Abstract
Purpose
To characterize small supernumerary marker chromosomes (sSMC) in infertile males
Research question
Are molecular cytogenetic methods still relevant for the identification and characterization of sSMC in the era of next-generation sequencing?
Methods
In this paper, we report five males with oligoasthenozoospermia or azoospermia with a history of recurrent pregnancy loss in partnership in four cases. R-banding karyotyping and fluorescence in situ hybridization (FISH) analysis were performed and showed sSMC in all five cases. Microdissection and reverse-FISH were performed in one case.
Results
One sSMC, each, was derived from chromosome 15 and an X-chromosome; two sSMC were derivatives of chromosome 22. The fifth sSMC was a ring chromosome 4 complemented by a deletion of the same region 4p14 to 4p16.1 in one of the normal chromosomes 4. All markers were mosaics except one of sSMC(22).
Conclusion
Through this study, we emphasize the necessity of a proper combination of high-throughput techniques with conventional cytogenetic and FISH methods. This could provide a personalized diagnostic and accurate results for the patients suffering from infertility or RPL. We also highlight FISH analyses, which are essential tools for detecting sSMC in infertile patients. In fact, despite its entire composition of heterochromatin, sSMC can have effects on spermatogenesis by producing mechanical perturbations during meiosis and increasing meiotic nondisjunction rate. This would contribute to understand the exact chromosomal mechanism disrupting the natural and the assisted reproduction leading to offer a personalized support.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For decades, the karyotype has been the “gold standard” method of human cytogenetics providing a global examination of the genome. Since the arrival of fluorescence in situ hybridization technique (FISH), the efficiency and accuracy of karyotype analysis by combining conventional cytogenetic with molecular technologies have improved considerably. Very quickly, considerable advances in molecular techniques have changed the approaches used for clinical diagnosis and the search for chromosomal abnormalities. Here we underline the development of array comparative genomic hybridization (aCGH) and next-generation sequencing (NGS) technology that allow a higher resolution and accuracy [1]. These technological advances have improved the diagnosis of chromosomal aberrations and the management of infertile patients [2]. In fact, chromosomal abnormalities are a main cause of infertility [3,4,5]. Among these abnormalities, one can find sex chromosome aberrations, inversions, translocations, and small supernumerary marker chromosomes (sSMC) [5,6,7,8]. sSMC are a group of structurally abnormal additional chromosomes which result in an abnormal phenotype only in nearly 30% of the cases. They can be seen in prenatal, in postnatal, and in patients with mental disabilities and developmental disorders as well as hypofertility [9]. It was shown that the prevalence of sSMC is three times higher in patients with infertility than in the general population. They are noticed in about 0.125% and 0.044%, respectively [10]. Moreover, this frequency seems to be sex-dependent with a higher rate in male 0.165% versus 0.022% in female [9]. Their origin and composition cannot be recognized easily, which make them a major problem in clinical cytogenetics [11], especially as markers related to infertility are usually present in phenotypically normal carriers. They are accidentally discovered, or due to recurrent miscarriages or familial history of malformed child [12, 13]. In addition, even in the case of sSMC being inherited from a healthy parent, infertility history could be related to them [10, 14].
Therefore, it is of great interest to consider sSMC when investigating infertility, in order to determine their risk for recurrent pregnancy loss (RPL) and further assisted reproduction therapy (ART). sSMC in infertile men can be derived from any chromosome with a high proportion of the acrocentric chromosomes, mostly chromosomes 14, 15, and 22 [7, 10], which are generally associated with oligozoospermia and azoospermia [7, 8].
In this study, conventional karyotyping and molecular cytogenetics methods were performed in order to characterize sSMC in four new cases associated to male infertility and spontaneous abortions in the female partner. In one case, the sSMC is related only to male infertility. The possible mechanisms of spermatogenesis interruption and marker diagnosis tools in ART are discussed.
Materials and methods
Five couples were referred due to reproductive problems including primary infertility and/or two or more spontaneous abortions (Table 1). The local ethical board of Farhat Hached Hospital approved this work and a written informed consent was taken from the couples. Semen analysis showed oligoasthenozoospermia (OAT) in two patients (P1, P3) and azoospermia in three patients (P2, P4, P5).
