Abstract
Purpose
This study was to evaluate if spent culture media (SCM) of embryos could be used as a non-invasive tool to achieve aneuploidy screening. Ploidy calls, as well as concordance rates between PGT-A results from trophectoderm (TE) and SCM, were compared. Clinical outcomes of single euploid transfers were also evaluated.
Methods
The study was conducted from March 2017 to June 2018 in a university-based ART center. SCM of day 3 to the day(s) of TE biopsy of all biopsied blastocysts were collected for testing. PGT-A results of SCM were compared with the standard results of TE, with clinical relevance and outcomes examined.
Results
NiPGT-A using SCM gave a sensitivity of 81.6%, specificity of 48.3%, positive predictive value of 82.6%, and negative predictive value of 46.7% in ploidy calling. The concordance rates for autosomes and sex determination were 62.1% and 82.4%, respectively. There were 14 single embryo transfer cycles of euploids as determined by TE biopsy. Clinical outcomes not only confirmed 3 false positive results from SCM but also reflected the true ploidy status of the transferred embryo in one case. If ploidy calls were dichotomized without mosaic embryos, the sensitivity and NPV would increase to 91.0% and 66.7% (p = 0.60 and p = 0.25), respectively.
Conclusions
Cell-free DNA found in SCM could provide ploidy information of an embryo as in PGT-A from its TE. Given its potential to reflect the comprehensive chromosomal profile of the whole embryo, more research based on clinical outcomes is required to determine if SCM could be a reliable selection tool in PGT-A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vitro fertilization (IVF) has helped many couples to complete their families. Despite over three decades of clinical application, singleton live birth rate still varies from around 20% to slightly above 40%, depending on different age groups and the cycle types being scrutinized [1,2,3]. Delay in child-bearing age may be one of the culprits, but reliance on morphological assessment alone in embryo selection is likely to be another. Although embryo morphology is an easy and non-invasive assessment of developmental potential, morphologically normal embryos can carry chromosomal abnormalities [4, 5]. Aneuploidy, or an abnormal number of chromosomes, occurs when chromosomes do not properly segregate during cell division. The chance of aneuploidy increases with advanced maternal age [6], and whether ovarian stimulation in IVF also contributes to aneuploidy is still debatable [7,8,9,10]. In recent years, preimplantation genetic testing for aneuploidies (PGT-A) has integrated into many IVF programs to improve success rates by identifying suitable embryo(s) for transfer based on its ploidy status. Several published studies demonstrated improved implantation and pregnancy rates when PGT-A was used [11,12,13]. However, there remains much unknown for PGT-A to be applied as a universal screening test for all IVF patients. One of the main concerns is that the process of PGT-A involves an embryo biopsy procedure, which is invasive and may have potential detrimental effect on the embryo developmental potential [14]. While trophectoderm (TE) biopsy alleviates this concern, it is possible that the genetic constitution of TE may not represent that of the inner cell mass (ICM) [15, 16], although there were also evidence showing high concordance between TE and ICM [17, 18]. Therefore, a non-invasive method that can evaluate the ploidy status of an embryo as a whole would be ideal in embryo selection.
The finding of genomic DNA in human blastocoele fluid by Palini et al. [19] has provided the ground work for non-invasive PGT-A (NiPGT-A). In their study, they demonstrated the successful amplification of TBC1D3 and TSPY1, which are multi-copy genes on chromosome 17 and the Y chromosome, respectively. They proposed that blastocoele fluid could be obtained and used in PGT for some monogenic diseases or for sex determination. Since then, a few studies used blastocentesis to obtain blastocoele fluid for NiPGT-A and compared with TE biopsies [20,21,22]. However, blastocentesis is still a minimally invasive procedure during which a needle for intracytoplasmic sperm injection (ICSI) needs to puncture into the blastocoelic cavity to aspirate its fluid content. Technical variability might lead to varying degree of concordance in ploidy status even when cell-free DNA is present. The procedure may also take up any loose cells or dislodged cellular material trapped in the cavity, leading to discordant results.
