Abstract
Purpose
Tyrosine kinase inhibitors (TKIs) such as imatinib are commonly used chemotherapeutics, but the effects of long-term treatments on reproductive outlook for cancer survivors are unknown. The purpose of this study was to examine the effects of long-term imatinib treatments on follicle development and embryo quality. Since prospective studies are not possible in healthy humans, we have incorporated a commonly used mouse model.
Methods
Adult female mice were treated with daily IP injections of imatinib for 4–6 weeks. Liquid chromatography-mass spectrometry was used to measure imatinib in serum and ovarian tissues. At the end of treatments, females were superovulated and mated to yield fertilized embryos. Oocytes and embryos were collected from oviducts, assessed for development by microscopy, and fertilized embryos were cultured in vitro. Blastocysts were fixed and stained for differential cell counts.
Results
Long-term imatinib treatments caused a shift in follicle development, with imatinib-treated females having fewer primordial follicles, but an increase in primary and secondary follicles (P < 0.05). There was no effect on ovulation or fertilization rates. However, blastocysts from imatinib-treated females had fewer total cells (P < 0.05) and a significant shift from inner cell mass to increased trophectoderm cells.
Conclusion
This pilot study indicates that long-term TKI treatments may have significant impact on ovarian reserve and embryo developmental capacity. More studies are needed in other model systems to determine the long-term impact of TKIs in patients. Knowing the potential effects of chemotherapeutics on reproductive outlook is critical for quality of life and more research is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The harmful effect of chemotherapeutics on future fertility is a significant concern for female cancer survivors [1, 2]. While the collateral impact of chemotherapy is well founded for older generation chemotherapies, the same is not true of newer pharmaceuticals such as tyrosine kinase inhibitors (TKIs). Meanwhile, the American Society of Clinical Oncology recommends that clinicians carefully consider and discuss the risks and benefits of all cancer treatments and their potential to impact future fertility [3]. TKIs have become first-line treatment for many cancers [4]. For women of reproductive age, the question of whether TKIs have a significant deleterious effect on future fertility is a critical question but unfortunately little is known regarding the impact of TKIs on ovarian reserve and fertility.
Unlike conventional gonadotoxic chemotherapeutics, targeted molecular therapy with TKIs were thought to have no overt gonadotoxic effects [5]. However, TKIs have been associated with a variety of endocrine-related side effects including disruption of the thyroid, adrenal function, bone remodeling, and gonadal dysfunction. The mechanisms underlying these harmful “off-target” side effects suggest that ovarian function may also be adversely impacted [6].
Imatinib was designed to target the fusion protein BCR-ABL, which is a primary mutation driving chronic myeloid leukemia. However, it is also a potent inhibitor of other tyrosine kinases, especially ABL1, ABL2, KIT, and platelet-derived growth factor receptor (PDGFR) [7,8,9]. While the role of ABL in the ovary is unknown, both KIT and PDGFR are expressed by the ovary and the developing germ cells [10,11,12]. Within the ovary, KIT-ligand and PDGF have been shown to independently promote primordial follicle activation, transition of primordial to primary follicle, oocyte growth, granulosa cell proliferation, and follicle survival [13,14,15]. ABL kinase is also expressed in oocytes; however, its function is unknown [16]. It has therefore been hypothesized that imatinib treatment may inhibit the normal biological functioning of oocytes and the subsequent proper embryo development.
The adverse clinical impact of imatinib on fertility was first brought to light in a case report describing amenorrhea and premature ovarian insufficiency in a 28-year-old woman on long-term imatinib therapy [17]. A subsequent report then highlighted a woman with severely compromised ovarian response to gonadotropin stimulation while on imatinib, with a rebound to a normal ovarian response after discontinuing imatinib [18].
In this study, we have utilized a mouse model with varying exposures to imatinib to analyze the effect of long-term imatinib treatment on fertility by observing the ovulatory response to controlled ovarian stimulation and subsequent preimplantation embryo development, in addition to ovarian follicle counts to assess possible effects on ovarian reserve and follicle growth. We hypothesize that imatinib treatment may diminish ovarian reserve, decrease oocyte recruitment, and lead to a lower number and quality of oocytes and preimplantation embryos.