Karyotype
Conventional R-banding karyotypes were performed on metaphase spreads prepared from phytohemagglutinin (PHA)-stimulated peripheral blood lymphocytes according to standard protocols with a resolution of nearly 400-band level. Cultures were incubated for 72 h, and at least 15 R-banded metaphases were analyzed for each patient [15, 21] and more cells were analyzed in mosaic cases. Also, cytogenetic analyses of cultured lymphocytes revealed a normal karyotype for the five female partners of P1 to P5 (data not shown).
FISH
FISH was performed using commercial probes: whole chromosome painting WCP15, WCPX, WCPY, WCP22, and WCP18; centromere-specific regions D15Z1, DYZ3, and DXZ1 (Vysis®, Downers Grove, IL, USA and Kreatech®); subcentromere-specific probes Sc15q11.2; and specific probes for the acrocentric chromosomes Midi54 (homemade probes) and SE14/22 (Cytocell®, Oxford Gene Technology, Cambridge, UK). Probe was applied to metaphase slides according to standard procedures. Chromosomes were evaluated using an Axioskop Zeiss® fluorescence microscope, and images were captured with a CCD camera (Cytovision, Applied Imaging®).
In one case (P3), centromere-specific multicolor FISH (cenM-FISH; homemade probe-set) using all available human centromere-specific probes, labeled with five different fluorochromes and hybridized simultaneously, was performed according to standard protocol [22].
Microdissection and reverse-FISH
Microdissection was done in one case (P3) as described by Kosyakova et al [19]. Reverse-FISH was performed using the microdissection-derived probe in a standard FISH-setting.
Array CGH
The array comparative genomic hybridization (aCGH) was performed in one case (P3) as previously described [23]. Agilent® oligonucleotide array was performed according to the manufacturer’s instructions (Agilent Human Genome CGH Microarray kit 44K®).
Results
An sSMC was discovered in R-banding of cases P1 to P5. Clinical and cytogenetics results are summarized in Table 1. In P1, P2, P3, and P5, the sSMC was in a mosaic state.
In case P1, the marker derived from an X-chromosome and was present in 16% of the 222 analyzed cells with a basic karyotype 47,XYY. Semen analysis showed a low rate of sperm that was classified as OAT according to the WHO criteria.
The sSMC of case P2 was an inverted-duplicated-shaped derivative from centromere-near region of chromosome 15; the sSMC was present in 18% of the analyzed cells.
Case P3 had in karyotyping a small ring-shaped sSMC in about 27% of the analyzed metaphases. FISH using specific probes for centromeric regions did not resolve the case. After microdissection followed by reverse-FISH, the sSMC was identified to be derived from 4p14 to 4p16.1; the identical region was deleted on one of the two normal chromosomes 4. However, this result could not be confirmed by aCGH.
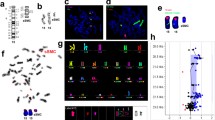
The sSMC in cases P4 and P5 derived from chromosome 22 (Fig. 1a–l). One of them was present in 100% of the analyzed cells (P4), and the other was present in a very low level of mosaicism 3% (3/100) in R-banding, which could be corrected to 15% in the analyzed metaphases after FISH.
FISH results on metaphase spread (a) and on nucleus (b) using centromeric probes for X (green) and Y (red) chromosomes for patient 1. Conventional karyotype showing a small supernumerary marker chromosome for patient 2 (c), FISH results using WCP15 (green) and WCPX (d); SNRPN probe (red) and PML (green) showing two spots on both normal chromosome 15 (e); subcentromere 15q11.2 probe (red) and MiDi54 (green) confirming the inverted-duplicated shape (f) for patient 2. Conventional karyotype showing a small supernumerary ring (g), FISH results using MIDI54 (green) and subcentromeric probe 14/22 (h) and Cent-FISH results showing no hybridization for the ring (i) and reverse-FISH results after microdissection showing a ring 4 and a deletion 4p (j) in patient 3. FISH results using whole chromosome painting (WCP) of chromosomes 22 in green and WCP15 in red showing a small marker derived from chromosome 22(arrow) (k), Tuple 1 probe (red) and ARSA (green) showing 2 spots on both normal chromosomes 22 (l) in patient 4
Discussion
Human infertility is a multifactorial disease that must be explored with precaution, taking into account all risk factors including hormonal, infectious, anatomical, and especially genetic ones [24]. About 15% of male infertility is due to genetic factors including chromosome abnormalities [25]. Patients carrying sSMC do not have obvious clinical features. However, the abnormal pairing and segregation of chromosomes during meiosis may produce unbalanced gametes with abnormal chromosome numbers or structures, which can lead to infertility. Actually, chromosomes are highly organized in the genome in specific positions called chromosome territories [26] in diploid cells as well as in sperm cells. Subsequently, any misconfiguration of their usual localization would disrupt the nuclear architecture of the cell resulting in impaired synapsis and low recombination frequency and more importantly illegitimate attraction with XY bivalent [14]. Curiously, it has been shown that at pachytene stage, in normal male meiosis, acrocentric bivalents, especially 15 and 22, are preferentially close to the XY pair [16, 27]. However, this well-regulated chromosomal distribution could be disrupted in the presence of chromosomal rearrangements such as sSMC. Indeed, in sSMC carriers, the additional chromosomes are attracted to their homologous sister chromosomes [28, 29]. Interestingly, the same results were shown in sperm cells of sSMC(15) carriers for instance [14]. Furthermore, a repositioning of the XY pair near the sSMC is then raised [28]. This close proximity, repositioning, and interaction would result in alteration of synapsis between the X- and Y-chromosomes, which could either, at least, disturb or arrest the male meiosis resulting in infertility in sSMC carriers.
