Abstract
Tetrasomy 9p (ORPHA: 3310) (i(9p)) is a rare chromosomal imbalance. It is characterized by the presence of a supernumerary chromosome incorporating two copies of the short arm of chromosome 9 and is usually present in a mosaic state postnatally. Depending on the level of mosaicism, the phenotype ranges from mild developmental delay to multiple congenital anomalies with severe intellectual disability. Here, we report on a patient diagnosed with i(9p) mosaicism after the recurrent failure of in vitro fertilization. Although the patient’s clinical phenotype was normal, the level of mosaicism varied greatly from one tissue to another. A sperm analysis evidenced subnormal spermatogenesis with chromosomally balanced spermatozoa and no risk of transmission to the offspring. Although individuals with i(9p) and no clinical manifestations have rarely been described, the prenatal diagnosis of this abnormality in the absence of ultrasound findings raises a number of questions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetrasomy 9p is a rare chromosome imbalance that was first described by Ghymers et al. in 1973 [1]. It is defined by the presence of an isochromosome for the short arm of chromosome 9 (i(9p)) in a homogenous or mosaic state. Tetrasomy 9p is frequently caused by nondisjunction during meiosis II and then duplication of the short arm and loss of the long arm [2]. Short arm isochromosomes have also been reported for chromosomes 5, 8, 10, 12, 18, and 20, and all of these conditions are associated with a severe phenotype.
Tetrasomy 9p is a rare event, although there are a few literature reports of prenatally and postnatally diagnosed mosaic and non-mosaic cases [3]. The clinical manifestations associated with i(9p) are highly variable and include intellectual deficiency and developmental delay (in 73% of cases), growth delay (39%), intra-uterine growth retardation (28%), skeletal anomalies (55%), genital/urinary tract anomalies (43%), and ophthalmologic anomalies (42%). Microcephaly is present in 20% of cases, and dysmorphic features include abnormal ears (69%), hypertelorism (46%), micro-retrognathia (45%), and cleft lip/palate (32%) [3]. It has been suggested that the severity of handicap is related to the degree of mosaicism [4,5,6]. Only a few patients with a normal phenotype have been described. Here, we report a patient with tetrasomy 9p mosaicism but no clinical manifestations other than moderate oligozoospermia with chromosomally balanced sperm. We compared our clinical, cytogenetic, and molecular findings with previous reports on patients with a normal phenotype.
Clinical report
A 41-year-old man and his 37-year-old wife were referred for cytogenetic assessment after the failure of four in vitro fertilization (IVF) attempts and eight embryo transfers. The indication for IVF was moderate oligoasthenozoospermia. The patient did not have any dysmorphic features and had a high educational level. A clinical examination did not evidence any Blaschko’s lines on the back or trunk. There was no family history of miscarriages and/or genetic abnormalities. The female partner’s medical history included pelvic endometriosis, myomectomy, and curettage for a first-trimester miscarriage.
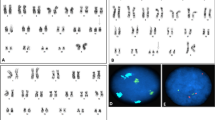
GTG-banding karyotyping (performed according to standard procedures) of a blood sample revealed a mos 47,XY,+i(9)(p11)[6]/46,XY[18] karyotype consistent with mosaicism for tetrasomy 9p. After blood culture, a FISH analysis of metaphase chromosomes (using a centromeric probe for chromosome 9 (9p11-q11 Alpha Satellite DNA, Abbott Molecular, Abbott Park, IL, USA) confirmed the karyotype results and the level of mosaicism.
After genetic counselling, a FISH analysis using the same probe was performed on uncultured blood (n = 100), urine (n = 100), and buccal mucosa (n = 16) samples. The level of mosaicism ranged from 6% (in the buccal mucosa) to 70% (in the urine).
Array comparative genomic hybridization (aCGH, using an Agilent Human SurePrint G3 Human CGH array Kit 8x60k) was performed on blood samples, according to the manufacturer’s instructions. The data were processed using Agilent Feature Extraction software and Workbench Lite 6.0 software (both from Agilent Technologies). The array CGH analysis of peripheral blood confirmed the presence of a mosaic i(9p) lacking a long arm sequence in 60% of the cells (Fig. 1).
To evaluate the risk of transmission to the offspring, we analyzed the patient’s sperm. After decondensation of the sperm chromatin with a 1 N NaOH solution, the same FISH probe was used to evaluate i(9p) inheritance. The results were essentially normal, since the spermatozoa (n = 600) did not carry the extra chromosome. Given the patient’s normal clinical phenotype, these findings indicate that many tissues can have a low level of i(9p) mosaicism.