Materials and methods
Animal model
Female (CF1) mice aged 6–7 weeks and males (B6D2F1) aged 10 weeks were purchased from Envigo USA (formerly Harlan) and housed in a temperature- and light cycle-controlled animal facility. All experiments were conducted in accordance with the “Guide for the Care and use of laboratory Animals” and preapproved by the University of Southern California Institutional Animal Care and Use Committee.
The model included three treatment groups: group 1—400 mg/kg intraperitoneal (IP) imatinib injected daily (100 μl) for 4 weeks; group 2—imatinib IP continued daily for 6 weeks. Since mice metabolize imatinib quickly, treated mice also received imatinib ad lib in their drinking water to ensure a base minimum was maintained [19]. To prevent the possibility of a direct interaction between IP-injected hormones and imatinib, the last imatinib injection was given on the day before superovulation regimen was begun. Imatinib in drinking water was also stopped on the last day of imatinib injections. Control mice received daily IP injection of 100 μl of sterile water and no imatinib. Imatinib was purchased from Tocris BioScience (#5906, Minneapolis, MN, USA) and prepared daily, by mixing in sterile water followed by 0.22 μm filtration. Water bottles were refreshed daily and completely changed for fresh once each week. Thirty-four female mice were assigned to treatments (n = 10 mice/imatinib treatment and 7 controls/treatment (time)). Unfortunately, the embryos from 3 females (one per treatment) were lost due to lab accident on one collection day; thus, the results include data from 9 females/imatinib treatment and 13 controls.
After 4 or 6 weeks of imatinib, female mice were treated for superovulation. Mice were injected IP with 5 IU of equine chorionic gonadotropin (eCG; P.G.600, NADA#140-856, Merck Animal Health, USA) and 48 h later, ovulation was induced with 5 IU human chorionic gonadotropin (hCG; Chorulon, NADA#140-927, Merck Animal Health, USA). After hCG, each female was caged with a mature male of proven fertility. At 72 h after hCG, all females were euthanized by isoflurane inhalation (VetOne by Fluriso, India) and cervical dislocation. Blood was drawn via cardiac puncture while the mice were heavily sedated, immediately prior to cervical dislocation. Ovaries, oviducts, and uteri were collected from each female. Ovaries were fixed in 10% histology-grade formalin and processed for histological assessment. Oviducts and uterus transferred to flushing and holding medium (FHM made in-lab [20]) and transported to the embryology lab for cultures.
The daily dose of imatinib commonly prescribed for patients ranges from 400 to 600 mg/day/patient, with some patients receiving 400 mg twice daily (800 mg/day) [21]. In our animal model, we used the dose that is commonly used in rodent model studies 400 mg/kg/day (~ 16 mg/day/mouse). To verify that our model was providing safe and appropriate serum concentrations of imatinib, we ran the mass spec analysis over-time. The concentration of imatinib in serum averaged 3600 ng/ml, 1000 ng/ml, 50 ng/ml, and 10 ng/ml at 2 h, 4 h, 8 h, and 16 h respectively [19]. In CML patients, trough serum levels above 1000 ng/ml are considered therapeutic and patients are maintained at these levels for life. Patients given 400 mg daily dose attain daily serum levels that average 1403 ng/ml (ranging from 138 to 3011 ng/ml [22]). Cardiotoxicity has been reported at prolonged imatinib levels greater than 5000 ng/ml [23]). Therefore, the highest daily serum levels of imatinib in our mouse model are well below toxicity levels.
