Abstract
Purpose
To evaluate whether miR-148a-3p overexpression is associated with disrupted decidualization of recurrent implantation failure (RIF).
Methods
Endometrial miRNA and mRNA expression profiles during the implantation window derived from women with and without RIF were identified using microarray and RT-qPCR. Immortalized human endometrial stromal cells (HESCs) were cultured for proliferation and in vitro decidualization assays after enhancing miR-148a-3p expression or inhibiting putative target gene homeobox C8 (HOXC8) expression. RT-qPCR, western blot, and luciferase reporter assays were used to confirm the relationship between miR-148a-3p and HOXC8 gene.
Results
MiR-148a-3p was significantly upregulated in RIF endometrial tissues. Forced expression of miR-148a-3p notably attenuated HESC in vitro decidualization. Mechanistic studies revealed that miR-148a-3p directly bounds to the HOXC8 3′ untranslated region (3′UTR) and suppressed HOXC8 expressions in both mRNA and protein levels. Further investigations demonstrated that inhibition of HOXC8 in HESCs induced similar effects on decidual process as those induced by miR-148a-3p overexpression.
Conclusion
Taken together, our findings suggested that elevated miR-148a-3p might account for flawed decidualization in RIF by negatively regulating HOXC8, raising the possibility that miR-148a-3p might be a novel therapeutic target in RIF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite impressive advances in in vitro fertilization-embryo transfer (IVF-ET) and its derivative techniques, there are still many infertile couples suffering from recurrent implantation failure (RIF). RIF, defined as failure to achieve a clinical pregnancy following more than three transfers with high-quality embryos or the transfer of ten or more embryos in multiple cycles, is an intriguing clinical dilemma in human-assisted reproduction treatment [1, 2]. Although the underlying pathogenesis of RIF remains poorly characterized, it is increasingly apparent that this heterogeneous disease originates from both asynchronous (displaced) and pathological (disrupted) window of implantation (WOI) [2, 3]. As a nutritive and immunoprivileged matrix essential for establishing and maintaining window of receptivity, both displaced and disrupted decidualization give rise to embryo implantation failure [4,5,6,7,8].
Decidualization, characterized by the endometrial stromal cells transformation into specialized secretory decidual cells, is a prerequisite for underpinning endometrial receptivity and placental development [4, 9, 10]. In response to the increasing levels of progesterone and local cyclic AMP production instead of blastocyst implantation, human endometrial decidualization initiates at the postovulatory phase during regular menstruation cycle [4]. Of course, successful decidualization in human reproduction also depends on blastocyst attachment and invasion [11]. Normal decidual process is fundamental to establish and maintain pregnancy, and suboptimal decidualization has been demonstrated to implicate in adverse ripple effects during human pregnancy such as RIF, recurrent miscarriage, preeclampsia, preterm birth, and fetal death [4, 11,12,13]. To date, an extremely large number of molecular mediators have been identified to characterize the decidual progress, including steroid hormones, transcription factors, growth factors, adhesion molecules, cytokines, and chemokines [4, 14, 15]. However, the detailed molecular mechanisms underlying decidual process remain elusive. The remarkable morphological and biochemical changes during the decidual response remind us that epigenetic regulation may be involved in this highly dynamic event.
As important epigenetic modulators, microRNAs (miRNAs) are increasingly recognized to intertwine with various essential biological processes in mammalian fertility, ranging from gametogenesis to embryo implantation [16]. MiRNAs are endogenous, evolutionarily conserved, small non-coding RNA molecules of 21–24 nucleotides that normally suppress gene expression through RNA-RNA interactions with the 3′ untranslated region (3′UTR) of their target messenger RNAs (mRNAs) [17,18,19]. Compelling evidence has highlighted the importance of miRNAs in a spectrum of physiological and pathological processes including proliferation, differentiation, apoptosis, immunity, and tumorigenesis [17, 20, 21]. For example, the initially discovered miRNAs lin-4 and let-7 coordinate developmental timing in Caenorhabditis elegans by degrading their target developmental regulators [22, 23]. In the last decades, more and more miRNAs have been reported to participate in human endometrial stromal decidualization under different physiological or intervened conditions [24,25,26,27]. In addition, it has become apparent that aberrant miRNA expression could represent an important contributor to impaired endometrial decidualization and subsequent embryo implantation defects [28,29,30]. However, little is known about how miRNAs mediate decidual process in both physiologic and pathologic conditions.