Discussion
Here, we reported on an unusual case of mosaic i(9p) in a healthy, clinically normal adult. To the best of our knowledge, this is the seventh literature report on a patient with mosaic i(9p) and normal mental development [7,8,9,10,11] (Table 1) but is the first in which a sperm analysis was performed to evaluate the risk of inheritance.
For the case reports detailed in Table 1, the reasons for investigation were variously skin lesions (n = 1), infertility (n = 4), a family study (n = 1), and male infertility in the partner (n = 1). In the first case [7], karyotyping was prompted by the observation of Blaschko’s lines, a feature that is known to be associated with mosaicism [7]. In contrast, i(9p) was discovered fortuitously in two cases [11]. Both of the women had an unremarkable family medical history, and karyotyping was prompted respectively by a family study for a pericentric inversion of chromosome 7 and by azoospermia in the woman’s partner. In four cases (three men and a woman), the cytogenetic analysis was performed as part of infertility treatment [8,9,10].
It is known that karyotype rearrangements predispose to infertility as a result of meiotic errors [12]. Reciprocal translocations, inversions, and heterochromatin variants of chromosome 9 have been reported in infertile individuals [13]. Changes in heterochromatin content are associated with infertility, and it has been suggested that large heterochromatic blocks can destabilize chromosomal pairing and cause meiotic arrest [14]. Hence, the observation of four infertile patients with mosaic i(9p) suggests that the isochromosome has an impact on human fertility. However, considering that (i) the main indications for karyotyping are mental retardation, development abnormalities, and infertility, and (ii) the patients did not suffer from the first two types of impairment, the remaining patients were either infertile or had been assessed as a result of infertility in the partner. Hence, is i(9p) really involved in infertility, or is there bias in the patients studied to date? Our oligozoospermic patient’s spermatozoa did not carry i(9p). One can hypothesize that i(9p) leads to spermatogenesis arrest for spermatogonia carrying the isochromosome, and that only spermatogonia with a normal karyotype complete spermatogenesis. In our opinion, the diagnosis of i(9p) was prompted by the clinical manifestations only for the patient with skin lesions. In the other six cases, the diagnosis could be considered to be fortuitous.
The phenotypic variability of i(9p) is largely unexplained. i(9p) shows a strong trend towards tissue-limited mosaicism [5, 8, 15,16,17,18,19,20]. This mosaicism is predominantly detected in the peripheral blood, and is often absent or only present at a low frequency in the skin and the buccal mucosa [19]. However, most of the literature reports studied a single tissue, and a high proportion of the patients presented mental retardation and/or development abnormalities. In such cases, i(9p) was considered to be the cause of the abnormal phenotype. In the seven “normal phenotype” cases, the level of i(9p) mosaicism ranged from 6 to 100%. With a view to explaining the phenotype, one other tissue was analyzed in four cases, and three other tissues were analyzed in the present case. As expected, the level of mosaicism varied markedly from one tissue to another. In our case, mosaicism was observed in only 6% of buccal mucosa cells, and i(9p) was not found in the spermatozoa. Hence, the level of mosaicism in blood does not appear to be a good predictor of mental retardation. In our present case (and probably also in the six other cases with a normal phenotype), the mosaicism level in brain tissue was probably null.
This tissue-limited mosaicism can make prenatal diagnosis very difficult. In the literature, false-negative prenatal results have been reported for patients with an abnormal phenotype [5, 18]; i(9p) mosaicism in the blood was identified postnatally. In contrast, our report and the six previous reports on cases with normal phenotype suggest that a prenatal diagnosis of i(9p) should be considered with caution. If an invasive procedure is prompted by ultrasound findings, the i(9p) might well be causative. In contrast, the presence of i(9p) in patients without any ultrasound findings (i.e., when the invasive procedure is prompted by advanced maternal age or abnormal laboratory results) raises a number of questions. Is the sole presence of the isochromosome sufficiently predictive of a poor prognosis for the pregnancy? Indeed, this category of patients might be excluded after noninvasive prenatal testing, and so i(9p) might be undiagnosed. It has been suggested that when a low level of i(9p) mosaicism is detected after amniocentesis, fetal blood sampling is valuable for confirmation [21]. Furthermore, the application of molecular cytogenetic techniques (such as aCGH and interphase FISH on uncultured amniocytes) may usually detect mosaic i(9p) without being influenced by the effect of long-term amniocyte culture [22].