On the other hand, sampling spent culture media (SCM) for cell-free DNA seems to be more promising due to its non-invasive nature. Indeed, using polymerase chain reaction (PCR) methodology, embryonic DNA can be detected in SCM [23] and one early study investigated the use of SCM in diagnosing alpha-thalassemia-SEA [24]. A few pilot studies [25,26,27,28] also showed feasibility of NiPGT-A using SCM and demonstrated ploidy consistency between TE biopsy and the corresponding SCM of the same embryo. However, the actual sample size dedicated to ploidy comparison was small, and additional PCR cycles were sometimes included in the whole genome amplification (WGA) process for SCM but not for TE samples. These differences might introduce bias in ploidy determination and comparison, challenging the result validity. Here, we reported our prospective study in which 168 SCM samples were collected from 48 PGT cycles of 37 patients. To the best of our knowledge, this study constituted one of the largest data set on the subject. Our objectives were as follows: (1) to conduct the study with close adherence to standard routines in the IVF and PGT laboratories, (2) to evaluate non-invasive PGT-A (NiPGT-A) using SCM in a clinical setting by comparing ploidy status and chromosomal concordance rates with the current PGT-A using TE, and (3) to correlate reproductive outcomes of single euploid transfers as determined by TE with NiPGT-A results.
Materials and methods
Study design and patient population
We conducted this prospective study at the Assisted Reproductive Technology Unit of the Chinese University of Hong Kong from March 2017 to June 2018. All patients undergoing PGT for monogenic diseases (PGT-M), structural rearrangement (PGT-SR), and/or aneuploidies (PGT-A) were recruited. In cases where PGT-M or PGT-SR were indicated, PGT-A would be performed for those embryos unaffected by the disease or being carriers in a recessive condition. PGT-A were not performed for disease-affected embryos. Patients with no blastocyst for biopsy were excluded. The study was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee, CREC Ref. No. 2015.472. A research licence (no. R3004) was also obtained from the Council on Human Reproductive Technology of Hong Kong.
Ovarian stimulation and oocyte retrieval
Ovarian stimulation was achieved as previously described [29]. Briefly, a long down-regulation protocol with buserelin nasal spray (Suprecur, Hoechst, Germany) or a short protocol with a gonadotropin releasing hormone (GnRH) antagonist (Cetrotide, Merck Serono, Germany) was used. Highly purified human menopausal gonadotropin (Menopur, Ferring, USA) or recombinant follicle-stimulating hormone (rFSH, Gonal-F, Serono, Switzerland; or Puregon, MSD, USA) at doses ranging from 150 to 450 IU were given daily. When 3 or more follicles ≥ 18 mm in diameter were seen, 5,000 IU of hCG (Pregnyl, MSD, USA) or 0.2 mg triptorelin (Decapeptyl, Ferring, Sweden) was administered as ovulation trigger, followed by transvaginal oocyte retrieval 36 h later and subsequent ICSI.
Fertilization and embryo culture
Cumulus cells were removed from oocytes with hyaluronidase (Vitrolife, Goteborg, Sweden). After denudation, all mature oocytes were given at least an hour in culture. Insemination by ICSI took place 4–6 hours post-retrieval. Fertilized oocytes were cultured in G-1 medium (Vitrolife, Goteborg, Sweden) supplemented with 10% serum substitute supplement, SSS (Irvine Scientific, Santa Ana, USA). On the morning of day 3 development, each embryo was cleaned and rinsed using a 170-μm pipette (Flexipets, Cook Medical, Bloomington, USA). A hole of approximately 10 μm in diameter was drilled on the zona pellucida by using a non-contact laser (Saturn 5 Active Laser System, Research Instrument, UK). After assisted hatching, each embryo was individually cultured in G-2 medium (Vitrolife, Goteborg, Sweden) supplemented with 10% SSS until blastocyst stage. All embryos in this study were cultured using sequential culture media in 30-μl microdrops under oil, with incubation conditions of 37.0 °C and 6% CO2/5% O2 balanced in N2.
Trophectoderm biopsy
Blastocyst grading was performed using the criteria established by Gardner and Schoolcraft [30]. Blastocysts were categorized into good, fair, or poor quality based on the simplified SART embryo scoring system [31]. The grade is good when the ICM/TE is AA or AB; the grade is fair when the ICM/TE is BA, BB, or BC; and the grade is poor when the ICM/TE is CB or CC. TE biopsy would be performed when an embryo attained at least one grade B, or better, for either its ICM or TE on day 5 or day 6 of development. Blastocyst(s) of CC grade were biopsied when there was no good-quality blastocyst within the same cohort. In a typical biopsy procedure, 5–8 cells were removed from the TE by laser. The cell sample was rinsed and tubed for PGT while the biopsied embryo was cryopreserved by vitrification (Vit Kit–Freeze, Irvine Scientific, Santa Ana, USA).