Herein, we utilized miRNA and mRNA microarray assays to establish simultaneous miRNA and mRNA expression profiles in the endometrial tissues at the time of embryo implantation from RIF patients and further characterized the regulatory role of altered miRNAs in RIF pathogenesis. Our results illustrated that significantly elevated miR-148a-3p in RIF women versus controls may contribute to implantation failure by suppressing endometrial stromal cell decidualization as a direct inhibitor of gene homeobox C8 (HOXC8).
Materials and methods
Participants and tissue collection
A total of 31 infertile female patients who underwent in vitro fertilization or intracytoplasmic sperm injection (IVF/ICSI) cycles due to male factors were recruited at the Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University and included in this study. The study group consisted of 18 RIF patients without achieving clinical pregnancy after > 3 transfers with good quality embryos. Thirteen women who achieved live birth within the first three transfer cycles were enrolled as the control group. Before embryo transfer, the embryo morphology for cleavage or blastocyst stage was assessed according to the Puissant criteria or Gardner blastocyst scoring system, respectively [31]. The morphologic good-quality blastomeres with 7–10 cells and scores of ≥ 3 or blastocysts with scores of ≥ 4 BC would be selected for transfer. All 20–40-year-old volunteers had a regular ovulatory menstrual cycles (ranging from 25 to 35 days) and did not receive any hormone drugs or intrauterine contraceptive devices within 3 months prior to endometrial biopsy. The exclusion criteria for both groups were as follows: couples with abnormal chromosomal karyotypes, diminished ovarian reserve (antral follicle count (AFC) < 5 follicles or basal follicle-stimulating hormone (FSH) level > 12 IU/L), poor ovarian response in the previous treatment cycles (≤ 3 oocytes retrieved with a conventional stimulation protocol), known diseases correlated with endometrial pathology (i.e., congenital uterine malformation, hysteromyoma, endometrial polyp, endometriosis, intrauterine adhesions, hydrosalpinx), and autoimmune disturbances and endocrine dysfunctions.
All women were instructed to monitor the follicles development via transvaginal ultrasonography in our center. Then, the urinary luteinizing hormone (LH) levels were started to be quantified daily when the diameter of the dominant follicle exceeded 14 mm. Ovulation was ultimately determined according to ultrasound examination combined with an obvious urinary LH surge. The day of ovulation was defined as day 0, and the WOI phase was deemed to be the day of ovulation + 7 (day 7). Endometrial biopsy specimens were derived from the fundal area of the uterine cavity on day 7 after ovulation using a Pipelle catheter (Unimar Inc., Wilton, Conn) as detailed [32]. Endometrial tissues from patients and controls were immediately processed and divided into two parts. One part for RNA extraction was snap-frozen in liquid nitrogen and stored at − 80 °C. The other was fixed in 4% paraformaldehyde, dehydrated in graded ethanol, and embedded in paraffin for histological evaluation.
Hematoxylin and eosin staining
For the histological analysis, paraffin samples were serially sliced into 5 μm sections. After being deparaffinized with xylene II and rehydrated in descending graded ethanol (100%, 100%, 100%, 95%, 80%, and 70%, respectively), the slices were stained in hematoxylin (Beyotime, Shanghai, China) for 3 min, differentiated in 1% hydrochloric acid ethanol for 10 s, and stained in eosin (Beyotime, Shanghai, China) for 30 s. Then, the slices were dehydrated in ascending graded ethanol (95%, 100%, 100%, and 100%, respectively), cleared in xylene II, and sealed with neutral balsam. Finally, the slices were observed under a microscopy and histological scoring was performed according to the Noyes criteria [33].
MiRNA and mRNA microarray assays
The Exiqon miRCURY™ LNA Array (v18.0, Exiqon, Vedbaek, Denmark) and Arraystar Human LncRNA Microarray (v4.0, Arraystar, Inc., Rockville, MD, USA) were utilized to profile miRNA and mRNA expression patterns, respectively, in the endometrial tissues from 8 RIF patients and 10 controls. RNA extraction, RNA labeling and array hybridization, and data analysis were carried out as described in detail previously by KangChen Bio-tech (Shanghai, China) [34, 35]. To denote significantly different expressions of miRNAs and mRNAs, a volcano plot filtering with a fold change > 2.0 and a P value < 0.05 was performed between RIF samples and the matched control samples. We further filter and select the miRNAs and mRNAs for investigation considering expression levels, miRNA-mRNA conjoint analysis, and previously published studies [36,37,38,39,40].
