Abstract
Purpose
To identify biomarkers that prospectively predict IVF cycle cancellation.
Methods
In this prospective study, sera were obtained prior to any intervention, from women about to undergo an IVF cycle. The sera were assayed by ELISA for levels of insulin-like growth factor (IGF)-1, IGF-2, IGF binding protein (BP)-1, and soluble fms-like tyrosine kinase (sFLT-1). The cancellation or progression of the IVF cycle was subsequently obtained by chart review. Associations between serum components and outcome were analyzed by the Mann-Whitney test. Receiver operator curves were constructed to evaluate the strength of the correlations between biomarkers and cycle cancellation, as assessed from the area under the curve (AUC).
Results
A total of 205 women were included. Twenty-seven (13.2%) cycle cancellations due to poor response were recorded. Women with a cancelled cycle had reduced anti-Mullerian hormone (AMH) values (p < 0.001) and antral follicle count (p = 0.003). There were no significant differences between the two groups with regard to age and BMI. Median concentrations of IGF-1 and sFLT-1 were elevated in sera from women whose IVF cycles were cancelled as compared to those with ongoing cycles (p = 0.015 and p < 0.001, respectively); AUC for IGF-1 and sFLT-1 were 0.67 and 0.75, respectively. Concentrations of sFLT-1 remained significantly higher in patients with cancelled cycles even after controlling for AMH levels. There were no differences in IGF-2 and IGFBP-1 levels between the two groups.
Conclusions
Measurement of circulating IGF-1 and sFLT-1 levels prior to initiation of an IVF cycle has the potential to identify women whose cycles have an increased likelihood to be subsequently cancelled.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimal ovarian response to controlled ovarian hyperstimulation is a key to a successful in vitro fertilization (IVF) cycle; failure to respond and recruit an adequate number of follicles [1] is the main reason for cancellation [2]. Response to gonadotropin stimulation is often difficult to predict and can vary substantially from person to person and frequently in the same individual between different cycles. Individual patient characteristics including age, menstrual cycle length, body mass index, and outcomes of previous IVF cycles have been used in the past to select a treatment protocol [3,4,5,6,7]. Additionally, in recent years basal follicle stimulating hormone (FSH) levels as well as anti-Müllerian hormone (AMH) levels and antral follicle count (AFC) have been suggested as possible predictive markers of ovarian response [8, 9].
The insulin-like growth factor (IGF) system has been implicated in folliculogenesis and oocyte maturation [10, 11]. In our previous study, serum IGF-1 levels were shown to increase during stimulation, in women with PCOS as well as in controls. In the same study, IGF-1 levels, from day 3 to day 28, were negatively correlated with the number of immature oocytes [12]. In addition, studies have indicated that higher levels of IGF-1 in follicular fluid are associated with a higher rate of successful cycles [13, 14]. Elevated serum IGF-1 levels have also been positively associated with cycles that resulted in conception [15]. Baseline IGF-1 serum levels have also been suggested to be a good predictive marker for a live birth following an IVF cycle [16]. The above indicates a potential role for IGF-1 in ovarian response and ultimately the IVF success rate.
Other members of the IGF system, IGF-2, and IGF binding protein 1 (IGFBP-1), have also been shown to influence follicular development and embryogenesis. Specifically, trophoblasts have been reported to express IGF-2 mRNA, with levels being highest in the first trimester. Studies have also indicated that IGF-2 interacts with IGFBP-1 in the decidua, modulating trophoblast proliferation [17,18,19,20]. Furthermore, baseline serum levels of IGFBP-1 were shown to be significantly higher in women who had a live birth following an IVF cycle, in comparison to those with unsuccessful outcomes [16]. IGFBPs were also found to be elevated in follicular fluid containing mature oocytes, in comparison to fluid with immature oocytes [21].
Finally, folliculogenesis has been shown to be predominantly influenced by angiogenesis; well-vascularized follicles have been reported to result in high-quality oocytes and embryos [23]. Well-vascularized follicles have been associated with increased follicular fluid EG-VEGF (endocrine gland-derived vascular endothelial growth factor) and IGF-1 levels [23]. In addition, LH-induced ovarian follicular angiogenesis has been shown to be mediated by changes in the expression of VEGF and soluble FMS-like tyrosine kinase-1 (sFLT1) [22]. Given the importance of perifollicular vascularization in the production of high-quality oocytes during the LH surge, it stands to reason that high levels of sFLT1, a strong antagonist of VEGF, would inhibit the ovarian response and thus predispose to cycle cancellation.