Embryo culture and processing
The tract (oviduct and uterus) was flushed with FHM medium and all oocytes and embryos were collected and examined for stage of development, using 40x magnification on a Zeiss Axio inverted microscope. All cleaving embryos were transferred into culture drops and cultured to 120 h post-hCG, as previously published [20]. Embryo development was examined daily. At the end of culture, all blastocysts were fixed in 4% formalin in PBS, stored at 4 °C, and used for cell counts. Fixed blastocysts were permeabilized in PBS with 0.1% Triton X-100 and Hoechst DNA dye which labels all nuclei. Embryos were co-labeled with CDX2 primary antibody (#PA5-20891, ThermoFisher Scientific, USA) followed by Alexa-568 goat anti-rabbit (#A-11011, ThermoFisher Scientific, USA) secondary antibody. The CDX2 specifically identifies trophoblast (TE) cells. Cells not labeled with CDX2 are classified as inner cell mass (ICM) cells. Blastocysts were examined on a Zeiss Axio inverted microscope. To count cells, small groups of blastocysts (< 10/slide) were mounted onto glass slides and covered with mounting medium (Vectashield antifade mounting medium, Vectorlabs.com). To prevent crushing of embryos, a small dab of Vaseline was placed on the edge of the coverslip before placing it over the embryos. The coverslips were sealed with clear nail polish and allowed to dry in a dark box overnight. Slides were then mounted onto the inverted Zeiss microscope with 40x objective, and the cells counted for each embryo, while carefully focusing up and down through the blastocyst. On the first pass through each blastocyst, we counted all blue cells (Hoechst 33258 = total cells; excitation 352 nm, emission 455 nm filter set) and then red cells (CDX2 = TE cells; excitation 579 nm, emission 603 nm). The total number of inner cell mass cells was calculated from the total number of blue minus total number of red cells.
Follicle counts
To obtain an estimate of the total number, and growth distribution of follicles in ovaries, six of the fixed ovaries, one/female within treatment, were randomly grouped and embedded in paraffin using standard methods. This created one block of 6 ovaries for each treatment and enabled us to follow the follicle counts from consecutive sections from each ovary. Sections were cut at 8 μm thickness and every 5th section was mounted onto a single slide and processed for standard H&E staining. Each ovarian section was carefully examined at 20x and 40x magnification. Only those follicles that contained a visible oocyte nucleus were counted. By skipping to every 5th section and only counting follicles with an oocyte nucleus, we avoided counting any follicles more than once per ovary. Follicles were classified as primordial, primary, secondary, pre-ovulatory, or post-ovulatory (ovulatory-sized follicles that had failed to ovulate, as evidenced by the presences of the oocyte, blood cells, and luteinizing granulosa cells). Since donor females were superovulated with hCG, it would be expected that all or most ovulatory-sized follicles would have ovulated.
Liquid chromatography-mass spectrometry
One ovary from each female mouse was transferred to 1.5-ml tubes, flash frozen on dry ice, and stored at – 80 °C. After all ovaries were collected and frozen, they were removed from the freezer and each ovary was transferred to a 2-ml screw cap RNAse/DNAse-free tube containing 300 μl of DPBS and 1.5-mm molecular-grade zirconium beads. Ovaries were homogenized on a BeadBug Microtube Homogenizer (Benchmark Scientific, Sayreville, NJ, USA) at maximum speed for 2 × 30 s. Afterwards, the liquid was pipetting into new 1.5-ml tubes, flash frozen on dry ice, and shipped to the Oregon Health and Sciences University for analysis of imatinib and its primary metabolite N-desmethyl imatinib. The methods for the liquid chromatography-mass spectrometry (LC-MS/MS) have been published previously [19]. Ovary tissue and serum were processed simultaneously. The serum concentrations have been previously reported [19].
Statistical analysis
Data were analyzed using mixed-effects analysis of variance (ANOVA), with treatment group as the fixed effect and individual mouse treated as a random effect nested within treatment. All analyses were carried out using Stata 14.2 (StataCorp, College Station, TX).
Results
Imatinib concentration in ovary
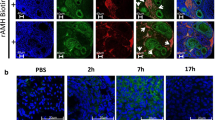
To determine the levels of imatinib attained in our mouse model and the rates of metabolism from imatinib to its primary metabolite, N-desmethyl imatinib, we used an LC-MS/MS analysis on ovarian tissue of mice treated either by IP injection or ad lib in drinking water. Imatinib is metabolized rapidly in rodent models (Fig. 1a). The primary metabolite of imatinib, N-desmethyl imatinib, was detected at approximately 1/10th the concentration of imatinib, but was retained at the higher concentration for a longer period of time (Fig. 1b). Levels of imatinib and N-desmethyl imatinib attained after 7 days ad lib in drinking water were roughly equivalent to the levels remaining in ovary at 8 h post-injection. The concentration of imatinib and N-desmethyl imatinib in the ovarian tissues was approximately 5–10% lower than the concentration detected in serum [19].