Here, we report five couples consulting for genetic exploration of infertility and for reproductive counseling (Table 1).
It is well-known, as previously reported in two multicentric reviews in more than 200 infertile patients, that the most frequent sSMC related to fertility were derived from chromosomes 14, 15, and 22. While the most common indications were OAT and azoospermia [7, 10].
In this report, the first patient had mos47,XYY[15]/48,XYY,+mar[3] karyotype.
47,XYY is a known syndrome with a 1:1000 incidence and seems to have no real impact on fertility since the abnormal spermatocytes undergoing meiosis are generally excluded at the first meiosis checkpoint [30, 31]. Even though variable degrees of infertility have been reported [32], this syndrome (47,XYY) seems to be more frequent among infertile patients than in the general population [33,34,35,36]. In this particular case, fertility could be reduced in the presence of the extra chromosome. In fact, as described earlier in case of sSMC(15), this could have a mechanical effect disturbing the meiosis process or results in spontaneous abortion in the female partner as reported in the present patient.
The second sSMC shown here was inverted-duplicated-shaped chromosome 15. It did not encompass the Prader Willi and Angelman syndrome critical region (PW/ASCR), a region responsible for the pathological clinical features of the inv-dup(15) syndrome. In fact, as in the general population [9], sSMC 15 are the most frequent ones in subfertil group exhibiting oligozoospermia or azoospermia [9, 10].
Two other markers were derived from chromosome 22 in patients P4 and P5. The sSMC was present in a low level of mosaicism in P4. Generally, markers derived from chromosome 22 could be correlated to Emmanuel syndrome (OMIM 609029) or Cat Eye syndrome (OMIM 115470) depending on trisomy or tetrasomy of the proximal region 22 or rather unspecific clinical manifestations, whereas sSMC derived from chromosome 22 related to fertility issues are generally not associated to other clinical features. Indeed, around 16 related infertility cases are reported to date (http://ssmc-tl.com/chromosome-22.html#W). Most of them are inv-dup(22)(q11.1) containing pericentromeric near region, apparently harmless but linked to spermatogenesis failure and RPL [7]. Therefore, sSMC(22) should be considered while investigating infertile couples in order to give a better genetic counseling prior to any pregnancy.
In case P3, the marker was a ring shape and derived from the short arm of chromosome 4 (p14p16.1). The cells with sSMC have a balanced karyotype; those which lost the sSMC, have a partial monosomy 4p14 to 4p16.1.We could explain the mosaicism here by the instability of acentric chromosomes which could be lost during mitosis leading to genomic imbalance [37]. Terminal deletion of 4p14 region is responsible for Wolf-Hirschhorn syndrome (WHS), a syndrome characterized by typical dysmorphic features and severe intellectual impairment accompanied by growth retardation [17]. For this patient, apparently with normal phenotype, who complains only from infertility, the most likely reason explaining this presentation could be a mosaicism with a higher proportion of complemented cells in authentic conditions. A tissue mosaicism could also explain the patient’s phenotype.
The 4p deletion was not visible on the conventional karyotype. Array CGH 44K was also unable to detect both rearrangements in the same DNA cells (supernumerary ring 4p and deletion 4p). In this particular case, the coexistence of the terminal deletion played the role of a cancelation of the overdose within the sSMC. This chromosomal anomaly could not be delineated by aCGH, a tool enabled to characterize such a balanced rearrangement.
