Abstract
Purpose
To determine whether luteal support with intramuscular injection of human chorionic gonadotropin 1 day post-LH surge in natural cycle frozen embryo transfer (nFETs) increases ongoing pregnancy rates (OPR).
Methods
Retrospective cohort study of women who underwent natural cycle FET with transfer of a single day-5 or − 6 euploid blastocyst between January 2017 and December 2018 at an academic medical center were divided into two groups based on whether they received hCG 1 day post-LH surge. Patients with uterine factor infertility were excluded.
Results
A total of 529 nFET cycles were included. The OPR was significantly higher in the treatment group than in the non-treatment group when controlling for potential confounders such as embryo morphology (69.9% versus 57.4%, p = 0.0119, aOR1.724, 95% CI 1.13–2.65). There were no significant differences observed in the rates of first trimester loss (aOR 1.05, 95% CI 0.032–2.96) or biochemical pregnancy (aOR 0.79, 95% CI 0.31–1.76). Odds ratios were adjusted for patient’s age, body mass index, peak endometrial thickness, gravidity, and parity.
Conclusion
The current data suggest that the hCG booster given to patients within 1 day post-LH surge results in improved cycle outcomes compared to patients who do not receive the booster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the advent of improved embryo culture techniques and the reemergence of preimplantation genetic testing as a frequent adjunctive modality in in vitro fertilization, frozen embryo transfer cycles have become increasingly common in assisted reproductive technology. Depending on whether patients are ovulatory and on the preferences of the treating physician, patients may undergo programmed replacement cycles in which exogenous estrogen and progesterone orchestrate implantation, or rather undergo natural frozen embryo transfer (nFET) with reliance upon endogenous hormones. nFET has been shown to have similar success rates to programmed FET, while allowing the patient to avoid the risks and inconvenience of intramuscular progesterone injections during the first trimester [1]. The success of nFET is predicated on normal endogenous hormonal production, which can be buttressed with various forms of luteal phase support, including estrogen, progesterone, and human chorionic gonadotropin (hCG).
A pure nFET cycle does not involve the addition of any exogenous hormones. It has long been the practice in our clinic to supplement nFET with vaginal progesterone suppositories starting the evening after embryo transfer. Less well studied, however, is the practice of administering a single hCG bolus after the endogenous luteinizing hormone (LH) surge. This approach has the added benefit of augmenting production of not only progesterone but also estrogen from the corpus luteum [2]. One of the authors of this study has been using multiple 1500-IU hCG doses as an adjunct to the natural cycle luteal phase—on days 18 and 21—with an improvement in luteal phase progesterone levels and excellent outcomes (Zev Rosenwaks, personal communication). Increasingly over the last 18 months, we have anecdotally recognized a potential benefit of such an injection in conjunction with vaginal progesterone administration initiated after transfer, and in this study have sought to more formally decipher whether this regimen offers a significant clinical benefit to our patients undergoing nFET as compared to the use of vaginal progesterone alone.
Materials and methods
Cycle selection
The Weill Cornell Medicine institutional review board approved this study. All nFET cycles in which a single euploid embryo, confirmed via preimplantation genetic testing for aneuploidy (PGT-A), was transferred from January 2017 through December 2018 were reviewed for potential inclusion. Only cycles after 2016 were included as this is when the center’s PGT-A platform was updated to next-generation sequencing (NGS) from the previous microarray platform. Only the first FET cycle for each patient was included to eliminate repeated measures bias. Patients with a history of uterine factor infertility or known tubal disease without surgical correction were excluded from the study. Endometrial pathology was ruled out within 12 months of FET via saline sonogram or hysteroscopy.