Culture medium collection
Culture medium in which an embryo had grown from day 3 until the day of biopsy was collected. After a blastocyst was removed from its culture dish for TE biopsy as described above, 20 μl of its spent culture medium was collected into RNase- and DNase-free PCR tubes, using sterile dual-filter pipette tips (Eppendorf, New York, USA). Each pipette tip was discarded after collecting one SCM sample to avoid cross contamination. Care was taken to avoid merging of culture microdrops with each other. Culture medium of a blank microdrop, which had not been exposed to any embryo, was also collected from the same dish as negative control. All samples were then stored at − 20 °C until ready for analyses, with storage period ranged from 2 days to up to 3 months before whole genome amplification (WGA).
PGT procedures
Trophectoderm (TE) biopsies underwent WGA using the PicoPLEX WGA Kit (Rubicon Genomics, Ann Arbor, USA), following manufacturer’s instructions. WGA products of TE samples were then subjected to standard PGT-A procedures as in clinical routine. Microarray comparative genomic hybridization (aCGH, Agilent Technologies) had been clinically applied in our unit since year 2015 and switched to semi-conductor sequencing platform, Ion Proton (Life Technologies, Carlsbad, CA, USA), beginning from January 2018. For cycles where PGT-M or PGT-SR was indicated, Sanger sequencing and linkage analysis were concomitantly performed for validation.
For NiPGT-A, each SCM sample was amplified by SurePLEX (Illumina Inc., Santa Clara, CA, USA) using a single aliquot of 3 μL as the input volume, following SurePLEX protocol without modification. WGA success was evaluated by loading 5 μL of the amplified products on a 2% agarose gel. Samples that failed amplification did not proceed to library construction. Library of the WGA product of SCM was then constructed by VeriSeq PGS Library Prep Kit according to the manufacturer’s protocol. The 6 base-pair index sequence was added for the downstream dual-indexed single-end 36 base-pair sequencing. Each library was quantified for quality control before pooling by Qubit High Sensitivity dsDNA kit (Life Technologies, Carlsbad, CA, USA) for optimal molar concentration for sequencing. Sequencing was performed by MiSeq Reagent Kit v3-PGS on MiSeq System (Illumina Inc., Santa Clara, CA, USA).
Before variant calling, fastQ files were quality controlled by both FastQC (Babraham bioinformatics) and lane metrics on BaseSpace (Illumina Inc., Santa Clara, CA, USA). Sequencing data were aligned by BaseSpace and copy number variations (CNV) were called by BlueFuse Multi software (Illumina, USA).
PGT result interpretation
For clinical reporting, standard PGT-A results as obtained from TE were used. The detection limit for segmental aneuploidy or CNV was ≥ 10 Mb. When segmental or numerical aneuploidies were detected, the aberrations were always reported and the embryo would not be recommended for transfer. In the case of mosaicism, a mosaic level of ≥ 30% would be reported; this cut-off was established by our in-house validation using cell line mixtures at 7 artificial mosaic ratios: 0%, 16.7%, 33.3%, 50%, 66.7%, 83.3%, and 100%. Cell line mixture of 33.3% provided reproducible results with good calling rates; and therefore, we set the detection limit of mosaicism at 30%. Embryos carrying mosaic aberration(s) would not be recommended for transfer.
For SCM results, due to the small fragment size and very minute DNA concentration, the window size for analysis was larger because sequencing data for SCM were relatively noisier than for TE; and therefore, the detection limit was down to sub-chromosomal level. The same cut-off of 30% was used for mosaicism. When comparing concordance between SCM and TE, segmental CNV was taken into consideration but not to the exact coordinates of the variation. Similarly, mosaic chromosome was compared in terms of its presence or absence but not its percentage level.
For the purpose of comparing ploidy status between TE and SCM, a negative result referred to a euploid result or a result with < 30% mosaicism; a positive result referred to an aneuploid or ≥ 30% mosaic result, which could include a whole chromosome gain or loss and segmental CNVs.