It is important to consider that this type of marker chromosomes is more problematic for the diagnosis and management of the infertile carriers than the other types of SMCs. In fact, although carriers of such rearrangement are balanced, they might have an increasing risk of producing severe unbalanced gametes resulting in a partial trisomy (due to the ring 4) or a partial monosomy due to 4p deletion [38].
Only 38 cases of sSMC derived from chromosome 4 were reported in the sSMC database, among them two rings chromosome 4. One of them was found in an infertile man with asthenoteratozoospermia and no other clinical findings (http://ssmc-tl.com/chromosome-4.html, case04-O-p12/1-1). Supernumerary ring 4 in children are generally associated with developmental delay and other features depending on the size of the duplicated region [39]. Cases of supernumerary ring marker originated from McClintock mechanism as expected here and related to male infertility are very rare. As best as we know, only seven cases are reported to date involving chromosomes 1, 6, 8, 13, 15, and Y (Table 2). Interestingly, ART had been proposed in three previously reported cases. In one case, microsurgical testicular sperm extraction was discussed but not done [40]. In the second case, Y chromosome microdeletion studies showed a deletion of the AZF region, excluding the ART’s chances of success [41]. In the last case [18], the authors suggested the preimplantation genetic diagnosis (PGD) as a power tool to screen unbalanced embryos with supernumerary ring 8, in order to transfer a balanced one. Such approach could be a pertinent alternative for infertile couples carrying small and rare SMC and should be more approved to provide a personalized genetic counseling for next generations.
The clinical implication of chromosome markers in the general population as well as in the subfertil one is still problematic for geneticists and clinicians. Indeed, in 30% of the cases, sSMC are inherited from one of the parents with a higher incidence in healthy fertile mothers [42]. Contrariwise, in men, the marker may be lost in sperm cells by a natural normal gamete selection [3, 10] or lead to infertility similarly to the cases presented here.
Although sSMC were reported in men suffering from unexplained infertility or recurrent abortion in their female partners, the underlying mechanism by which they interfere with fertility still enigmatic [7, 10]. Previous case-control studies showed that men referred as having severe azoospermia had a higher incidence of chromosomal abnormalities than other groups of fertile or patients of other groups [43, 44]. Likewise, in previously reported studies, the sperm count was considerably lower in infertile men with chromosomal abnormalities, particularity in sSMC cases, than those exhibiting normal karyotypes [3]. Moreover, segregation studies of sSMC in infertile carriers using sperm-FISH, mostly described for sSMC(15) [20, 45,46,47,48] and for chromosomes 20, 22, and 14 [49,50,51], have shown that more than 80% of sperm were either normal or balanced. At these conditions, the marker was present in less than the expected fraction (50%) with a variable frequency ranging from 6 to 26% [49,50,51]. This suggests either a possible exclusion of the marker in the germ line or a nondisjunction leading to a testicular mosaicism [46, 50]. Even so, as in 47,XYY cases with oligozoospermia, heterochromatic sSMC were supposed to interact with the XY bivalent during meiosis disturbing the normal spermatogenesis process [3, 50]. The mosaic form involving a supernumerary chromosome could be then the result of a nondisjunction during the first stage of fetal development [52] leading to the mosaic karyotype seen in patient P1. Thus, mosaic cases involving other chromosomal rearrangements should be studied with more attention as they may interfere with the spermatogenesis process [32, 33, 52]. Clearly, these studies support the idea that chromosomal abnormalities, including marker chromosomes, could lead to infertility by interrupting the spermatogenesis process during meiosis I, resulting in OAT and azoospermia [5, 7] even when they are present in a very low level of mosaicism as seen here or RPL by producing unbalanced gametes [3, 53].
Unlike intellectual disabilities, developmental delay, and autism spectrum disorders where the use of aCGH and NGS as first-tier test is recommended, in the case of infertility with excepted chromosomal rearrangements involving heterochromatic material, low level of mosaicism, and balanced rearrangements, these techniques show real limitations. In fact in a review of 237 infertile patients with marker chromosomes, aCGH failed in more than 80% of the cases to detect sSMC [7] as seen here in the 4th case. Clearly, conventional cytogenetic techniques and FISH are still the techniques of choice to characterize sSMC. This justifies, to date, largely their application in the panoply of genetic examinations.