Clinical protocols
Controlled ovarian hyperstimulation, oocyte maturation trigger, oocyte retrieval, embryo culture, and embryo transfer were conducted per our routine protocols [3]. Patients were stimulated with gonadotropins (Menopur [Ferring]; Gonal-F [EMD-Serono]; and/or Follistim [Merck]) followed by pituitary suppression with GnRH-antagonist (Cetrotide 0.25 mg [EMD-Soreno] or Ganirelix Acetate 0.25 mg [Organon]). Less commonly, patients were down-regulated with GnRH-agonists (Lupron [Abbott Pharmaceuticals]) followed by stimulation with gonadotropins. Pretreatment with E2 patches (Climara 0.1 mg, Bayer HealthCare) or birth control pills (Ortho-Novum, Janssen Pharmaceuticals) were used in some cases before initiating gonadotropin therapy.
hCG (Pregnyl [Schering-Plow]; Novarel [Ferring Pharmaceuticals]; or Profasi [EMD-Sereno]) was used to trigger follicular maturation when, in general, two follicles attained > 17 mm. Patients on antagonist protocols deemed to be at high risk of developing OHSS were triggered with either 4 mg leuprolide acetate or a combination of 4 mg of leuprolide acetate and 1500 IU hCG. Oocyte retrieval was performed under sedation 35–37 h following trigger. As PGT-A was to be performed, intracytoplasmic sperm injection was employed in all cycles. Embryos were biopsied on day 5 or day 6, and only euploid embryos confirmed via PGT-A were transferred. The best morphologically graded euploid embryo available was selected for transfer. Embryo transfer was performed by the physician assigned to perform such procedures for the entire practice on that day as per a rotating schedule; this may or may not have been a patient’s primary physician.
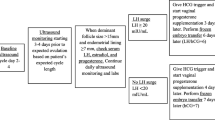
Only patients undergoing nFET were included in this study. Transvaginal ultrasonography was performed to assess follicle development and endometrial thickness in the mid-follicular phase. Serum estradiol and LH levels were measured starting 3–4 days prior to suspected ovulation date and continued until the LH surge was detected. The LH surge was defined by an LH level > 17 IU/L during the follicular phase with a subsequent dropping estradiol level thereafter. Patients were divided into two groups depending on whether they received injection of hCG. hCG use was determined by individual physician preference, rather than by a set protocol. Group A received one bolus dose of hCG (typical dose 3300 IU) 1 day after identification of the LH surge (dose range: 2500 IU n = 8; 3300 IU n = 105; 5000 IU n = 26; 10,000 IU n = 7). In general, patients with a higher BMI were administered higher doses of hCG. Group B received no hCG. Both groups started vaginal progesterone supplementation the evening after embryo transfer. Serum E2, P4, and hCG levels were drawn 10 days post-FET. If positive, a repeat hCG level was drawn 48 h later and pelvic ultrasound was performed at 5.5 weeks to confirm intrauterine pregnancy. Follow-up ultrasounds were done to assess fetal viability. Patients were referred out for antenatal care at 8–10 weeks’ gestation.
Laboratory procedures and blastocyst grading
Embryos were cultured individually in microwells of an EmbryoScope (Vitrolife, Sweden) with an integrated time-lapse system. Blastocysts were graded according to three of the following morphological parameters: inner cell mass (ICM), trophectoderm, and degree of expansion with hatching stage according to the Gardner criteria [4]. ICM morphology was graded into three categories: A – tightly packed cells; B – loosely packed cells; C – cells are not identifiable. Trophectoderm morphology was divided into three groups: A – many cells forming a cohesive epithelial layer; B – cells of uneven size; C – few large cells squeezed to the side. The degree of expansion and hatching stage were the following: 1 – the blastocoel constitutes less than 50% of the nonexpanded embryo; 2—the blastocoel fills more than 50% of the embryo 3—the blastocoel nearly fills the whole blastocyst; 4 – a very thin zona pellucida surrounding an expanded blastocyst; 5—the blastocyst is hatching; 6—the blastocyst has completely hatched out of the zona pellucida. In order to adjust for blastocyst grading, blastocysts were divided into four groups according to their morphological grading before cryopreservation: excellent (≥ 3AA), good (3-6AB, 3-6BA, 1-2AA), average (3-6BB, 3–6 AC, 3-6CA, 1-2AB, 1-2BA), and poor (1–6 BC, 1-6CB, 1-6CC, 1-2BB) [5, 6]. Vitrification was performed on day 5 or day 6 based on the development of each embryo.