In the present study, we investigated whether baseline levels of IGF-1, IGF-2, IGFBP-1, and sFLT-1 would differ between women whose IVF cycles were cancelled and those with an adequate ovarian response reaching oocyte retrieval.
Materials and methods
In this prospective study, serum samples were obtained from 205 women between July 2015 and February 2017 prior to the initiation of an IVF cycle (day 2). Subsequently, cycle cancellation due to poor response or IVF cycle progression was established by chart review. Weill Medical College Institutional Review Board approval was obtained prior to initiation of this study (IRB number 1502015953), and all subjects gave informed written consent.
IVF protocol
Study patients underwent standard ovarian stimulation protocols [31]. Stimulation was initiated on day 2 of the IVF cycle using exogenous gonadotropins. Pituitary suppression was achieved with either GnRH-antagonist or GnRH-agonist. The response to stimulation was assessed with serial transvaginal ultrasounds and estradiol levels. Cancellation of cycles was determined by the attending physician, blinded to the study. Cancellation in general was when two or less follicles were present. The final oocyte maturation was triggered with human chorionic gonadotropin (hCG) and/or GnRH-agonist when a minimum of 2 follicles reached ≥17 mm diameter. Oocyte retrieval was performed by ultrasound-guided transvaginal follicular puncture.
Embryo transfer was performed 3 or 5 days after retrieval. In general, 1–2 embryos were transferred, but the number of embryos differed according to maternal age and outcomes of previous IVF cycles. On the day after oocyte harvesting, intramuscular progesterone was initiated. It was discontinued either 2 weeks later after negative pregnancy test or after documentation of fetal heart activity.
IGF and sFLT-1 system assays
All sera were stored at –80 °C until assayed. The sera were assayed for IGF-1, IGF-2, IGFBP-1, and sFLT-1 by commercially available ELISA kits (IGF-1, IGFBP-1, and sFLT-1 from R&D Systems, Minneapolis, MN, IGF-2 from Alpco, Salem, NH). Values were converted to pg/ml or ng/ml by reference to a standard curve that was run in parallel to each assay. The lower limits of sensitivity were 31.25 pg/mL for both IGF-1 and IGFBP-1, 0.45 ng/mL for IGF-2, and 13.3 pg/ml for sFLT-1. Intra-assay and inter-assay variability for each assay was <10%.
Statistical analysis
The association between the above markers and cancellation of IVF cycle was calculated by the Mann-Whitney test since the data were not normally distributed. Normality of distribution was tested by the Shapiro-Wilk test. Receiver operator curves (ROC) were constructed to evaluate the strength of the correlations between the biomarkers and cycle cancellation, as assessed from the area under the curve (AUC). Cutoff values were determined by calculation of the Youden index. Sensitivity and specificity were also determined. A separate ROC curve was constructed by logistic regression to determine whether a combination of assays would better predict the cancellation of an IVF cycle. A P value of <0.05 was considered statistically significant. SPSS Statistics (IBM Corp, Armonk, NY) was used for the analysis and figures.
Results
A total of 205 women were included in this study. Of these, 27 (13.2%) had a cycle cancellation due to poor ovarian response. Baseline demographic characteristics of study subjects are presented in Table 1. There were no significant differences between the two groups with regard to age and body mass index. However, women with a cancelled cycle had lower levels of anti-Müllerian hormone when compared to those with an ongoing cycle [0.4 (0-2) vs. 1.4 (0-12) ng/ml], as well as a lower antral follicle count [5 (3-7) vs. 9.5 (1-47)] (p< 0.003). The cause of infertility (male factor, immunologic, idiopathic, low ovarian reserve/advanced maternal age, anovulation/polycystic ovary, endometriosis, uterine anomaly, tubal ligation, hydrosalpinx, bilateral tubal occlusion, salpingectomy) did not differ between the two groups (p > 0.05).
Associations between serum levels of IGF-1, IGF-2, IGFBP-1, and sFLT-1 and cancellation or progression of the IVF cycle are shown in Table 2 and Figs. 1 and 2. Baseline median (range) serum concentrations of IGF-1 were increased in women with a cancelled cycle when compared to women with an ongoing cycle [21.5 (1–39) vs. 11.9 (1–35) ng/ml, p = 0.015] (Fig. 1). In addition, median levels of sFLT-1 were elevated in women whose cycles were subsequently cancelled [1.1 (0–30) vs. 0.18 (0–21) ng/ml, p < 0.001], (Fig. 2). Median levels of IGF-2 in baseline sera were not different between the two groups [430.3 (357–819) vs. 471.7 (0–763) ng/ml p = 0.16]. IGFBP-1 concentrations also did not differ between the two groups [17.3 (2–50) vs. 22.5 (0–64) ng/ml, p = 0.53].