Concentration of imatinib and the metabolite N-desmethyl imatinib in ovarian tissue. The concentration of imatinib (a) and N-desmethyl imatinib (b) in ovaries was measured by LC-MS/MS. Female mice were either injected IP with imatinib (100 mg/kg) or fed imatinib ad lib (continuous) in drinking water (1 mg/ml) for 7 days. Imatinib was rapidly metabolized and barely detectable after 16 h. Concentrations of N-desmethyl imatinib were 10% lower than imatinib at 2 h post-injection and were retained in the tissue for a longer time, but declined by 16 h post-injection
Ovulation rates
There was no significant difference in ovulation rate (the total number of eggs and embryos) collected per donor (Table 1). Two females (18%) in each imatinib treatment produced no culture-grade embryo, while all control females had culture-grade embryos. The average number of eggs and embryos (ovulation rate) per female ± SEM was 33 ± 4, 18 ± 7, and 34 ± 10 for groups 0, 4, and 6 weeks, respectively (Table 1). All embryos that had cleaved to at least 4 cells by the time of collection were cultured to 120 h post-hCG by which time, they should have progressed to the blastocyst stage. The percent of culture-grade embryos (≥ 4 cells) at the time of collection was numerically lower in the imatinib groups, but was not statistically different (89% ± 3.9, 68% ± 13.9, and 68% ± 13.3, respectively; P = 0.10).
Ovarian reserve and follicle development
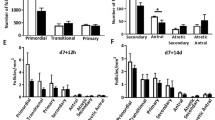
To compare the populations of follicles at various stages of development, we calculated percentages of the total follicles. Adult females treated long-term (4–6 weeks) with imatinib had significantly fewer total follicles (Table 2, Fig. 2a), including fewer primordial follicles (Fig. 2b). There was an equally significant increase in the percent of primary and secondary follicles, but no differences in the pre-ovulatory or post-ovulatory antral follicles. Taken together, these data indicate an increase in primordial follicle activation, a shift towards growing follicles, and a reduction in ovarian reserve following long-term imatinib treatments.
Imatinib impacts follicle reserve and primordial follicle activation. The total number of follicles remaining in the ovaries of imatinib-treated mice was significantly lower than age-matched controls (a). There were fewer primordial follicles (b) and a significant increase in the percent of growing follicles: primary (c) and secondary follicles (d). There were no differences in the number of pre-ovulatory (small) antral follicles (e) nor post-ovulatory antral follicles (f; large antral follicles that had failed to ovulate, still contained oocytes with follicular atresia and/or luteinization)
Embryo development
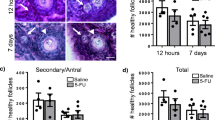
Long-term imatinib treatment had a significant impacted embryo quality (Fig. 3). The percent of embryos developing to blastocysts ranged from zero to 100% in the imatinib groups, while the controls were all above 75% blastocysts. The overall percent of embryos that developed to blastocyst was not statistically different between treatments (P = 0.07; Fig. 3a). However, embryos from imatinib-treated females had fewer cells per blastocyst (Fig. 3b) and a shift in cell differentiation, with fewer inner cell mass cells and increased trophectoderm cells (Fig. 3c, d).
Long-term imatinib treatment lead to reduced embryo developmental potential. All blastocysts were fixed at 120 h post-hCG and labeled for differential cell counts. Embryo development was assessed by percent of cultured embryos that developed to blastocysts (a), the total number of cells per blastocyst (b), and the percentage of cells that were either ICM (c) or TE (d). The ratio of ICM:TE was significantly lower in the imatinib-treated mice (p = 0.01). The number of blastocysts counted: 34, 46, and 40 for treatments 0, 4, and 6, respectively
Discussion
The findings from this pilot study suggest a deleterious effect with long-term imatinib exposure in a murine model. We observed a decrease in the proportion of primordial follicles and an increase in growing follicles in mice treated long-term with imatinib. This indicates a reduction of the total ovarian reserve. In addition, imatinib treatment lead to a decrease in the quality of preimplantation embryos as evidenced by a disturbance in the ratio of inner cell mass cells to trophectoderm cells. Long-term imatinib resulted in blastocysts with significantly fewer cells than controls, which indicate lower embryo developmental potential [24, 25]. Evidence of high concentrations of imatinib and N-desmethyl imatinib were detected in the ovarian tissue and support the causal associations seen in our study. To our knowledge, this is the first study to directly observe the response of controlled ovarian stimulation and the subsequent competence of the preimplantation embryo in mice undergoing long-term treatment with imatinib.