Outcome variables assessed
The primary outcome of this study was ongoing pregnancy rate. Secondary outcomes included positive pregnancy test and first trimester miscarriage. Ongoing pregnancy rate was defined as the proportion of transfers resulting in clinical intrauterine pregnancies at 10–12 weeks’ gestation (as identified by a transvaginal ultrasound). First trimester loss rate was defined as the proportion of clinical pregnancies resulting in first trimester losses. The biochemical rate was defined as the proportion of transfers resulting in a transient elevation in the serum b-hCG level without sonographic evidence of a gestational sac. Data on ectopic pregnancy incidence was included. The following demographic characteristics were extracted from the medical record: age at transfer, gravidity, parity, body mass index (BMI), blastocyst grading, and peak endometrial thickness.
Statistical analysis
All data analyses were conducted with R-Studio statistical software. Continuous variables were expressed as mean ± standard deviation and were tested for normality; Student’s t test was used for parametric data. Categorical variables were compared by Chi-square and Fisher’s exact tests. Odds ratios (OR) with 95% confidence intervals (CI) were calculated and adjusted for patient age at time of transfer, embryo quality as assessed by blastocyst grade, BMI, gravidity, parity, and peak endometrial thickness. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
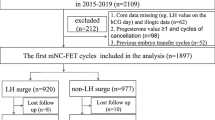
A total of 1555 natural cycle FETs were performed at the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine during the study period of January 2017 through December 2018. Of these, 822 cycles were excluded for not having PGT-A prior to transfer, 44 PGT-A cycles for transferring more than 1 embryo, 9 cycles for uterine factor infertility, and 151 cycles for repeated measures bias.
Of the 529 FETs included, 146 received the hCG booster (Group A) and 383 did not (Group B) (Table 1). The demographic characteristics of patients in Group A and Group B are summarized in Table 1. There were no statistically significant differences in age at transfer, BMI or distribution of embryo quality across groups. Primary infertility diagnoses were similar between groups. Patients without hCG treatment had statistically higher (but not clinically meaningful) gravidity and parity as well as thicker peak endometrial thickness.
Group A versus group B
The ongoing pregnancy rate was significantly higher in the hCG treatment group (69.9%) versus the control group (57.4%) (p = 0.0119), with a calculated odds ratio of 1.717 (95% CI 1.149–2.600). When adjusting for patient’s age at transfer, embryo quality, BMI, peak endometrial thickness, gravidity, and parity, the ongoing pregnancy rate was significantly higher in patients receiving hCG (aOR 1.724, 95% CI 1.13–2.65) (Table 2). The rates of ongoing pregnancy were 87.5%, 66.7%, 76.9% and 71.4% in patients receiving dosages of 2500 IU, 3300 IU, 5000 IU and 10,000 IU, respectively. A chi-square test of independence found that the relation between hCG dosage and ongoing pregnancy rate was not significant, X2 (1, n = 145) =2.315, p = .5096.
The multivariate logistic regression revealed that, in addition to hCG use, embryo quality was significantly associated with outcome. Stratifying for transferred embryo quality, the use of hCG led to higher ongoing pregnancy rates in all embryo grade categories: excellent- (84% vs 61%), good- (73% vs 67%), average- (72% vs 57%), and poor-quality embryos (58% vs 52%). However, only the average-quality group reached statistical significance (p = 0.036, aOR 2.03, CI 1.11–3.84), likely due to the small sample size of the remaining groups.
The rate of first trimester loss in the treatment group (3.4%) versus the control group (3.7%) was not statistically significant (p = 0.90, OR 0.93, CI 0.23–2.49). When adjusting for the factors above, the differences remained insignificant (aOR 1.05, 95% CI 0.032–2.96). Similarly, the rate of initial positive pregnancy test was not different between the treatment (5.5%) and control groups (7.3%) (p = 0.58, OR 0.73, CI 0.31–1.58), as well as when adjusted (aOR 0.79, 95% CI 0.31–1.76).
