that will remain the same across cycles. Therefore, the euploidy rate is unlikely to change significantly between two cycles less than a year apart. Therefore, the increase in number of euploid embryos is likely due to increase in the number of mature oocytes and thus an increase in the number of blastocysts available for biopsy. Our study shows that in the control IVF-PGT-A, the mean number of euploid embryo was 0.8 per cycle versus 2 euploid embryos in the GH cycle. This result is the more clinically relevant measure, since it represents the opportunity for an embryo transfer, and thus ultimately at pregnancy.
Many studies have shown that different stimulation strategies are needed for women categorized as poor and over-responders. Our study focused on a group of women who do not fit either category and constitute a group of suboptimal responders, for which scarce data is available on the best approach. Prior literature using the definition of suboptimal responders as having an oocyte yield of 4–9 oocytes have shown that 43–50% of IVF cycles fall in this category and that increasing the oocyte yield will significantly increase the pregnancy rates in these women [21, 22]. Therefore, our study provides a preliminary evidence for a strategy which could have major impact on optimization of ovarian stimulation for such suboptimal responders.
One strength of the study is that the patients were treated in a single clinic with the IVF cycles managed by a single physician. Therefore, improvements in a subsequent cycle would not be attributable to improved cycle management by a different physician. Likewise, all cycles were performed in a single IVF laboratory, so differences in culture/biopsy techniques which have been shown to influence euploidy rates is minimized. Another strength is the same patient is undergoing identical stimulation with the same fixed gonadotropin dosing in the initial and subsequent cycles, except for the addition of GH. Therefore, patient variables which could confound such a study (age, infertility diagnosis, ovarian reserve parameters,) are greatly minimized.
There are several limitations of our study. One major limitation is in the design of our study which is retrospective and observational; however, our study provides preliminary evidence to further investigate the role of adjuvant GH in women who are non-POR prospectively. Another limitation is the data was collected retrospectively, and therefore, our study is subject to inherent recall bias. Our sample size was relatively small, although adequately powered to detect large differences in our primary outcome measures; it rendered us unable to detect smaller differences in our secondary outcomes such as number of MII oocytes retrieved.
The novelty of our study is that it investigates the use of GH in IVF cycles where PGT-A was performed to determine the impact on ploidy of embryos. Our study also investigates the use of adjuvant GH in a new population of women who had unexpected suboptimal outcomes in an initial IVF/PGT-A cycle, but who were not POR/poor prognosis patients. We thus provide preliminary data to support further investigation of use of GH in a broader population of patients.
References
Homburg R, et al. Growth hormone facilitates ovulation induction by gonadotrophins. Clin Endocrinol. 1988;29(1):113–7.
Hart RJ, Rombauts L, Norman RJ. Growth hormone in IVF cycles: any hope? Curr Opin Obstet Gynecol. 2017;29(3):119–25.
Serafim MK, et al. Impact of growth hormone (GH) and follicle stimulating hormone (FSH) on in vitro canine preantral follicle development and estradiol production. Growth Hormon IGF Res. 2015;25(2):85–9.
Hull KL, Harvey S. Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int J Endocrinol. 2014;2014:234014.
Ipsa E, et al. Growth hormone and insulin-like growth factor action in reproductive tissues. Front Endocrinol (Lausanne). 2019;10:777.
Mendoza C, et al. Follicular fluid markers of oocyte developmental potential. Hum Reprod. 2002;17(4):1017–22.
Mendoza C, et al. Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum Reprod. 1999;14(3):628–35.
Younis JS, et al. The effect of growth hormone supplementation on in vitro fertilization outcome: a prospective randomized placebo-controlled double-blind study. Fertil Steril. 1992;58(3):575–80.
Kolibianakis EM, et al. Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in-vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2009;15(6):613–22.
Duffy JM, et al. Growth hormone for in vitro fertilization. Cochrane Database Syst Rev. 2010;1:CD000099.
Ferraretti AP, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–24.
Nardo LG, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586–93.
Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012–8.e1.
Moon KY, et al. Nomogram to predict the number of oocytes retrieved in controlled ovarian stimulation. Clin Exp Reprod Med. 2016;43(2):112–8.
Pantos K, et al. Influence of advanced age on the blastocyst development rate and pregnancy rate in assisted reproductive technology. Fertil Steril. 1999;71(6):1144–6.
Franasiak JM, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–663.e1.
Garcia-Velasco JA, et al. High doses of gonadotrophins combined with stop versus non-stop protocol of GnRH analogue administration in low responder IVF patients: a prospective, randomized, controlled trial. Hum Reprod. 2000;15(11):2292–6.
Cakmak H, et al. A novel “delayed start” protocol with gonadotropin-releasing hormone antagonist improves outcomes in poor responders. Fertil Steril. 2014;101(5):1308–14.
Ob’edkova K, et al. Growth hormone co-treatment in IVF/ICSI cycles in poor responders. Gynecol Endocrinol. 2017;33(sup1):15–7.
La Marca A, et al. Female age, serum antimüllerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2017;108(5):777–783.e2.
Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod. 2015;30(9):2005–8.
Alvaro Mercadal B, et al. Characterization of a suboptimal IVF population and clinical outcome after two IVF cycles. Gynecol Endocrinol. 2018;34(2):125–8.
Funding
None
Funding
Not applicable
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
IRB approval was granted through the UT Austin Institutional Review Board.
Consent to participate
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Skillern, A., Leonard, W., Pike, J. et al. Growth hormone supplementation during ovarian stimulation improves oocyte and embryo outcomes in IVF/PGT-A cycles of women who are not poor responders. J Assist Reprod Genet 38, 1055–1060 (2021). https://doi.org/10.1007/s10815-021-02088-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02088-2