Cell culture and treatment
The hTERT immortalized human endometrial stromal cells (HESCs) originated from the American Type Culture Collection (ATCC® CRL-4003™; Manassas, VA, USA) were kindly supplied by Prof. Haibin Wang (Xiamen University, Xiamen, China). The cells were cultured in phenol red-free DMEM/F-12 medium (Gibco, Grand Island, NY, USA) supplemented with 10% charcoal-stripped fetal bovine serum (CS-FBS; Biological Industries, Kibbutz Beit Haemek, Israel). The human embryonic kidney 293T (HEK293T) cells were incubated in DMEM High Glucose (HyClone, South Logan, UT, USA) containing 10% fetal bovine serum (FBS; Biological Industries). The cells were maintained at 37 °C in humidified air with 5% CO2 and passaged every 2–3 days by dissociation with trypsin (HyClone).
For enhanced miR-148a-3p expression in HESCs, the recombinant lentivirus containing full-length precursor miR-148a-3p (abbreviated as miR-148a-3p) was purchased from Hanbio Biotechnology Co., Ltd. (Shanghai, China). The empty lentiviral vector which only expressed a fluorescent reporter gene, ZsGreen, was used as a negative control (miR-NC). Gene HOXC8 was knocked down in HESCs by using short hairpin RNA (shRNA) lentiviral vectors. The effective target sequence for HOXC8 shRNA was 5′-GAGACGCCTCCAAATTCTATGGCTA-3′. The lentiviral vectors with HOXC8 shRNA sequence (abbreviated as shHOXC8) and empty vector (shR-NC) were also constructed by Hanbio Biotechnology Co., Ltd. (Shanghai, China). To generate the stable HESC line expressing miR-148a-3p or suppressing HOXC8, HESCs were plated into 6-well plates and cultured overnight. When cells reached 40% confluence, the medium was removed and replaced with the complete medium supplemented with lentiviral particles and polybrene (Hanbio Biotechnology Co., Ltd.). Three days after infection, the transduction efficiency of HESCs with ZsGreen signals was evaluated by inverted fluorescence microscope. Expression of ZsGreen above 90% was considered successful. Thus, the stably transfected cells were expanded and harvested for subsequent assays.
Reverse transcription and quantitative real-time PCR
Total RNA was extracted from endometrial biopsy specimens or cultured HESCs utilizing TRIzol Reagent (TaKaRa, Dalian, China). MiRNAs were reverse-transcribed with Mir-X™ miRNA First-Strand Synthesis Kit (TaKaRa), and total RNA was reversely transcribed into cDNA with PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa) according to the manufacturer’s protocol. Reverse transcription and quantitative real-time PCR (RT-qPCR) were employed with SYBR® Premix Ex Taq™ Kit (TaKaRa) on a LightCycler® 480 System (Roche, Basel, Switzerland). Small nuclear RNA RNU44 and β-actin served as endogenous controls for miRNA and mRNA, respectively. Relative expression was calculated using the 2−ΔCt method (ΔCt = Ct miRNA/gene of interest − Ct internal control) in endometrial tissues or the 2−ΔΔCt calculation [ΔΔCt = (Ct miRNA/gene of interest − Ct internal control) sample A − (Ct miRNA/gene of interest − Ct internal control) sample B] in cultured cells [41]. The RT-qPCR primers are listed in Supplemental Table 1, and the reverse primer for miRNA was provided by the Mir-X™ miRNA First-Strand Synthesis Kit. Each sample was analyzed in triplicate.
Western blotting
The proteins extracted from cultured cells with sodium dodecyl sulfate (SDS) loading buffer were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), and then probed with antibodies. The antibodies used were as follows: anti-HOXC8 (Sigma-Aldrich, St. Louis, MO, USA, H1791, 1:1000), anti-proliferating cell nuclear antigen (anti-PCNA, a proliferation marker for evaluating cell proliferation; Santa Cruz Biotechnology, Santa Cruz, CA, USA, sc-56, 1:1000), anti-β-actin (as an internal reference; Cell Signaling Technology, Danvers, MA, USA, 41970s, 1:5000), Peroxidase-conjugated Affinipure Goat Anti-Mouse (Proteintech, Chicago, IL, USA, SA00001-1, 1:5000), and Peroxidase-conjugated Affinipure Goat Anti-Rabbit (Proteintech, SA00001-2, 1:5000). The immunoreactive bands were visualized with Immobilon™ Western Chemiluminescent HRP Substrate (Millipore) in ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA, USA).