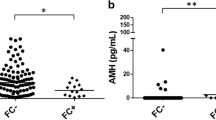
To investigate if sFLT-1 and IGF-1 correlated with the most commonly used predictor of ovarian response, AMH, we compared levels of the aforementioned markers between women with low, normal, or high levels of AMH. Women presenting with low levels of AMH (≤1 ng/ml, n = 65) had higher concentrations of sFLT-1 (median 4.2 (0–25.4) ng/ml) than did women with normal (>1-4 ng/ml, n = 68) or elevated (>4 ng/ml, n = 14) AMH level [1.3 (0-37) and 0 (0–15) ng/ml, respectively, p = 0.012] (Fig. 3). IGF-1 levels did not significantly differ between these groups.
In addition, differences in levels of sFLT-1 between patients with a cancelled cycle and patients who proceeded with their IVF cycle remained significant after controlling for levels of AMH. In patients with an AMH < 1 ng/ml, sFLT-1 levels were higher in the cancelled cycle group [1.1 (0–1.2) ng/ml, n = 9] (Fig. 4) when compared to the ongoing cycle group [0.3 (0–2.5) ng/ml, n = 48] (p = 0.033). This trend was consistent in patients with AMH levels of 1 ng/ml or higher (Fig. 5). In this subgroup of patients, women with a cancelled cycle also had elevated levels of sFLT-1 when compared to women with an ongoing cycle [0.8 (0.3–29) vs 0.14 (0–5.1) ng/ml, p = 0.001, n = 10 vs n = 64 ] These differences were significant despite the small number of patients in each group following this stratification and the removal of two significant outliers.
We examined the predictive value of each individual marker and combination of markers by calculating ROC curves. Cutoff points were also calculated for both individual markers, by calculating the Youden index. This analysis showed the area under the curve (AUC) for IGF-1 to be 0.672 (p = 0.017, Fig. 6) (cutoff point of 18 ng/ml sensitivity 68%, specificity 67%), while area under the curve for sFTL-1 was 0.758 (p = 0.015, Fig. 7) (cutoff point 0.39 ng/ml 94% sensitivity, specificity 58%), indicating that baseline elevated levels of IGF-1 and sFLT-1 might be good predictors of a cancelled cycle. In addition, the AUC for AMH was higher than both sFLT-1 and IGF-1, at 0.831 (p < 0.001) (Fig. 8). Furthermore, AUC for AFC was lower than that for sFLT-1 (0.750, p = 0.003), while AUC for AFC and sFLT-1 combined was 0.743 (p = 0.006) (Figs. 9 and 10). We then proceeded to calculate the ROC curve for AMH and sFLT-1 combined. The resulting AUC was higher than that provided by each marker singularly, at 0.923 (p < 0.001) (Fig. 11).
Discussion
In the present study, we demonstrated that elevated IGF-1 and sFLT-1 levels in sera obtained prior to initiation of an IVF cycle may be predictive of subsequent cycle cancellation due to a poor ovarian response.
IGF-1 is a 70 amino acid polypeptide involved in folliculogenesis and oocyte maturation. More specifically, it has been suggested that IGF-1 may be necessary for the gonadotropin-induced stimulation of granulosa cells, thus enhancing follicular development [24,25,26,27,28]. In addition, studies have shown that preovulatory follicular fluid levels of IGF-1 are lower than and correlate with serum levels [29, 30]. This led us to hypothesize that serum IGF-1, and by extension IGF-1 in the follicular fluid, may affect folliculogenesis and oocyte maturation. Our finding of increased IGF-1 serum levels in women who subsequently had a cancelled cycle could be reflective of an enhanced endocrine response to a poor ovarian reserve.
In addition, IGF-2 has been shown to contribute to trophoblast invasion, acting synergistically with IGFBP-1 [18]. In a previous study, baseline serum IGF-2 levels were shown to be decreased in women with a live birth, when compared to women with adverse outcomes following an IVF cycle. However, in the present study, IGF-2 levels did not differ between women with a cancelled cycle and an ongoing cycle, suggesting that IGF-2 may not be involved in follicular development.
Another member of the IGF system, IGFBP-1, has been reported to be involved in oocyte maturation. Higher levels of IGFBP-1 have been measured in fluid containing mature oocytes, when compared to concentrations in fluid containing immature oocytes [21]. Our results however did not show any significant difference in baseline IGFBP-1 serum concentration between the two groups.