Conclusion
Taking all these facts into account, the identification and the characterization of sSMC’s content is mandatory in patients with fertility issues. The aim is to provide a genetic counseling regarding the risk of the occurrence in the offspring of unbalanced gametes and to offer an alternative solution for extremely affected patients. In this regards, chromosome segregation analysis using sperm-FISH would be worthwhile to determine the rate of unbalanced gametes and the disomy frequency for the most involved chromosomes in infertility cases. In addition, the characterization of the exact content of marker chromosomes in infertile men would guide the therapeutic strategies for the assisted medical reproduction such as PGD.
Our findings provide new evidence for the pathogenesis of infertility and widen the clinical spectrum of sSMC derived from chromosome 4, 15, and 22. The use of different molecular cytogenetic approaches is necessary to better characterize sSMC in the general population as well as in infertile men. The practice of PDG for infertile carriers of sSMC to analyze and possibly transfer normal/balanced and euploid embryos after in vitro fertilization could be of great interest in the area of reproductive medicine.
References
Weise A, Mrasek K, Pentzold C, Liehr T. Chromosomes in the DNA era: perspectives in diagnostics and. 2019;
Cariati F, D’Argenio V, Tomaiuolo R. The evolving role of genetic tests in reproductive medicine. J Transl Med [Internet]. 2019;17(1):1–33. Available from: https://doi.org/10.1186/s12967-019-2019-8
Chandley BYANNC, Edmondt P, Christie S, Gowans L, Fletcher J, Frackiewicz A, et al. Cytogenetics and infertility in man*. 1975
De Krom G, Arens YHJM, Coonen E, Van Ravenswaaij-Arts CMA, Meijer-Hoogeveen M, Evers JLH, et al. Recurrent miscarriage in translocation carriers: no differences in clinical characteristics between couples who accept and couples who decline PGD. Hum Reprod. 2015;30(2):484–9.
Mau-Holzmann UA, Institute. Somatic chromosomal abnormalities in infertile men and women. 2005;111:317–36.
Godo A, Blanco J, Vidal F, Sandalinas M, Garcia-Guixé E, Anton E. Altered segregation pattern and numerical chromosome abnormalities interrelate in spermatozoa from Robertsonian translocation carriers. Reprod Biomed Online [Internet]. 2015;31(1):79–88. Available from:. https://doi.org/10.1016/j.rbmo.2015.04.003.
Liehr T, Hamid Al-Rikabi AB. Impaired spermatogenesis due to small supernumerary marker chromosomes: the reason for infertility is only reliably ascertainable by cytogenetics. Sex Dev. 2018;
Armanet N, Tosca L, Brisset S, Liehr T, Tachdjian G. Small supernumerary marker chromosomes in human infertility. Cytogenet Genome Res. 2015;146(2):100–8.
Liehr T, Weise A. Frequency of small supernumerary marker chromosomes in prenatal, newborn, developmentally retarded and infertility diagnostics. Int J Mol Med. 2007;19(5):719–31.
Manvelyan M, Riegel M, Santos M, Fuster C, Pellestor F, Mazaurik ML, et al. Thirty-two new cases with small supernumerary marker chromosomes detected in connection with fertility problems: detailed molecular cytogenetic characterization and review of the literature. Int J Mol Med. 2008;21(6):705–14.
Liehr T, Claussen U, Starke H. Small supernumerary marker chromosomes (sSMC) in humans. Cytogenet Genome Res. 2004;107(1–2):55–67.
Hochstenbach R. Multiple small supernumerary marker chromosomes resulting from maternal meiosis I or II errors. Mol Syndr. 2015;6:210–21.
Liehr T. Familial small supernumerary marker chromosomes are predominantly inherited via the maternal line [2]. Genet Med. 2006;8(7):459–62.
Olszewska M, Wanowska E, Kishore A, Huleyuk N. Genetic dosage and position effect of small supernumerary marker chromosome ( sSMC ) in human sperm nuclei in infertile male patient. Nat Publ Gr [Internet]. 2015;(October):1–14. Available from: https://doi.org/10.1038/srep17408
Papanikolaou EG, Vernaeve V, Kolibianakis E, Van Assche E, Bonduelle M, Liebaers I, et al. Is chromosome analysis mandatory in the initial investigation of normovulatory women seeking infertility treatment? Hum Reprod. 2005;20(10):2899–903.