When assessing concordance of SCM with TE results at the chromosomal level, autosomal and sex chromosomes were independently compared. In autosomal concordance, a result from SCM must exactly match that from TE to be in complete concordance, i.e., implying a true positive or a true negative result (Fig. 1). In partial concordance, SCM identified a gain on a chromosome or chromosomal segment while TE identified a loss of the same chromosome or chromosomal segment, or vice versa; also included as partial concordance were SCM that identified some but not all the chromosomal variations as identified by TE. In autosomal discordance, SCM identified a chromosomal variation when there was none (false positive), or SCM was not able to identify any chromosomal variation when TE identified at least one (false negative); also, SCM could identify CNV(s) involving totally different chromosomes when compared with those identified by TE.
For sex chromosomes X and Y, results between SCM and TE could only be concordant or discordant. If SCM showed an XO result while TE showed an XX result, this would be classified as discordant but not partial concordant.
Detection of maternal contamination by QF-PCR
Quantitative fluorescence-polymerase chain reaction (QF-PCR) was performed on SCM samples that were suspected to contain maternal cell contamination. The WGA products of SCM and of the corresponding TE biopsy, as well as the corresponding parental genomic DNA, were tested with five short tandem repeat (STR) markers on chromosomes 13 (D13S796), 18 (D18S535), 21 (D21S11, D21S1435), and X (DX981). QF-PCR protocol was as previously described by our group [32]. The volume of input DNA for SCM was adjusted to 2 μl.
Euploid embryo transfer
Euploid embryo was thawed (Vit Kit–Thaw, Irvine Scientific, Santa Ana, USA) and the transfer was performed by synchronization with a day 5 endometrium of a natural cycle or hormonal replacement treatment (HRT) cycle. All transfers were performed under ultrasound guidance. Pregnancy test was performed 9–12 days after embryo transfer, and was considered positive when serum β-hCG was ≥ 20 IU/L. Luteal phase support was omitted in natural cycles while progesterone in the form of Endometrin (Ferring, USA) would continue to be administered until 8–10 weeks of gestation in HRT cycles.
Outcome measures
Primary outcome measures were concordance rates for aneuploidy or segmental CNVs and accuracy of ploidy calls. Secondary outcome measures included the reproductive outcomes after euploid embryo transfer. For reproductive outcome measurement, miscarriage is defined as a loss of an intrauterine pregnancy detected clinically or by ultrasound, and less than 20 weeks of gestation; ongoing pregnancy is a viable pregnancy with fetal cardiac activity and of ≥ 12 weeks of gestation; live birth is defined as live delivery after ≥ 24 weeks of gestation.
Statistical analysis
Concordance rates between TE and SCM were calculated for aneuploidy and sex determination. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for euploid versus aneuploid calls. Chi-square test was used to assess categorical parameters. All statistical analyses were performed using IBM SPSS Statistics Version 22. A p value of < 0.05 was considered statistically significant.
Results
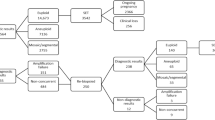
A total of 168 SCM samples were collected from 48 PGT cycles of 37 patients during the study period. They had a mean age of 36.8 ± 3.9 years of age (range 28–44 years), and their PGT indications were shown in Table 1. Overall, there were 49 euploids (29.2%) and 119 aneuploids (70.8%) based on their PGT-A results from TE biopsies. The blastocysts of day 5 and day 6 did not differ in aneuploidy rates: 68.9% (62/90) vs. 73.1% (57/78), p = 0.671. SCM samples of these blastocysts were collected on day 5 or day 6, corresponding to the day when TE biopsies were performed.
After whole genome amplification, 150 of the 168 SCM samples (89.3%) were successfully amplified, followed by library preparation and next-generation sequencing. There were 116 samples (77.3%) with over 250,000 reads after filtering, allowing interpretable results. The other 34 samples had low reads and noisy results, likely due to low DNA amount or fragmented DNA. The mean DNA concentration was significantly higher in samples with interpretable results (20.0 ng/μl, range 2.7–59.6 ng/μl) than in samples with failed amplification or low reads (11.3 ng/μl, range 2.3–42.4 ng/μl), p = 0.001. Moreover, SCM collected on day 6 (84.6%, 66/78) had a significantly higher chance of producing interpretable results than those collected on day 5 (55.6%, 50/90), p < 0.0001. The mean DNA concentration from day 5 SCM (15.5 ng/μl, range 2.3–45.2 ng/μl) was significantly lower than that from day 6 SCM (20.6 ng/μl, range 2.7–59.6 ng/μl), p = 0.02. Blank media that were collected from the same dish did not show amplification after WGA, implying the absence or an undetectable level of background DNA pre-existing in the culture media.