Serum sFLT-1 levels were higher in women who subsequently had a cancelled cycle, when compared to women who succeeded in proceeding with their IVF cycle. This could possibly be explained by its effect on perifollicular angiogenesis [22]. As a soluble receptor for VEGF, sFLT-1 and its balance with VEGF has been shown to be a major player in ovarian follicular angiogenesis as well as the pathogenesis of ovarian hyperstimulation syndrome. Therefore, our results could point to an unfavorable sFLT-1 and VEGF balance at baseline, sub-optimal ovarian follicle angiogenesis, and thus a poor ovarian response to COH and cancellation of the IVF cycle.
As expected, low levels of AMH, a marker with a known correlation with ovarian reserve, had a high predictive value for cycle cancellation (AUC = 0.831). In addition, AFC was also a good predictor (AUC = 0.75). However, the combined ROC curve for sFLT-1 and AMH resulted in a higher AUC than all the markers tested for, indicating that the addition of sFLT-1 to the assays currently performed in clinical practice could improve our ability to effectively predict a possible cycle cancellation (Fig.11).
In addition, sFLT-1 levels remained significantly higher in women with a cancelled cycle when compared to women with an ongoing cycle, even after controlling for levels of AMH <1 ng/ml. Despite the small numbers in the resulting groups, this could point to a possibly significant value for sFLT-1 as an independent predictor of cycle cancellation.
The development of a simple serum assay that could be performed at baseline to predict which patients are at increased risk for cycle cancellation would be invaluable. This would provide clinicians with the opportunity to identify such patients in advance and thus take appropriate measures to individualize and optimize their treatment. The significant differences in serum levels of IGF-1 and sFLT-1 at baseline between the two study groups could be reflective of an idiopathic predilection to poor ovarian response due to imbalance of these factors.
One of the strengths of this study was its prospective design; however the small number of patients that subsequently had a cancelled cycle (n = 27) is a major limitation. Further studies with an increased patient population should be carried out to determine more specific and effective cutoff values and possibly shed light into the underlying mechanisms.
Conclusions
In conclusion, measurement of serum levels of IGF-1 and sFLT-1 prior to initiation of an IVF cycle could prospectively predict subsequent IVF cycle cancellation.
Capsule
Baseline serum levels of IGF-1 and sFLT-1 were elevated in women that subsequently had a cycle cancellation, when compared to women who proceeded with their IVF cycle.
References
Badawy A, Wageah A, El Gharib M, Osman EE. Prediction and diagnosis of poor ovarian response: the dilemma. J Reprod Infertil. 2011;12(4):241–8.
Garcia JE, Jones GS, Acosta AA, Wright G. Human menopausal gonadotropin/human chorionic gonadotropin follicular maturation for oocyte aspiration: phase I, 1981. Fertil Steril. 1983;39:167–73.
Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu HC, et al. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298–307.
Sharif K, Elgendy M, Lashen H, Afnan M. Age and basal follicle stimulating hormone as predictors of in vitro fertilisation outcome. Br J Obstet Gynaecol. 1998;105:107–12.
Chuang CC, Chen CD, Chao KH, Chen SU, Ho HN, Yang YS. Age is a better predictor of pregnancy potential than basal follicle-stimulating hormone levels in women undergoing in vitro fertilization. Fertil Steril. 2003;79:63–8.
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718.
Sills ES, Alper MM, Walsh AP. Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol. 2009;146(1):30–6. https://doi.org/10.1016/j.ejogrb.2009.05.008.
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;1:124–40.
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;6:685–718.
Oosterhuis GJE, Vermes I, Lambalk CB, Michegelsen HWB, Shoemaker J. Insulin-like growth (IGF)-I and IGF binding protein-3 concentration in fluid from human stimulated follicles. Hum Reprod. 1998;13:285–9.
Dor J, Ben-Shlomo I, Lunenfeld B. Insulin-like growth factor-I (IGF-I) may not be essential for ovarian follicular development: evidence from IGF-I deficiency. J Clin Endocrinol Metab. 1992;74:539–42.
Schoyer KD, Liu HC, Witkin S, Rosenwacks Z, Spandorfer SD. Serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3) in IVF patients with polycystic ovary syndrome: correlations with outcome. Fertil Steril. 2007;88:139–44.
Choi YS, Kim SH, Ki SY, et al. Efficacy of ER-alpha polymorphisms and the intrafollicular IGF system for predicting pregnancy in IVF-ET patients. Gynecol Obstet Invest. 2009;67:73–80.