A study by Bernard et al. reported that TKI (sunitinib) treatment decreased ovulation rates, as seen by the lower number of corpora lutea (CLs) in superovulated mice, even though the number of developing follicles in that study was not affected by treatment [5]. Sunitinib is a broader spectrum inhibitor that targets numerous receptor TKIs including some similar to imatinib (KIT and PDGFR). The findings of our study were based on actual rates of ovulation and in vitro embryo developmental potential following long-term imatinib exposure. An earlier, retrospective study reported that imatinib did not affect the numbers of follicles; however, in that study, animals were treated only with imatinib ad lib in drinking water [26]. Our analysis of imatinib in serum and ovarian tissues demonstrated that ad lib imatinib in drinking water yields a much lower concentration of imatinib in serum and ovaries ([19], Fig. 1). Based on our findings, we hypothesize that follicle activation and a “burnout” effect may be implicated in the diminished ovarian reserve patterns that we observed [27].
Even though imatinib was not overtly ovo-toxic, there was evidence of gonadotoxicity with a significant reduction in embryo quality. The underlying reason may be explained by a decrease in the oocyte’s developmental competence after exposure to imatinib. The biological plausibility of this theory is supported by our data demonstrating a difference in the cellular differentiation of the preimplantation embryo. More specifically, we demonstrate a decrease in the proportion of inner cell mass cells to trophectoderm cells. We also observed a decreased total number of cells in the blastocysts from females exposed long-term to imatinib. Taken together, these objective and qualitative measures point to an increased likelihood of a compromised embryo after exposure to imatinib.
The change seen in the development of the blastocyst is likely a direct result of oocyte dysfunction caused by imatinib. Cellular function during the early cleavage stage is predominantly driven by the oocyte. There is little to no transcription of mRNA from the zygotic genome. Therefore, maternally supplied mRNAs and proteins during folliculogenesis are crucial for the proper development of the embryo. It remains uncertain what specific molecular interaction among TKI-treated mice was detrimental to the emerging follicles and whether this is also potentially genotoxic.
TKIs are presumed to be teratogenic as seen in animal studies and case reports have been published that detailed pregnancies in women taking imatinib that resulted in serious abnormalities or spontaneous abortions [28]. Because of the teratogenic potential from imatinib, oncologists recommend a washout period prior to pregnancy. However, due to the high rate of CML recurrence when imatinib is withheld, a prolonged period without treatment places a serious risk on the woman. The longer the time without imatinib treatment prior to pregnancy, the higher the likelihood of CML returning during pregnancy, thus creating a serious dilemma for the mother, to resume treatment during pregnancy or risk her own life by delaying treatment. Because of this dilemma, there are currently no official guidelines on length of pre-pregnancy washout is safe for imatinib. Extended studies are needed to determine if stopping imatinib for a prolonged washout period would improve egg and embryo quality and how to balance this washout with the potential for cancer to return during pregnancy.
With the limited data on balancing maternal safety and fetal teratogenicity, many oncologists recommend a planned pregnancy only after a stable major molecular response (24 months of undetectable BCR-ABL1) has been achieved so that the medication may be stopped at the diagnosis of pregnancy [29,30,31]. However, only 1% of patients reached a stable major molecular response at 30 months of the initiation of TKI, and 36% at 8 years [32]. This equates to a potential delay in fertility for many years. The evidence from this study extends this concern by demonstrating that long-term TKI treatment may impact fertility potential. TKIs have been revolutionary in improving the prognosis for CML patients, with patients living to their previously anticipated life expectancy before their cancer diagnosis [33]. Furthermore, the GIMEMA registry of CML reveals that approximately 25% of patients at diagnosis are between the reproductive ages of 21–40; therefore, issues relating to future fertility are a serious concern for cancer survivors’ quality of life [29].