The overall concordance between TE and SCM samples for autosomal chromosomes was 62.1% (72/116). There were 27 SCM samples (23.3%) in complete concordance with TE samples, i.e., showing euploid results or aneuploid results for the same gain or loss of the same autosome(s). There were 45 SCM samples (38.8%) showing partial concordance only. In this subgroup, both SCM and TE gave aneuploid calls, but SCM had fewer or additional chromosomal gains or losses, in among which 12 samples (26.7%) showed reciprocal gain or loss as compared with TE (Fig. 1). There were 44 discordant cases (37.9%) between SCM and TE. Sixteen were false negative results, where SCM indicated euploidy while TE indicated aneuploidy; 15 were false positive results, where SCM indicated aneuploidy while TE indicated euploidy; and 13 results in which both SCM and TE called for aneuploidy but based on completely different chromosome(s) (Fig. 1).
Pertaining to sex chromosomes alone, due to the lack of such information on the clinical PGT report from one of the couples with 8 embryos, only 108 samples (93.1%) could be compared between SCM and TE. SCM correctly identified 43 embryos with XX, 42 embryos with XY, and 4 embryos with XO, equivalent to 82.4% (89/108) concordance. Fifteen of the 19 discordant samples could not detect the Y chromosome present as shown in the TE biopsies, likely attributable to maternal cell contamination (MCC). Four discordant samples were due to copy number difference in the X chromosome. In the 15 sex-discordant samples, i.e., TE biopsies showing XY while SCM showing XX, we used five short tandem repeat (STR) markers to check for possible MCC. Although some markers were non-informative probably due to the very low amount of embryonic DNA and also small DNA fragment size, 3 of the samples indeed showed possible MCC (Supplementary Table 1).
Embryo morphology was also taken into consideration for concordance rates between TE and SCM. In the 116 SCM samples collected, there were 63 corresponding blastocysts (54.3%) with good/fair quality and 53 (45.7%) with poor quality. The concordance rates in blastocysts of good/fair quality versus those of poor quality were 58.7% vs. 66.0% for autosomes and 77.6% vs. 88.0% for sex chromosomes, respectively. The difference in concordance rates was not statistically significant.
Overall, the use of SCM from blastocysts of day 5 and day 6 in aneuploidy detection gave a sensitivity of 81.6%, specificity of 48.3%, positive predictive value (PPV) of 82.6%, and a negative predictive value (NPV) of 46.7% (Table 2). The same was compared between SCM collected on day 5 versus day 6, with no significant statistical difference found in sensitivity, specificity, PPV, and NPV when the embryos needed an extra day before reaching blastocyst stage.
There were 29 euploids (25.0%) based on TE results among the 116 embryos that had PGT-A results from both TE and SCM samples. Fifteen of them were thawed for 14 single euploid transfer cycles, resulting in 3 live births, 3 ongoing pregnancies, and 5 miscarriages (Table 3). Figure 2 showed the original aCGH profiles of TE biopsies for 4 of the transferred euploids compared with their NiPGT-A profiles, with Fig. 2a–d being euploids 2, 4, 9, and 15 of Table 3, respectively. The implantation rate (78.6%) and live birth/ongoing pregnancy rate (42.9%) were comparable with those reported in literature. If the PGT-A results from TE for these same 14 embryos were blinded and transfer decision were to be based on NiPGT-A results from SCM, there would only be 7 euploid transfers, with an implantation rate of 71.4% (5/7) and a live birth/ongoing pregnancy rate of 42.9% (3/7) (Table 3). In spite of the small sample size, the clinical outcomes of this hypothetical scenario were compatible with those of standard PGT-A.