Cell proliferation assays
Cell counting kit 8 (CCK8) and 5-ethynyl-2′-deoxyuridine (EdU) assays were adopted to evaluate cell proliferation in lentivirus-miR-148a-3p and miR-NC transduced HESCs. For CCK8 assay, the two cell groups were seeded into 96-well plates (2000 cells/well) in decuplicate and cell viability was detected at 1, 2, 3, and 4 days after seeding by employing the CCK8 reagent (Beyotime, Shanghai, China). Proliferating HESCs were further assessed after a 48-h incubation in a 96-well plate in triplicate using Cell-Light™ EdU Apollo® 567 In Vitro Imaging Kit (RiboBio, Guangzhou, China). The number of EdU-incorporated cells was counted in five random fields per well under a fluorescence microscopy. The EdU-positive cell rate was calculated with (EdU add-in cells/Hoechst-stained cells) × 100%.
In vitro decidualization
A well-established in vitro decidualization procedure was performed to estimate whether miR-148a-3p or HOXC8 deregulation result in aberrant decidualization in HESCs. To induce decidualization in vitro, HESCs were cultured in 2% CS-FBS phenol red-free DMEM/F-12 medium with 1 μM medroxyprogesterone-17-acetate (MPA; Sigma-Aldrich) and 0.5 mM N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (db-cAMP; Sigma-Aldrich) for 6 days in a humidified 5% CO2 incubator at 37 °C. The culture media were renewed every 2 days, and the induced decidual cells were collected for RNA isolation at 2, 4, and 6 days after decidualization, respectively. The classical decidualization marker prolactin (PRL) and insulin-like growth factor–binding protein-1 (IGFBP1) mRNA levels during in vitro decidual process were quantified by RT-qPCR.
Dual-luciferase reporter assay
To construct plasmids for dual-luciferase reporter assays, wild-type HOXC8 complementary DNA (containing the putative miR-148a-3p binding sites) and a mutant HOXC8 sequence (mutant in miR-148a-3p binding sites) were synthesized and inserted into pmirGLO vector (Promega, Madison, WI, USA). In addition, miR-148a-3p mimics (double-stranded RNA oligonucleotides) and negative control (NC mimics) were obtained from GenePharma Inc. (Shanghai, China). For reporter assays, the HEK293T cells were co-transfected with wild-type (HOXC8 WT) or mutant (HOXC8 Mut) reporter plasmid together with miR-148a-3p mimics or NC mimics by Lipofectamine® 2000 Reagent (Invitrogen, Carlsbad, CA, USA). The dual-luciferase reporter assay system (Promega) was applied to calculate the relative luciferase activity 2 days post-transfection according to the manufacturer’s instructions. Each sample was implemented in triplicate.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences, version 20.0 (SPSS 20.0; SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was first introduced for evaluating the distribution of continuous variables. Normally distributed data were shown as mean ± standard error of the mean (SEM), and non-normal data were expressed as median (quartile interval). Considering data distribution, two-tailed Students’ t test or Mann-Whitney U test was appointed for continuous variables. Categorical variables were given as numbers (percentages) and analyzed with chi-square test. All biological assays were repeated at least three times. A P value < 0.05 was considered as statistically significant.
Results
Clinical and histological characteristics of all participants
Table 1 summarizes the clinical characteristics of women recruited in this study. No significant differences were observed in the clinical features between RIF and control group, especially age, body mass index (BMI), ovarian reserve levels, and infertility duration. A portion of each endometrial sample was evaluated histologically and dated to be receptive, mid-secretory phase endometrium. The stromal cells in the endometrium on day 7 after ovulation from both RIF and controls are mounting a decidual response, characterized by cytoplasmic expansion and rounding of cells. No differences in histological evaluation were showed in patients and controls (Fig. 1a, b).
MiR-148a-3p was significantly elevated in endometrial tissues from RIF patients
To hunt for transcripts that potentially persecute embryo implantation, miRNA and mRNA expression profiles in the endometrial specimens collected from 8 RIF patients and 10 matched controls were revealed by microarray analysis. Our primary Exiqon array data were deposited in the Gene Expression Omnibus database under accession number GSE121219. A total of 157 miRNAs and 294 mRNAs exhibited distinct expression patterns in RIF patients as opposed to controls (fold change > 2.0 and P value < 0.05; data not shown). To systematically filter transcripts for further investigation, the collaborative miRNA-mRNA network involved in pregnancy establishment and maintenance was generated by a miRNA-mRNA conjoint analysis. Based on bioinformatic analysis and previous data, miR-148a-3p, as one of most upregulated miRNAs uncovered by the microarray, and its target gene HOXC8 were selected for further research (Fig. 2a, b). The endometrial tissues from 18 RIF and 13 controls were assessed via RT-qPCR to confirm the microarray results on miR-148a-3p and HOXC8. In consonance with microarray data, miR-148a-3p was significantly increased in RIF patients (fold change = 2.65, P < 0.01; Fig. 2c), whereas no obvious difference was found in HOXC8 expression (Fig. 2d).