Long-term imatinib treatment negatively impacts the ovarian reserve, oocyte, and embryo competence. This murine model found links between imatinib and a decreased fertility potential. Studies investigating the longitudinal impact of TKIs on ovarian reserve and fertility are still needed. In keeping with the American Society of Clinical Oncology, it is critical that patients receiving long-term imatinib have a discussion regarding the potential harms of imatinib on their future fertility and their options for fertility preservation. Until further evidence is available, it is advisable to encourage fertility preservation prior to initiation of TKIs.
References
Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4831–41.
Zeltzer LK. Cancer in adolescents and young-adults psychosocial-aspects—long-term survivors. Cancer. 1993;71:3463–8.
Oncology ASoC. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:5790.
Goel N, Malik R, Rathi B, Bhaskaran S, Rajaram S, Mehta S, et al. Pregnancy with metastatic gastrointestinal stromal tumor (GIST) on imatinib chemotherapy: an oncologist’s nightmare and obstetrician’s dilemma. J Gastrointest Cancer. 2013;44:115–7.
Bernard V, Bouilly J, Kramer P, Carre N, Schlumberger M, Visser JA, et al. The tyrosine kinase inhibitor sunitinib affects ovulation but not ovarian reserve in mouse: a preclinical study. PLoS One. 2016;11:e0152872.
Steegmann JL, Cervantes F, le Coutre P, Porkka K, Saglio G. Off-target effects of BCR-ABL1 inhibitors and their potential long-term implications in patients with chronic myeloid leukemia. Leuk Lymphoma. 2012;53:2351–61.
Rausch JL, Boichuk S, Ali AA, Patil SS, Liu L, Lee DM, et al. Opposing roles of KIT and ABL1 in the therapeutic response of gastrointestinal stromal tumor (GIST) cells to imatinib mesylate. Oncotarget. 2017;8:4471–83.
Melaiu O, Catalano C, De Santi C, Cipollini M, Figlioli G, Pelle L, et al. Inhibition of the platelet-derived growth factor receptor beta (PDGFRB) using gene silencing, crenolanib besylate, or imatinib mesylate hampers the malignant phenotype of mesothelioma cell lines. Genes Cancer. 2017;8:438–52.
Zitvogel L, Rusakiewicz S, Routy B, Ayyoub M, Kroemer G. Immunological off-target effects of imatinib. Nat Rev Clin Oncol. 2016;13:431–46.
Yoon SJ, Kim KH, Chung HM, Choi DH, Lee WS, Cha KY, et al. Gene expression profiling of early follicular development in primordial, primary, and secondary follicles. Fertil Steril. 2006;85:193–203.
Sleer LS, Taylor CC. Cell-type localization of platelet-derived growth factors and receptors in the postnatal rat ovary and follicle. Biol Reprod. 2007;76:379–90.
Carlsson IB, Laitinen MP, Scott JE, Louhio H, Velentzis L, Tuuri T, et al. Kit ligand and c-Kit are expressed during early human ovarian follicular development and their interaction is required for the survival of follicles in long-term culture. Reproduction. 2006;131:641–9.
Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12:61–9.
Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006;131:1007–15.
Pascuali N, Scotti L, Abramovich D, Irusta G, Di Pietro M, Bas D, et al. Inhibition of platelet-derived growth factor (PDGF) receptor affects follicular development and ovarian proliferation, apoptosis and angiogenesis in prepubertal eCG-treated rats. Mol Cell Endocrinol. 2015;412:148–58.
McGinnis LK, Carroll DJ, Kinsey WH. Protein tyrosine kinase signaling during oocyte maturation and fertilization. Mol Reprod Dev. 2011;78:831–45.
Christopoulos C, Dimakopoulou V, Rotas E. Primary ovarian insufficiency associated with imatinib therapy. N Engl J Med. 2008;358. United States:1079–80.
Zamah AM, Mauro MJ, Druker BJ, Oktay K, Egorin MJ, Cedars MI, et al. Will imatinib compromise reproductive capacity? Oncologist. 2011;16:1422–7.
Salem W, Li K, Krapp C, Ingles SA, Bartolomei MS, Chung K, et al. Imatinib treatments have long-term impact on placentation and embryo survival. Sci Rep. 2019;9:2535.