Original aCGH profiles of TE biopsies and NiPGT-A profiles of SCM for embryos in Table 3; a euploid no. 2, b euploid no. 4, c euploid no. 9, and d euploid no. 15
Discussion
Our study reported a large series of non-invasive PGT-A using SCM, utilizing a common and well-established NGS protocol without making modification to the amplification cycles in WGA. As compared with PGT-A using TE, our NiPGT-A results showed a concordance of 73.3% (85/116) and 82.4% (89/108) on autosomal and sex chromosomes, respectively. As a screening test for aneuploidy, NiPGT using SCM demonstrated a sensitivity of 81.6%, specificity of 48.3%, PPV of 82.6%, and NPV of 46.7%. Although this high concordance with PGT-A results was undermined by the high non-informative and false positive rates, our data suggested that NiPGT-A using SCM might be improved to achieve similar efficacy as routine PGT-A in identifying euploid embryos and potentially enhancing pregnancy outcomes. We contributed the largest sample size in among recent studies that utilized NGS methodologies on SCM, and our results gave high sensitivity and PPV (Table 4). Although there should be room for improvement, PGT-A results from TE biopsy but not the actual pregnancy outcomes were used as reference to evaluate performance; and therefore, we also presented our clinical outcomes with PGT-A results from TE and NiPGT-A results from SCM side-by-side (Table 3). Although this preliminary comparison on clinical outcomes was small, it is intriguing that a euploid based on TE is not always equal to a live birth. For example, there was one miscarriage (euploid no. 12, Table 3) with whom the product-of-conception was available and tested to have mosaic trisomy 21 while its corresponding SCM showed a mosaic result involving trisomy 12 and monosomy 21. The PGT-A result inconsistencies between TE and SCM certainly deserve a re-evaluation of the predictive value of the different sampling methods. We believe healthy live birth should be a more appropriate endpoint to use.
Earlier studies of PGT-A involving single centers demonstrated improved ongoing pregnancy rates, reduced miscarriage rate, and a shorter time-to-pregnancy [11, 12, 33]; however, a recent multi-center randomized controlled trial (RCT) showed usefulness only in patients 35 years of age or above [34]. Since the same NGS platform was used for PGT-A in this RCT, the subtle differences in biopsy techniques, embryo handling and culture conditions, cryopreservation methods and cryosurvival, and patient social and/or fertility background across centers could likely cause the variation in outcomes. Interestingly, our data from the small number of transferred euploids (Table 3) appeared to have a high incidence of miscarriage (5/13 or 38.5%), which could possibly be related to the sample size, the biopsy process, and the limitation of sampling TE but not the ICM, and also patients’ etiologies, such as recurrent miscarriage, that require PGT. Thus, a sampling method that is consistent and not technically demanding will be ideal to truly realize the potential benefit from PGT-A. Collecting SCM for PGT-A appears to be quick and easy without having to inflict any trauma on the embryos, if such methodology can be proven to be sound and robust.
Although two other recent studies [27, 28] also reported on the successful amplification of cfDNA from SCM, they employed additional amplification cycles in order to maximize yield for subsequent sequencing. As the amount of DNA present in SCM was very low, variation to a validated WGA protocol might introduce bias to the process and affect downstream interpretation of results [35]. Since we had 18 samples that failed amplification and 34 samples that had low reads, it remained unclear whether increasing the number of amplification cycles could enhance the total quantity of DNA for sequencing; on the contrary, the increased cycles of amplification might lead to higher error rate, leading to a decrease in the number of mapped reads. Perhaps NGS results derived from the recommended number and additional number of amplification cycles on the same SCM aliquots should be compared for potential improvement in accuracy and noise reduction.
Since it is our goal to use SCM as a non-invasive embryo selection tool, we did not modify our clinical routine so to keep all steps practically applicable. We did assisted hatching on day 3 of embryo development for all our PGT cycles, and we collected culture media on day 5 or day 6 when TE biopsies were performed. In the recent publication by Ho et al. [28], the group confirmed that assisted hatching on either day 3 or day 5 did not affect the concentration of cfDNA found in SCM, and we had similar findings through a separate series of SCM collected in ICSI cycles without PGT-A (unpublished data). In our ICSI series, embryo handling and culture conditions were the same as PGT-A cases except that we did not perform assisted hatching on day 3. The SCM collected from blastocysts without assisted hatching could still produce interpretable NGS results. This would imply that NiPGT-A, if clinically applied, would not require additional intervention to current laboratory routine other than having to collect SCM from usable blastocysts. On the other hand, published data has not yet addressed if SCM could be collected from embryos on day 6. In such case, an embryo would have been in the same media from day 3 to day 6, or a culture period of approximately 72 h. We found that SCM from embryos that were only at morula stage on day 5 but formed blastocysts on day 6 had a better chance of producing PGT-A results and gave similar concordance rate as SCM from day 5 blastocysts. This might be directly related to the longer time during which the embryo could release cfDNA.