Microarray validation of miR-148a-3p and HOXC8 expressions by RT-qPCR. a, b Altered expressions of miR-148a-3p and HOXC8 in RIF relative to control were uncovered by microarray assays. c, d RT-qPCR was employed for validating the miR-148a-3p and HOXC8 expressions. RT-qPCR reverse transcription and quantitative real-time PCR, RIF recurrent implantation failure. Data were expressed as mean ± SEM (a, b) or median with interquartile range (c, d). a, b Two-tailed Students’ t test. c, d Two-tailed Mann-Whitney U test. **P < 0.01, ***P < 0.001
MiR-148a-3p overexpression suppressed HESC in vitro decidualization
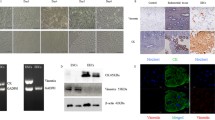
To gain insights into the contributions of miR-148a-3p to HESCs, a stable miR-148a-3p-overexpressing HESC line (Supplemental Fig. 1a) was generated for in vitro cell proliferation and decidualization assays. For cell proliferation analysis, CCK8, western blotting for PCNA and EdU assays were carried out. There were no observable differences in the cell viabilities (Fig. 3a), PCNA protein levels (Fig. 3b), and EdU-positive cell ratios (Fig. 3c, d) among miR-148a-3p and miR-NC groups, which disclosed that upregulated miR-148a-3p did not interfere with HESCs proliferation. Afterwards, a reliable in vitro induced decidualization system was resorted to unearth whether increased miR-148a-3p expression affect HESC decidualization. The mRNA levels of two decidual markers PRL and IGFBP1 were significantly downregulated in decidualized HESCs after treatment with miR-148a-3p (Fig. 3e, f). These results added novel evidence showing that miR-148a-3p adversely disturbed HESC decidual process in vitro.
MiR-148a-3p overexpression impaired HESC in vitro decidualization. a For CCK8 assay, the cell index was tested at days 1, 2, 3, and 4 following seeding of HESCs overexpressing miR-148a-3p or miR-NC. b Western blotting assay assessed the PCNA protein levels upon miR-148a-3p overexpression in HESCs. c, d EdU cell proliferation assay was utilized for calculating the S-phase cell percentages in lentivirus-miR-148a-3p-HESCs. S-phase cells were incorporated by EdU (red). e, f The stable HESCs were subjected to in vitro decidualization for 6 days. The relative expression levels of decidual markers PRL and IGFBP1 were analyzed by RT-qPCR in decidualized HESCs collected on 2, 4, and 6 days after induced decidualization in vitro. HESCs human endometrial stromal cells, CCK8 cell counting kit 8, NC negative control, PCNA proliferating cell nuclear antigen, EdU 5-ethynyl-2′-deoxyuridine, PRL prolactin, IGFBP1 insulin-like growth factor–binding protein-1, RT-qPCR reverse transcription and quantitative real-time PCR. Data were shown as mean ± SEM. **P < 0.01, ***P < 0.001, two-tailed Students’ t test
HOXC8 was a direct functional target of miR-148a-3p
HOXC8 was potential target gene of miR-148a-3p through in silico analysis. Despite RIF and control women displayed parallel HOXC8 expression levels, the HOX family member, HOXC8, was still selected as a potential target of miR-148a-3p in accordance with previous studies [39, 40]. RT-qPCR and western blotting assays were performed to validate the regulation of miR-148a-3p on HOXC8 and exhibited that forced expression of miR-148a-3p markedly reduced the HOXC8 mRNA and protein levels in HESCs (Fig. 4a, b). Subsequently, the wild-type and mutated 3′UTRs of HOXC8 were cloned into the dual-luciferase reporter vectors to explore whether miR-148a-3p straightforwardly target HOXC8 3′UTR. HEK293T cells co-transfected with miR-148a-3p mimics and reporter vector HOXC8-WT showed significantly decreased luciferase activity (Fig. 4c). These results indicated that HOXC8 was a direct target of miR-148a-3p.