Discussion
The present study was conducted to investigate the use of single dose hCG as luteal phase support in nFET cycles. We hypothesized that hCG supplementation after the LH surge would buttress endogenous steroidogenesis leading to improved pregnancy outcomes. Our findings show that the addition of an hCG bolus 1 day post-LH surge is associated with a statistically significant higher ongoing pregnancy rate. Furthermore, our data show that hCG use is not associated with higher rates of first trimester loss, biochemical pregnancies or ectopic pregnancies. These results were unchanged after adjustments for confounders, including embryo quality.
The majority of studies investigating the use of hCG in nFET have focused on its role as a trigger to prompt ovulation prior to an endogenous LH surge. In a randomized controlled trial (n = 124), Fatemi et al. found that nFET after spontaneous surge had improved outcomes compared to when hCG was given to trigger ovulation [7]. In that analysis, the study was terminated before its conclusion, as a significantly higher ongoing pregnancy rate (31.1% vs. 14.3%) was achieved when a spontaneous LH surge as opposed to an hCG trigger was used to time day-3 transfers. On the other hand, Weissman et al. found no difference in success rates with the use of an hCG trigger to time nFET as compared to waiting for the endogenous surge; however, the authors noted higher patient satisfaction with the practice of triggering, as less monitoring was required in these cycles [8].
There are few studies on the use of hCG as luteal phase support. Of note, two prior randomized controlled trials have not found a statistically significant difference in pregnancy outcomes with the addition of hCG as luteal phase support, however, we felt additional investigation was warranted based on variations with the study design and hCG administration in these trials. In a randomized controlled trial comparing various endometrial preparation methods in FET by Mandani et al., one subgroup (n = 117) underwent nFET with ovulation triggered by 10,000 IU hCG followed by 2500 IU hCG every 3 days during the luteal phase [9]. As described previously, hCG given to trigger ovulation has been found to be associated with poorer outcomes than spontaneous ovulation, leaving the impact of the luteal phase hCG less clear [7, 8]. Furthermore, our study analyzes the use of bolus hCG dosing rather than every few days during the luteal phase. Another randomized controlled study (n = 450) by Lee et al. investigated nFET cycles augmented with 1500 IU hCG on the day of FET and 6 days later; however, progesterone luteal phase support was not given [10]. Additionally, the aforementioned studies analyzed only non-PGT, cleavage-stage embryo transfers. Both blastocyst transfer and vaginal progesterone luteal support have been associated with higher ongoing pregnancy rates, and by limiting our study to only PGT-A embryos and controlling for luteal phase progesterone use, we eliminated major sources of potential confounding present in these prior studies [11, 12].
Given its long half-life, intramuscular hCG achieves circulating serum concentrations for approximately 8–9 days after administration. In the ovary, hCG activates the LH receptor; however, emerging research indicates that the downstream effects of LH and hCG differ [2]. Casarini et al. found that hCG binding led to more potent activation of the cyclic AMP-protein kinase a pathway, which stimulates progesterone production in granulosa cells; on the other hand, LH led to a more potent activation of cell cycle regulators ERK and AKT, important in folliculogenesis [13]. Demonstrations in vitro suggest that hCG promotes longevity of the corpus luteum via increasing levels of antiapoptotic BCL-2 and decreasing proapoptotic Bax [14].
In addition to its effects on the corpus luteum, research suggests that hCG may signal to the endometrium of future blastocyst implantation, foster growth and differentiation of trophoblast cells, and establish placental villous structures [15]. Given that hCG biosynthesis begins early on in embryo development, and hCG is detected in relatively high concentrations in the uterine cavity prior to implantation, the exogenous administration of hCG during in vitro fertilization may mimic the local effects of hCG when fertilization occurs naturally [16].