Our study also took a very strict approach to assess autosomal concordance rates. Only if the PGT-A results of SCM exactly matched with those of TE were classified as complete concordance. SCM results that revealed more or fewer chromosomal involvement, or those with reciprocal gains and losses, were classified as partial concordance. When both SCM and TE called aneuploidy but for different chromosome(s), such results were classified as discordance. On the basis of using PGT-A results of TE as the standard for comparison, the above three scenarios still called for the correct ploidy status of the embryo tested. As such, our use of SCM was capable of achieving a correct ploidy call, or general concordance with TE results, of 73.3% (85/116). This finding was similar to the 65.4% reported by an Australian group, when they collected the SCM of embryos incubated from day 3 to day 5 [36]. This group further proposed that SCM could be collected from a narrower growth period of day 4 to day 5 to improve ploidy concordance, because moving the embryos to fresh media on day 4 and collecting the media on day 5 might reduce maternal contamination from cumulus cells or polar body DNA and increase embryonic DNA in the SCM. However, refreshing culture medium on day 4 could be a major change in routine practice, whether the embryology laboratories use sequential or single-step culture media. The extra embryo handling outside of the incubator may impose unknown effect on developmental potential, and one must balance the risks over benefits in a clinical setting.
One of the limitations of our study is that in our cohort of 116 embryos, there were only 29 euploids (25%), implying that one false positive result from SCM would have a higher impact on the overall specificity than one false negative result on sensitivity. The proportion of euploids and aneuploids was discrepant, which might have led to the relatively low specificity (48.3%) and NPV (46.7%) from our SCM. We further examined the proportion of euploids and aneuploids in the 52 embryos with non-informative SCM. We found 20 euploids (38.5%) and 32 aneuploids (61.5%) that did not have enough cfDNA in their SCM, which was not statistically different to those with informative SCM. This comparison suggested that the amount of DNA released by an embryo into its culture environment was probably unaffected by its ploidy status, in agreement with the findings by Vera-Rodriguez et al. [27].
Another limitation we encountered was contamination of maternal origin, which has been the major hurdle for most studies trying to find non-invasive means for embryo selection [26,27,28, 36]. ICSI was used to fertilize the oocytes in our study to eliminate the chance of paternal contamination from sperm. To avoid or minimize the chance of MCC, we included an additional cleaning and rinsing step on day 3 before each embryo was transferred into its individual culture microdrop. When the embryo became a blastocyst on day 5 or day 6, each of the SCM was collected using a new sterile pipette tip. Blank media from the same dish under the same culture condition were also collected as negative control, and their lack of DNA amplification was strong evidence that contamination did not originate from the environment or the manufacturing of the media. Despite all measures, some cumulus cells might still be adhering to the zona pellucida (ZP) after oocyte denudation, and slow degeneration of the two polar bodies trapped inside the ZP might release maternal DNA [37]. As pointed out in the delicate experimental design of Vera-Rodriguez et al. [27], the maternal DNA fraction found in SCM could be as high as 86% when NGS did not even show a contaminated pattern. Using single nucleotide polymorphism (SNP) analyses, they found that the median fraction of embryonic DNA in SCM was only 8%. This finding may imply that when embryonic DNA could be present up to a certain fraction, above 14% for example, the effect of maternal contamination on the accuracy of the sequencing result might be negligible. If SCM were to become a useful non-invasive tool, there needs to be an effective way to either diminish/identify the maternal DNA or to enhance signals from cfDNA of the embryo. Indeed, 7 of our SCM were shown to be full maternal contamination; in other words, these SCM samples had female-euploid results while the TE of the corresponding embryos indicated male euploids. Should the noise from maternal contamination be resolved in the near future, our same set of samples would have much improved sensitivity and NPV (88.8% and 60.9%, respectively; Table 5).