HOXC8 was a direct functional target of miR-148a-3p. a, b Comparison of HOXC8 mRNA and protein expression levels in HESCs infected with lentivirus-miR-148a-3p versus miR-NC. c The putative miR-148a-3p-binding sites in HOXC8 mRNA 3′UTR and correlated mutant sequences were presented. The direct interaction between miR-148a-3p and HOXC8 was revealed by dual-luciferase reporter assay. d, e RT-qPCR quantified the PRL and IGFBP1 mRNA levels to evaluate the decidual responses in in vitro decidualized HESCs transduced with shHOXC8 or shR-NC lentiviruses after 2, 4, and 6 days. HESCs human endometrial stromal cells, NC negative control, 3′UTR 3′ untranslated region, WT wild type, Mut mutant, RT-qPCR reverse transcription and quantitative real-time PCR, PRL prolactin, IGFBP1 insulin-like growth factor–binding protein-1. Data were presented as mean ± SEM. ***P < 0.001, two-tailed Students’ t test
To further elucidate the potential involvement of HOXC8 in decidual progression, we brought the lentivirus-shHOXC8 infected HESC line (Supplemental Fig. 1b, c) into the in vitro model for decidualization. As expected, HOXC8 inhibition produced significant decreases in PRL and IGFBP1 mRNA levels during HESC decidual process (Fig. 4d, e), which claimed that HOXC8 was a functional target gene of miR-148a-3p.
Discussion
In the present study, we profiled miRNA and mRNA expression patterns in endometrial tissues derived from RIF patients and observed that miR-148a-3p showed a striking upregulation in RIF compared with normal controls. We further discovered that miR-148a-3p overexpression attenuated HESC in vitro decidualization by targeting HOXC8. These findings indicated that miR-148a-3p might induce a blunted decidualization response in RIF by directly repressing HOXC8, which provided new insights into epigenetic pathogenesis for RIF.
RIF is a predicament encountered in reproductive medicine, which frequently puzzles both physicians and patients. Although human embryo implantation defects have been widely studied in recent years, its precise molecular mechanism is not yet understood. More recently, new insights into miRNAs in RIF pathogenesis of endometrial origin have emerged, and several RIF-associated miRNAs are beginning to be characterized [30, 42, 43]. For example, Revel et al. identified 13 differentially expressed miRNAs in the secretory endometrium of RIF patients compared with fertile women and one of the upregulated miRNAs was miR-145 whose predicted targets were involved in cell cycle and cell adhesion pathways critical for implantation [30]. Then, Kang et al. revealed that miR-145 distorted embryo-epithelial juxtacrine communication at implantation by suppressing maternal type-1 insulin-like growth factor receptor (IGF1R) [43]. Despite this, to our knowledge, the miRNA-processing machinery in insufficient endometrial decidualization during the implantation window that may underlie human implantation failure remains to be explored. Here we described a specific endometrial miRNA-mRNA expression profile in RIF at the time of embryo implantation and, for the first time, uncovered a miR-148a-3p/HOXC8 regulatory circuit that might account for flawed decidualization in RIF.
MiR-148a-3p, a member of miR-148/152 family, is located at chromosome 7p15.2 and has previously been implicated in cellular differentiation and development [36,37,38]. It has been generally accepted that miR-148a-3p was underexpressed in numerous solid tumors and proposed as a tumor suppressor in oncogenesis, whereas its expression level and role in RIF pathogenesis remain enigmatic [36]. As outlined in our study, miR-148a-3p was expressed at a relatively high level in RIF women versus controls. Given the causative role of decidual program in RIF, in vitro decidualization modeling was used to investigate whether altered miR-148a-3p expression hamper HESC decidualization. Our data showed that forced expression of miR-148a-3p notably attenuated HESC in vitro decidualization, which indicated that miR-148a-3p might be a negative regulator for decidual response in RIF.
Following paired miRNA and mRNA expression profiles in our microarray results, HOXC8 was experimentally validated as a miR-148a-3p target. HOXC8 belongs to homeobox gene family, members of which encode homeodomain-containing transcription factors that play an essential role in embryonic morphogenesis and differentiation [44]. HOXC8 functions as a transcriptional activator or repressor to regulate numerous physiological processes, such as cell proliferation, differentiation, migration, and adhesion [45]. There is a growing realization that aberration in HOXC8 expression is responsible for tumorigenesis in various tumors [46, 47]. It is intriguing to note that diminished HOXA10 or HOXA11 expression has been reported to impede endometrium receptivity and embryo implantation in women with endometriosis, polycystic ovarian syndrome, fibroids, or hydrosalpinx, suggesting that maternal HOX gene expression is indispensable for human implantation process [39, 40]. Therefore, we postulated that HOXC8, a paralogous gene of HOXA10 and HOXA11, might also participate in modulating endometrium receptivity for implantation. No significant difference in endometrial HOXC8 expression was detected between RIF patients and controls, which might be attributed to complicated miRNA-mRNA regulatory networks or its moderate effect on endometrial function in vivo. Taken the crucial role of HOX family into consideration, we proceeded to interpret whether HOXC8 was a direct functional target of miR-148a-3p in vitro. As speculated, we confirmed that HOXC8 destroyed the HESC decidual program as a direct target gene of miR-148a-3p.