Strengths of our study include its relatively large sample size, incorporation of women of all age ranges, and representation of patients from the full spectrum of infertility diagnoses. Additionally, our study controlled for chromosomal aneuploidy, which is one of the largest causes of implantation failure. Although patients in the treatment group had a lower mean gravidity, parity, and peak endometrial thickness, they still demonstrated better outcomes with hCG administration. Of note, 9 patients (3.57%) in our study had a fresh transfer resulting from sibling oocytes generated from their PGT-A cycle (outcomes of which included live birth [n = 1], biochemical pregnancy [n = 2], first trimester loss [n = 2], and no pregnancy [n = 5]). Given the small sample size of this group and their distribution of outcomes in their subsequent FET cycle (ongoing pregnancy [n = 4], first trimester loss [n = 1], and no pregnancy [n = 4]), they were included in our analysis. We acknowledge as limitations, however, the retrospective nature of the study as presented as well as the variability in hCG dosing, which was subject to physician judgment according to patient BMI and clinical scenario.
Conclusions
This study provides evidence that nFET cycles in which the luteal phase is buttressed with both a single hCG injection after the endogenous surge as well as vaginal progesterone after transfer are associated with high clinical success rates with minimal negative impact on the patient experience. These findings may provide a basis for further larger randomized control trials and identification of optimal dosing parameters of hCG.
References
Ghobara T, Vandekerckhove P. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2017;7:CD003414.
Devoto A, Fuentes L, Koehn P, Cespedes P, Palomino A, Pommer R, et al. The Corpus Luteum: life cycle and function in natural cycles. Fertil Steril. 2009;92(3):1067–79.
Reichman D, Rosenwaks Z. GnRH antagonist-based protocols for in vitro fertilization. Methods Mol Biol. 2014;1154:289–304.
Gardner D, Balaban B. Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and ‘OMICS’: is looking good still important? Mol Hum Reprod. 2016;22(10):704–18.
Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovoc N, et al. Morphological grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107(3):664–70.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observation study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81.
Fatemi HM, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. 2010;94(6):2054–8.
Weissman A, Horowitz E, Ravhon A, Steinfeld Z, Mutzafi R, Golan A, et al. Spontaneous ovulation versus HCG triggering for timing natural-cycle frozen-thawed embryo transfer: a randomized study. Reprod BioMed Online. 2011;23(4):484–9.
Madani T, Ramezanali F, Yahyaei A, Hasani F, Lankarani NB, Yeganeh LH. Live birth rates after different endometrial preparation methods in frozen cleavage-stage embryo transfer cycles: a randomized controlled trial. Arch Gynecol Obstet. 2019;299(4):1185–91.
Lee VCY, Li RHW, Yeung WSB, Chung HP, Ng EHY. A randomized double-blinded controlled trial of hCG as luteal phase support in natural cycle frozen embryo transfer. Hum Reprod. 2017;32(5):1130–7.
Holden EC, Kashani BN, Morelli SS, Alderson D, Jindal SK, Ohman-Strickland PA, et al. Improved outcomes after blastocyst-stage frozen-thawed embryo transfers compared with cleavage stage: a Society for Assisted Reproductive Technologies Clinical Outcomes Reporting System Study. Fertil Steril. 2018;110(1):89–94.
Bjuresten K, Landgren BM, Hovatta O, Stavreus-Evers A. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertil Steril. 2011;95(2):534–7.
Casarini L, Lispi M, Longobardi S, Milosa F, La Marca A, Tagliasacchi D, et al. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular Signalling. PLoS One. 2012;7(10):e46682.
Sungino N, Suzuki T, Kashida S, Karube A, Takiguchi S, Kato H. Expression of Bcl-2 and Bax in the human corpus luteum during the menstrual cycle and in early pregnancy: regulation by human chorionic gonadotropin. J Clin Endocrinol Metab. 2000;85(11):4379–86.
Cole L. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102.
Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocinol. 2007;269(1–2):85–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures/conflict of interest
DER has nothing to disclose. CRS has nothing to disclose. ZR has nothing to disclose.
DER and CRS should be considered similar in author order.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reichman, D.E., Stewart, C.R. & Rosenwaks, Z. Natural frozen embryo transfer with hCG booster leads to improved cycle outcomes: a retrospective cohort study. J Assist Reprod Genet 37, 1177–1182 (2020). https://doi.org/10.1007/s10815-020-01740-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01740-7