Mendoza C, Ruiz-Requena E, Ortega E, Cremades N, Martinez F, Bernabeu R, et al. Follicular fluid markers of oocyte developmental potential. Hum Reprod. 2002;17:1017–22.
Dorn C, Reinsberg J, Kupka M, van der Ven H, Schild RL. Leptin, VEGF, IGF-1 and IGFBP-3 concentrations in serum and follicular fluid of women undergoing in vitro fertilization. Arch Gynecol Obstet. 2003;268:187–93.
Ramer I, Kanninen TT, Sisti G, Witkin SS, Spandorfer SD. Association of in vitro fertilization outcome with circulating insulin-like growth factor components prior to cycle initiation. Am J Obstet Gynecol. 2015;213:356.e1–6.
Han VKM, Bassett N, Walton J, Challis JRG. The expression of insulin-like growth factor (IGF) and IGF binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab. 1996;81:2680–93.
Pringle KG, Roberts CT. New light on early post-implantation pregnancy in the mouse: roles for insulin-like growth factor-II (IGF II)? Placenta. 2006;28:286–97.
Irving JA, Lala PK. Cell surface integrins on human trophoblast cell migration: regulation by TGF-beta, IGF-II, and IGFBP-1. Exp Cell Res. 1995;217:419–27.
Irwin JC, Giudice LC. Insulin-like growth factor binding protein-1 binds to placental cytotrophoblast a5b1 integrin and inhibits cytotrophoblast invasion into decidualized endometrial stromal cultures. Growth Horm IGF Res. 1998;8:21–31.
Kawano Y, Narahara H, Matsui N, Nasu K, Miyamura K, Miyakawa I. Insulin-like growth factor-binding protein-1 in human follicular fluid: a marker of oocyte maturation. Gynecol Obstet Invest. 1997;44:145–8.
Gutman G, Barak V, Maslovitz S, Amit A, Lessing JB, Geva E. Regulation of vascular endothelial factor-A and its soluble receptor sFLT-1 by luteinizing hormone in vivo: Implication for ovarian follicle angiogenesis. Fertil Steril. 2008;89:922–6.
Vural F, Vural B, Doger E, Cakiroglu Y, Cekmen M. Perifollicular blood flow and its relationship with endometrial vascularity, follicular fluid EG-VEGF, IGF-1, and inhibin-a levels and IVF outcomes. J Assist Reprod Genet. 2016;33:1355–62.
Davoren JB, Kasson BG, Li CH, Hsueh AJW. Specific insulin-like growth factor I– and II–binding sites on rat granulosa cells: relation to IGF action. Endocrinology. 1986;119:2155–62.
Adashi EY. The ovarian follicle: life cycle of a pelvic clock. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive endocrinology, surgery, and technology, vol. 1. Philadelphia: Lippincott-Raven; 1996. p. 211–34.
Davoren JB, Hsueh AJW, Li CH. Somatomedin C augments FSH-induced differentiation of cultured rat granulose cells. Am J Physiol. 1985;249:E26–33.
Adashi EY, Resnick CE, D’Ercole AJ, Svoboda ME, Van Wyk JJ. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev. 1985;6:400–20.
Chun SY, Billig H, Tilly JL, Furuta I, Tsafriri A, Hsue AJ. Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor I. Endocrinology. 1994;135:1845–53.
Eden JA, Jones J, Carter GD, Alaghband-Zadeh J. A comparison of follicular fluid levels of insulin-like growth factor-1 in normal dominant and cohort follicles, polycystic and multicystic ovaries. Clin Endocrinol. 1988;29:327–36.
Rabinovici J, Dandekar P, Angle MJ, Rosenthal S, Martin MC. Insulin like growth factor I (IGF-I) levels in follicular fluid from human revelatory follicles: correlation with serum IGF-I levels. Fertil Steril. 1990;54:428–33.
Huang JY, Rosenwaks Z. Assisted reproductive techniques. Methods Mol Biol. 2014;1154:171–231.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (include name of committee + reference number) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent
“Informed consent was obtained from all individual participants included in the study.”
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dimitrios Nasioudis and Evelyn Minis are first co-authors.
Rights and permissions
About this article
Cite this article
Nasioudis, D., Minis, E., Irani, M. et al. Insulin-like growth factor-1 and soluble FMS-like tyrosine kinase-1 prospectively predict cancelled IVF cycles. J Assist Reprod Genet 36, 2485–2491 (2019). https://doi.org/10.1007/s10815-019-01618-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01618-3