In addition, we assessed each endometrial biopsy tissue histologically and found no differences in histological endometrial dating between patients and controls. It is conceivable as histological dating of timed endometrial biopsy tissue alone lacks the sensitivity to evaluate endometrial development and is not related to fertility status [6, 48]. Our results further discovered that abnormal miRNA caused pathological (disrupted) decidualization in RIF, which also highlight the abovementioned point.
In conclusion, miR-148a-3p has appeared as a novel repressor of decidualization in vitro during the receptive period for embryo implantation, and overexpressed miR-148a-3p might answer for suboptimal decidual process in RIF as a negative regulator of HOXC8. Of note is that the molecular mechanism of HOXC8 as a transcription factor during decidualization process remains unclear in our study. Nonetheless, our findings strengthened the concept that miRNA-mediated decidualization was vital for human embryo implantation and raised the prospect that modulating disordered miRNAs might be helpful for improving implantation rates in reproductive treatment. So more profound mechanism is warranted for further investigation to explore this possibility.
References
Thornhill AR, deDie-Smulders CE, Geraedts JP, Harper JC, Harton GL, Lavery SA, et al. ESHRE PGD Consortium Best practice guidelines for clinical preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS). Hum Reprod. 2005;20(1):35–48.
Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil Steril. 2017;108(1):15–8.
Sebastian-Leon P, Garrido N, Remohí J, Pellicer A, Diaz-Gimeno P. Asynchronous and pathological windows of implantation: two causes of recurrent implantation failure. Hum Reprod. 2018;33(4):626–35.
Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905.
Ochoa-Bernal MA, Fazleabas AT. Physiologic events of embryo implantation and decidualization in human and non-human primates. Int J Mol Sci. 2020;21(6).
Kliman HJ, Frankfurter D. Clinical approach to recurrent implantation failure: evidence-based evaluation of the endometrium. Fertil Steril. 2019;111(4):618–28.
Zhu H, Hou CC, Luo LF, Hu YJ, Yang WX. Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions. Gene. 2014;551(1):1–14.
Berkhout RP, Lambalk CB, Repping S, Hamer G, Mastenbroek S. Premature expression of the decidualization marker prolactin is associated with repeated implantation failure. Gynecol Endocrinol. 2020;36(4):360–4.
Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine. 1997;6(3):301–7.
Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178(3):357–72.
Ruan YC, Chen H, Chan HC. Ion channels in the endometrium: regulation of endometrial receptivity and embryo implantation. Hum Reprod Update. 2014;20(4):517–29.
Macklon N. Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertil Steril. 2017;108(1):9–14.
Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–67.
Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55(5):795–810.
Vinketova K, Mourdjeva M, Oreshkova T. Human decidual stromal cells as a component of the implantation niche and a modulator of maternal immunity. J Pregnancy. 2016;2016:8689436.
Salilew-Wondim D, Gebremedhn S, Hoelker M, Tholen E, Hailay T, Tesfaye D. The role of microRNAs in mammalian fertility: from gametogenesis to embryo implantation. Int J Mol Sci. 2020;21(2):E585.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25(46):6163–9.
Chen X, Xie D, Zhao Q, You Z-H. MicroRNAs and complex diseases: from experimental results to computational models. Brief Bioinform. 2019;20(2):515–39.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74.
Seki N, Hata A. Noncoding RNAs: a new fine-tuner is a key player of human pathogenesis. J Hum Genet. 2017;62(1):1.
Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, et al. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122(4):553–63.
Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–6.
Estella C, Herrer I, Moreno-Moya JM, Quinonero A, Martinez S, Pellicer A, et al. miRNA signature and Dicer requirement during human endometrial stromal decidualization in vitro. PloS one. 2012;7(7):e41080.
Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod. 2010;82(4):791–801.
Sha AG, Liu JL, Jiang XM, Ren JZ, Ma CH, Lei W, et al. Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertility and sterility. 2011;96(1):150–5.e5.
Zhang Q, Zhang H, Jiang Y, Xue B, Diao Z, Ding L, et al. MicroRNA-181a is involved in the regulation of human endometrial stromal cell decidualization by inhibiting Kruppel-like factor 12. Reproductive biology and endocrinology : RB&E. 2015;13:23.
Chill HH, Dior UP, Kogan L, Revel A. microRNAs and endometrial pathophysiology. Adv Exp Med Biol. 2015;887:143–55.
Galliano D, Pellicer A. MicroRNA and implantation. Fertil Steril. 2014;101(6):1531–44.
Revel A, Achache H, Stevens J, Smith Y, Reich R. MicroRNAs are associated with human embryo implantation defects. Hum Reprod. 2011;26(10):2830–40.