Metzler-Guillemain C, Mignon C, Depetris D, Guichaoua MR, Mattei MG. Bivalent 15 regularly associates with the sex vesicle in normal male meiosis. Chromosom Res. 1999;7(5):369–78.
Battaglia A, Carey JC, South ST. Wolf – Hirschhorn syndrome: a review and update. 2015;223(August):216–23.
Cheng D, Yuan S, Yi D, Luo K, Xu F, Gong F, et al. Analysis of molecular cytogenetic features and PGT-SR for two infertile patients with small supernumerary marker chromosomes. 2019
Kosyakova N, Liehr T, Al-rikabi ABH. FISH-microdissection. In: Thomas Liehr (ed.), editor. Fluorescence in situ hybridization (FISH), Springer Protocols Handbooks. Springer Berlin Heidelberg; 2017. p. 81–100.
Guediche N, Tosca L, Terki AK, Bas C, Lecerf L, Young J. Array comparative genomic hybridization analysis of small supernumerary marker chromosomes in human infertility. Reprod Biomed Online [Internet] 2012;24(1):72–82. Available from: https://doi.org/10.1016/j.rbmo.2011.08.014
Dana M, Stoian V. Association of pericentric inversion of chromosome 9 and infertility in romanian population. Maedica (Buchar) [Internet]. 2012;7(1):25–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23118816%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3484792
Edited by Thomas Liehr. Fluorescence in situ hybridization (FISH) application guide. s; 2009.
Slimani W, Khelifa H Ben, Dimassi S, Chioukh F, Jelloul A, Kammoun M, et al. Clinical and molecular findings in nine new cases of tetrasomy 18p syndrome: FISH and array CGH characterization. 2019;1–7.
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62(March):2–10.
Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol [Internet]. 2018;15(6):369–84. Available from: https://doi.org/10.1038/s41585-018-0003-3
Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet [Internet]. 2001;2(4):292–301. Available from: http://www.nature.com/articles/35066075
Sarrate Z, Blanco J, Vidal F. Acrocentric bivalents positioned preferentially nearby to the XY pair in metaphase i human spermatocytes. Fertil Steril [Internet]. 2012;98(5):1241–5. Available from: https://doi.org/10.1016/j.fertnstert.2012.07.1110
Karamysheva T, Kosyakova N, Guediche N, Liehr T. Small supernumerary marker chromosomes and the nuclear architecture of sperm–a study in a fertile and an infertile brother. 2015;6368(1):32–6.
Klein E, Manvelyan M, Simonyan I, Hamid AB, Guilherme RS, Liehr T, et al. Centromeric association of small supernumerary marker chromosomes with their sister- chromosomes detected by three dimensional molecular cytogenetics. 2012;1–7.
Chandley AC, Fletcher J, JAR. Normal meiosis in two 47 , XYY men. Hum Genet. 1976;33:231–40.
Milazzo JP, Rives N, Macé B. Chromosome constitution and apoptosis of immature germ cells present in sperm of two 47 , XYY infertile males. 2006;21(7):1749–58.
Kim IW, Khadilkar AC, Ko EY, Edmund S, Sabanegh J. 47,XYY syndrome and male infertility. Rev Urol. 2013;15(4):188–96.
Boroujeni PB, Sabbaghian M, Dizaji AV, Moradi SZ, Almadani N, Lashkari FM. Clinical aspects of infertile 47 , XYY patients: a retrospective study. Hum Fertil [Internet]. 2017;0(0):1–6. Available from: https://doi.org/10.1080/14647273.2017.1353143
Zhang X, Liu X, Xi Q, Zhu H, Li L, Liu R, et al. Reproductive outcomes of 3 infertile males with XYY syndrome: retrospective case series and literature review. Med (United States). 2020;99(9).
El-Dahtory F, Elsheikha HM. Male infertility related to an aberrant karyotype, 47,XYY: four case reports. Cases J. 2009;2(1):1–4.
Gonzalez-Merino E, Hans C, Abramowicz M, Englert Y, Emiliani S. Aneuploidy study in sperm and preimplantation embryos from nonmosaic 47, XYY men. Fertil Steril. 2007;88(3):600–6.
Marshall OJ, Chueh AC, Wong LH, Choo KHA. Neocentromeres: new insights into centromere structure, disease development, and karyotype evolution. Am J Hum Genet. 2008;82(2):261–82.