Based on the literature data, the risk of transmission to the offspring seems quite low. Pregnancy has been reported in three cases. In the first case [8], the couple had a total of 7 pregnancies. The first two resulted from IVF and intracytoplasmic sperm injection, and both were twin pregnancies. The first pregnancy ended in spontaneous abortion at 8 weeks of gestation. In the second pregnancy, spontaneous delivery occurred at 24 weeks of gestation, both babies died immediately after birth, and the karyotype was normal. Five pregnancies were conceived naturally, with three miscarriages and two deliveries. Karyotyping was performed for three pregnancies; the result was normal for the surviving two children and was abnormal (trisomy 15) for one of the miscarriages. An influence of i(9p) can reasonably be ruled out for three of the five losses of pregnancy. In the second case [11] (a patient with a history of pericentric inversion of chromosome 7), a child was conceived naturally and was born healthy. In the present case, a miscarriage occurred after IVF but the spermatozoa did not carry the extra chromosome.
Interestingly, “normal” phenotypes have been reported for other mosaic isochromosome syndromes. A de novo 3% i(18p) mosaic was observed in a women with slight dysmorphia, and her daughter was subsequently found to have a 47,XX,i(18p) karyotype [23]. A 16% i(5p) mosaic was also described for a male after 5 years of infertility and two miscarriages. The couple had a healthy 8-year-old girl. After IVF, a healthy girl with a normal karyotype was born [24]. Lastly, an i(12p) mosaic has been described in a 4-year-old girl for whom karyotyping was prompted by the observation of hypopigmentation [25]. i(12p) is linked to the Pallister Killian syndrome, where the clinical phenotype is closely related to the frequency and tissue distribution of the isochromosome. In general, supernumerary isochromosome mosaicism (as for i(9p)) is currently considered to be associated with a poor prognosis, although few “normal” phenotypes have been described. There appears to be risk of supernumerary isochromosome inheritance in one in five cases described to date. For men, a sperm FISH analysis (as performed in the present case) will be of value in evaluating this risk. The main clinical problem relates to the prenatal diagnosis of a mosaic de novo isochromosome in the absence of any ultrasound findings.
Conclusion
We provided the first report on a sperm FISH analysis in a man with a mosaic i(9p) and a normal clinical phenotype. Despite a high percentage of aneuploid cells in some tissues, no clinical manifestations were noted, and no chromosomally unbalanced sperm were identified. This and the other cases of mosaic isochromosomes described in the literature challenge our current approach to genetic counselling and prenatal diagnosis—even though the emergence of noninvasive prenatal testing for conventional trisomies should enable us to avoid the identification of this type of abnormality in a fetus with no ultrasound findings. Furthermore, the level of mosaicism should not be considered as the sole prognostic factor in this situation. Given the impossibility of evaluating the level of mosaicism in the brain, amniocentesis and (postnatally) a skin biopsy might be the best sampling approaches.
Furthermore, in view of the female partner’s medical background, we hypothesize that recurrent IVF failure was not due to i(9)p.
References
Ghymers D, Hermann B, Distèche C, Frederic J. Partial tetrasomy of number 9 chromosome, and mosaicism in a child with multiple malformations. Humangenetik. 1973;20:273–82.
Dutley F, Balmer D, Baumer A, Binkert F, Schinzel A. Isochromosome 12p and 9p: parental origin and possible mechanisms of formation. Eur J Hum Genet. 1998;6:140–4.
El Khattabi L, Jaillard S, Andrieux J, Pasquier L, Perrin L, Capri Y, et al. Clinical and molecular delineation of tetrasomy 9p syndrome: report of 12 new cases and literature review. Am J Med Genet A. 2015;9999:1–10.
Schaefer GB, Domek DB, Morgan MA, Muneer RS, Johnson SF. Tetrasomy of the short arm of chromosome 9: prenatal diagnosis and further delineation of the phenotype. Am J Med Genet. 1991;38:612–5.
Grass FS, Parke JC Jr, Kirkman HN, Christensen V, Roddey OF, Wade RV, et al. Tetrasomy 9p: tissue-limited idic (9p) in a child with mild manifestations, a normal CVS result. Report review. Am J Med Genet. 1993;47:812–6.
Van Hove J, Kleczkowska A, De Bruyn M, Bekaert J, Fryns JP. Tetrasomy 9p: prenatal diagnosis and fetopathological findings in a second trimester male fetus. Ann Genet. 1994;37:139–42.