Zhang Q, Li G, Zhang L, Sun X, Zhang D, Lu J, et al. Maternal common variant rs2305957 spanning PLK4 is associated with blastocyst formation and early recurrent miscarriage. Fertility and Sterility. 2017;107(4):1034–40.e5.
Niklaus AL, Aubuchon M, Zapantis G, Li P, Qian H, Isaac B, et al. Assessment of the proliferative status of epithelial cell types in the endometrium of young and menopausal transition women. Hum Reprod. 2007;22(6):1778–88.
Noyes RW, Hertig AT, Rock J. Reprint of: Dating the endometrial biopsy. Fertility and Sterility. 2019;112(4 Suppl1):e93–e115.
Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124(2):175–84.
Cao C, Sun J, Zhang D, Guo X, Xie L, Li X, et al. The long intergenic noncoding RNA UFC1, a target of MicroRNA 34a, interacts with the mRNA stabilizing protein HuR to increase levels of β-catenin in HCC cells. Gastroenterology. 2015;148(2):415–26.e18.
Chen Y, Song YX, Wang ZN. The microRNA-148/152 family: multi-faceted players. Mol Cancer. 2013;12:43.
Li Y, Deng X, Zeng X, Peng X. The role of Mir-148a in cancer. J Cancer. 2016;7(10):1233–41.
Wang X, Liang Z, Xu X, Li J, Zhu Y, Meng S, et al. miR-148a-3p represses proliferation and EMT by establishing regulatory circuits between ERBB3/AKT2/c-myc and DNMT1 in bladder cancer. Cell Death & Disease. 2016;7(12):e2503.
Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17(2):242–53.
Taylor HS. The role of HOX genes in human implantation. Hum Reprod Update. 2000;6(1):75–9.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8.
Choi Y, Kim HR, Lim EJ, Park M, Yoon JA, Kim YS, et al. Integrative analyses of uterine transcriptome and microRNAome reveal compromised LIF-STAT3 signaling and progesterone response in the endometrium of patients with recurrent/repeated implantation failure (RIF). PLoS One. 2016;11(6):e0157696.
Kang YJ, Lees M, Matthews LC, Kimber SJ, Forbes K, Aplin JD. MiR-145 suppresses embryo-epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J Cell Sci. 2015;128(4):804–14.
Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10(5):361–71.
Lei H, Wang H, Juan AH, Ruddle FH. The identification of Hoxc8 target genes. Proc Natl Acad Sci U S A. 2005;102(7):2420–4.
Shah M, Cardenas R, Wang B, Persson J, Mongan NP, Grabowska A, et al. HOXC8 regulates self-renewal, differentiation and transformation of breast cancer stem cells. Mol Cancer. 2017;16(1):38.
Liu H, Zhang M, Xu S, Zhang J, Zou J, Yang C, et al. HOXC8 promotes proliferation and migration through transcriptional up-regulation of TGFbeta1 in non-small cell lung cancer. Oncogenesis. 2018;7(2):1.
Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, et al. Reprint of: Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertility and Sterility. 2019;112(4 Suppl1):e116–e24.
Acknowledgments
We thank Prof. Haibin Wang and colleagues at Xiamen University, Xiamen, China, for constructive insight and valuable advice. The authors expressed gratitude to all participants involved in this study.
Funding
The work was supported by National Key Research & Development Program of China (2016YFC1000202, 2018YFC1002804), Major Program of National Natural Science Foundation of China (81490743), General Program of National Natural Science Foundation of China (81671522, 81571406), Shandong Provincial Key Research and Development Program (2016GGH4522), and the Special Fund of Taishan Scholars Program of Shandong Province.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Junhao Yan designed the study and finally approved the version to be submitted; Qian Zhang and Tianxiang Ni performed the study, analyzed the data, and wrote the paper; Yujie Dang performed the study; Lingling Ding and Jingjing Jiang collected samples and analyzed the data; Jing Li, Mingdi Xia, and Na Yu analyzed the data; and Jinlong Ma and Zi-Jiang Chen revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The study was approved by the Institutional Review Board of Center for Reproductive Medicine, Shandong University. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Table 1.
Primers for RT-qPCR (DOCX 16 kb)
Supplemental Fig 1.
Lentivirus-mediated miR-148a-3p overexpression or HOXC8 inhibition in HESCs (PNG 230 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Ni, T., Dang, Y. et al. MiR-148a-3p may contribute to flawed decidualization in recurrent implantation failure by modulating HOXC8. J Assist Reprod Genet 37, 2535–2544 (2020). https://doi.org/10.1007/s10815-020-01900-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01900-9