Baldwin EL, May LF, Justice AN, Martin CL, Ledbetter DH. Mechanisms and consequences of small supernumerary marker chromosomes: from Barbara McClintock to modern genetic-counseling issues. Am J Hum Genet. 2008;82(2):398–410.
Zhang ZT, Qi WX, Liu CX, Yin SW, Zhao Y, Li-Ling J, et al. A small supernumerary marker chromosome resulting in mosaic partial tetrasomy 4q26-q31.21 in a foetus with multiple congenital malformations. J Genet [Internet]. 2019;98(1):19–22. Available from: https://doi.org/10.1007/s12041-019-1075-4, 2019
Song S, Park SH, Shin E, Jung JH, Shim SH, Kim DS. Case report male infertility associated with a supernumerary marker chromosome. 2017;35(3):205–8.
Kuroda S, Yumura Y, Hamanoue H, Yasuda K, Yamanaka H, Sanjo H, et al. A case of azoospermia patient with a chromosomal abnormality considered a ring y chromosome. Hinyokika Kiyo. 2014;60:583–6.
Liehr T. Small supernumerary marker chromosomes and tumors marker. In: Small supernumerary marker chromosomes (sSMC) [Internet]. 2012. p. 181. Available from: https://doi.org/10.1007/978-3-642-20766-2
Mierla D, Jardan D, Stoian V. Chromosomal abnormality in men with impaired spermatogenesis. Int J Fertil Steril. 2014;8(1):35–42.
Arafa MM, Majzoub A, Alsaid SS, El W, Al A, Elbardisi Y, et al. Chromosomal abnormalities in infertile men with azoospermia and severe oligozoospermia in Qatar and their association with sperm retrieval intracytoplasmic sperm injection outcomes. 2018;132–9.
Cotter PD, Ko E, Larabell SK, Rademaker AW, Martin RH. Segregation of a supernumerary del(15) marker chromosome in sperm. Clin Genet. 2000;58(6):488–92.
Paetzold U, Schwanitz G, Schubert RKVDV, Montag M. Sperm analyses , genetic counselling and therapy in an infertile carrier of a supernumerary marker chromosome 15. 2006;5–9.
Gabrielli I, Domenico B, Mesoraca A, Pietro C, Giorlandino C. A case report of a meiotic segregation study on a small supernumerary marker chromosome. J Prenat Med. 2007;1(3):41–4.
Oracova EVA, Musilova P, Kopecna O, Rybar R, Vozdova M, Vesela K, et al. Sperm and embryo analysis in a carrier of supernumerary inv dup (15) marker chromosome case report. 2009;30(3):233–9.
Perrin A, Nguyen MH, Delobel B, Guéganic N, Basinko A, Le Bris M-J, et al. Characterization and meiotic segregation of a supernumerary marker chromosome in sperm of infertile males: case report and literature review. Eur J Med Genet [Internet]. 2012;55(12):743–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23000562
Kirkpatrick G, Ren H, Liehr T, Chow V, Ma S. Meiotic and sperm aneuploidy studies in three carriers of Robertsonian translocations and small supernumerary marker chromosomes. Fertil Steril [Internet]. 2015;103(5):1162-1169.e7. Available from: https://doi.org/10.1016/j.fertnstert.2015.02.006
Wiland E, Jarmuż M, Kurpisz M. Segregation of the marker chromosome der ( 20 ) in the sperm of a male with karyotype 46, XY [96]/47, XY+mar [4]. Med Sci Monit 2005;11(3):9–15.
Robinson DO, Jacobs PA. The origin of the extra Y chromosome in males with a. Hum Mol Genet. 1999;8(12):2205–10.
Hajlaoui A, Slimani W, Kammoun M, Sallem A, Braham S, Bibi M, et al. Sperm fluorescent in situ hybridisation study of interchromosomal effect in six Tunisian carriers of reciprocal and Robertsonian translocations. Andrologia [Internet]. 2018; Available from: https://doi.org/10.1111/and.12949
Acknowledgments
We would like to thank all the technicians as well as the patients for their collaboration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The local ethical board of Farhat Hached Hospital approved this work, and a written informed consent was taken from the couples.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Slimani, W., Jelloul, A., Al-Rikabi, A. et al. Small supernumerary marker chromosomes (sSMC) and male infertility: characterization of five new cases, review of the literature, and perspectives. J Assist Reprod Genet 37, 1729–1736 (2020). https://doi.org/10.1007/s10815-020-01811-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01811-9