Sait SNJ, Wetzler M: Tetrasomy 9p with no apparent phenotype characteristics (abstract), 53th ASHG Annual Meeting: Nr 678 (2003).
McAuliffe F, Winsor EJ, Chitayat D. Tetrasomy 9p mosaicism associated with a normal phenotype. Fetal Diagn Ther. 2005;20:219–22.
Ogino W, Takeshima Y, Nishiyama A, Yagi M, Oka N, Matsuo M. Mosaic tetrasomy 9p case with the phenotype mimicking Klinefelter syndrome and hyporesponse of gonadotropin-stimulated testosterone production. Kobe J Med Sci. 2007;53:143–50.
Baronchelli S, Conconi D, Panzeri E, Bentivegna A, Redaelli S, et al. Cytogenetics of premature ovarian failure: an investigation on 269 affected women. J Biomed Biotechnol. 2011;370195.
Papoulidis I, Kontodiou M, Tzimina M, Saitis I, Hamid AB, Klein E, et al. Tetrasomy 9p mosaicism associated with a normal phenotype in two cases. Cytogenet Genome Res. 2012;136:237–41.
Mierla D, Jardan D, Stoian V. Chromosomal abnormality in men with impaired spermatogenesis. Int J Fertil Steril. 2014;8(1):35–42.
Kosyakova N, Grigorian A, Liehr T, Manvelyan M, Simonyan I, Mkrtchyan H, et al. Heteromorphic variants of chromosome 9. Mol Cytogenet. 2013;6:14.
Humphray S, Oliver K, Hunt AR, Plumb RW, Loveland JE, Howe KL, et al. DNA sequence and analysis of human chromosome 9. Nature. 2004;429:369–74.
Calvieri F, Tozzi C, Benincori C, De Merulis MV, Bellussi A, Genuardi M, et al. Partial tetrasomy 9 in an infant with clinical and radiological evidence of multiple joint dislocations. Eur J Pediatr. 1988;147:645–8.
Cazorla Calleja MR, Verdu A, Felix V. Dandy-Walker malformation in an infant with tetrasomy 9p. Brain Dev. 2003;25:220–3.
Cuoco C, Gimelli G, Pasquali F, Poloni L, Zuffardi O, Alicata P, et al. Duplication of the short arm of chromosome 9. Analysis of five cases. Hum Genet. 1982;61:3–7.
Eggermann T, Rossier E, Theurer-Mainka U, Backsch C, Klein-Vogler U, Enders H, et al. New case of mosaic tetrasomy 9p with additional neurometabolic findings. Am J Med Genet. 1998;75:530–3.
Lloveras E, Perez C, Sole F, Zamora L, Lladonosa A, Espinet B, et al. Two cases of tetrasomy 9p syndrome with tissue limited mosaicism. Am J Med Genet A. 2004;124:402–6.
Tang W, Boyd BK, Hummel M, Wenger SL. Prenatal diagnosis of tetrasomy 9p. Am J Med Genet A. 2004;126:328.
Chen C-P, Chang T-Y, Chern S-R, Lee C-C, Town D-D, Lee M-S, et al. Prenatal diagnosis of low-level mosaic tetrasomy 9p by amniocentesis. Prenat Diagn. 2007;27:383e5.
Chen CP, Wang LK, Chern SR, Wu PS, Chen YT, Kuo YL, et al. Mosaic tetrasomy 9p at amniocentesis: prenatal diagnosis, molecular cytogenetic characterization, and literature review. Taiwan J Obstet Gynecol. 2014;53(1):79–85.
Abeliovich D, Dagan J, Levy A, Steinberg A, Zlotogora J. Isochromosome 18p in a mother and her child. Am J Med Genet. 1993;46:392e3.
Venci A, Bettio D. Tetrasomy 5p mosaicism due to an additional isochromosome 5p in a man with normal phenotype. Am J Med Genet Part A. 2009;149A:2889–91.
Alesi V, Dentici ML, Restaldi F, et al. Unclassifiable pattern of hypopigmentation in a patient with mosaic partial 12p tetrasomy without Pallister–Killian syndrome. Am J Med Genet Part A. 2017;9999:1–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bellil, H., Herve, B., Herzog, E. et al. A high level of tetrasomy 9p mosaicism but no clinical manifestations other than moderate oligozoospermia with chromosomally balanced sperm: a case report. J Assist Reprod Genet 37, 573–577 (2020). https://doi.org/10.1007/s10815-020-01690-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01690-0