Biggers JD, McGinnis LK. Evidence that glucose is not always an inhibitor of mouse preimplantation development in vitro. Hum Reprod. 2001;16:153–63.
Petzer AL, Wolf D, Fong D, Lion T, Dyagil I, Masliak Z, et al. High-dose imatinib improves cytogenetic and molecular remissions in patients with pretreated Philadelphia-positive, BCR-ABL-positive chronic phase chronic myeloid leukemia: first results from the randomized CELSG phase III CML 11 “ISTAHIT” study. Haematologica. 2010;95:908–13.
Rezende VM, Rivellis AJ, Gomes MM, Dorr FA, Novaes MM, Nardinelli L, et al. Determination of serum levels of imatinib mesylate in patients with chronic myeloid leukemia: validation and application of a new analytical method to monitor treatment compliance. Rev Bras Hematol Hemoter. 2013;35:103–8.
Wolf A, Couttet P, Dong M, Grenet O, Heron M, Junker U, et al. Imatinib does not induce cardiotoxicity at clinically relevant concentrations in preclinical studies. Leuk Res. 2010;34:1180–8.
Kong X, Yang S, Gong F, Lu C, Zhang S, Lu G, et al. The relationship between cell number, division behavior and developmental potential of cleavage stage human embryos: a time-lapse study. PLoS One. 2016;11:e0153697.
Biggers JD, McGinnis LK, Raffin M. Amino acids and preimplantation development of the mouse in protein-free potassium simplex optimized medium. Biol Reprod. 2000;63:281–93.
Schultheis B, Nijmeijer BA, Yin H, Gosden RG, Melo JV. Imatinib mesylate at therapeutic doses has no impact on folliculogenesis or spermatogenesis in a leukaemic mouse model. Leuk Res. 2012;36:271–4.
Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra162.
Cole S, Kantarjian H, Ault P, Cortes JE. Successful completion of pregnancy in a patient with chronic myeloid leukemia without active intervention: a case report and review of the literature. Clin Lymphoma Myeloma. 2009;9:324–7.
Abruzzese E, Trawinska MM, Perrotti AP, De Fabritiis P. Tyrosine kinase inhibitors and pregnancy. Mediterr J Hematol Infect Dis. 2014;6:e2014028.
Ault P, Kantarjian H, O'Brien S, Faderl S, Beran M, Rios MB, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006;24:1204–8.
O'Brien S, Berman E, Borghaei H, Deangelo DJ, Devetten MP, Devine S, et al. NCCN clinical practice guidelines in oncology: chronic myelogenous leukemia. J Natl Compr Cancer Netw. 2009;7:984–1023.
Branford S, Yeung DT, Ross DM, Prime JA, Field CR, Altamura HK, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013;121:3818–24.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
Acknowledgments
A special thank you to Dr. Dennis Koop and Ms. Jenny Luo from the OHSU Bioanalytical Shared Resource/Pharmacokinetics Core for assistance with the LC-MS/MS analysis. The facility is part of the University Shared Resource Program supported by Oregon Health and Sciences University.
Funding
This research was supported by Grant No. IRG-58-007-54 from the American Cancer Society with pilot funding awarded to LKM; a fellowship research award from the Goldhirsh-Yellin Foundation to WS; and USC Norris Cancer Center Grant No. 2P30CA014089-43.
Author information
Authors and Affiliations
Contributions
All authors were involved in the initial planning of the research, reviewing frequent updates on the research progress, reviewing the data results, and writing the manuscript. LKM and WS treated the animals; LKM and IW conducted the cultures; WS counted follicles; LKM graded embryo development and counted blastocyst cells; SAI reviewed study designs and conducted the statistical analysis; IW, WA, and LKM drafted the manuscript; JRH, KC, and RJP reviewed all of the proposed research, critically evaluated the data, and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
All experiments were conducted in accordance with the “Guide for the Care and use of laboratory Animals” and preapproved by the University of Southern California Institutional Animal Care and Use Committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salem, W., Ho, J.R., Woo, I. et al. Long-term imatinib diminishes ovarian reserve and impacts embryo quality. J Assist Reprod Genet 37, 1459–1466 (2020). https://doi.org/10.1007/s10815-020-01778-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01778-7