Nevertheless, the increase in sensitivity of NGS technology not only gives better molecular resolution but also adds complexity to result interpretation. Mosaicism creates a new category of embryos that carry some potential of making healthy babies but with a lower implantation potential and a higher chance of miscarriage than euploid embryos [38,39,40]. Since mosaicism is common in early human embryo development, the sampling of only a few cells from the TE of a blastocyst is in itself a limitation [41,42,43]. As the clinical consequence of transferring a mosaic embryo is not well understood, we did not transfer any of the 9 embryos with mosaic TE but euploid SCM results in our study cohort. Since we could not evaluate the potential of mosaics to give healthy live births, we tried to exclude these 9 embryos and re-calculated the performance of SCM as compared with routine PGT-A. As expected, NiPGT-A sensitivity and NPV increased to 91.0% and 66.7%, respectively, although the improvement was not statistically significant (Table 5).
One of the putative advantages of using SCM instead of TE biopsy on aneuploidy screening is its usefulness in assessing blastocysts of poor quality. In performing TE biopsy, it is not unusual to forego a blastocyst with poor morphology when there are a few blastocysts of good to fair quality. Poor-quality embryos contain very few cells in the ICM and/or TE, and degeneration or cellular fragments are not uncommon. When these blastocysts are biopsied, their PGT-A results often reveal aneuploidy or no result due to the suboptimal amount and quality of cells biopsied. However, the quality of an embryo has weak correlation with its ploidy status; a euploid of poor morphology still has an implantation potential comparable to that of good morphology [4, 5]. In examining morphology of the blastocysts in our study, we found that SCM provided similar concordance rates in blastocysts of good/fair quality and of poor quality. Therefore, SCM could theoretically offer complementary information beside morphology in usable blastocysts which might otherwise be discarded.
In addition to NiPGT-A, SCM may potentially serve as a non-invasive tool to PGT-M and PGT-SR. We collected 20 μl of SCM from each embryo, with which only 3 μl was adequate for PGT-A. Although we focused on PGT-A in this study, approximately half of the 168 SCM samples were collected from cycles with PGT-M or PGT-SR indications. Therefore, we have substantial volume of SCM remaining for further PGT-M and PGT-SR investigation. So far, there has only been one proof-of-concept study by Wu et al. using SCM to diagnose α-thalassemia-SEA [24].
With clinical outcomes of 14 single euploid transfers available, we were surprised that 7 of them had aneuploid results from SCM. Among these “false positive results” from SCM, we followed up on euploids no. 3 and no. 4 during their pregnancies; however, both mothers declined amniocentesis and only reported that their babies were delivered full-term and healthy. On the other hand, euploid no. 12 (Table 3) was a miscarriage, with its product-of-conception (POC) showing a mosaic gain of chromosome 21, whereas NiPGT-A showed a mosaic loss of the same chromosome. Assuming this embryo was already a euploid/aneuploid mosaic at the time of biopsy, the TE obtained might have only sampled the euploid cell line while SCM might have reflected a more comprehensive scope of the embryo. However, this interpretation should be taken with caution since this was only one single case with a third source of testing material available. POC from the other 4 miscarriages was not obtainable or available for further testing. More research is warranted to correlate SCM findings and clinical outcomes.
So far, we have been relying on TE result as the “gold standard” as it is the best available reference point for comparison. However, we performed NGS on 24 donated aneuploids or embryos affected by single gene disorders from our study to verify the discordant results between TE and SCM. By utilizing the whole embryo to obtain at least 2 biopsies from the TE alone and 1 biopsy containing ICM, we found that only 9 of the 24 embryos (37.5%) had consistent result across the several biopsy locations within the same embryo. This implied that TE results could only be accurate at the site of biopsy. On the other hand, all studies including ours unanimously demonstrated that cfDNA found in SCM could reflect the chromosomal profile of an embryo. With consideration given to the potential origin of cfDNA found in SCM, such as cell apoptosis and degeneration of arrested cell(s), SCM might perform better in reflecting the “average” ploidy status of the embryo as a whole at the time of collection.
It is very clear that the ultimate goal of NiPGT is to allow de-selection of inviable embryos and selection of embryo(s) compatible with successful pregnancy. It is important to note that NiPGT, or any other non-invasive methods such as those used in prenatal testing, cannot offer a diagnosis with exact certainty; an invasive intervention is always recommended in high-risk subjects. Unlike embryo morphology, NiPGT can objectively prioritize the order of embryos for intrauterine transfer based on their ploidy status. This strategy shortens the time to pregnancy, avoids the invasive and traumatic biopsy procedures, saves the potential damage to preimplantation embryos, and gives a woman undergoing IVF a good chance to transfer embryo(s). We foresee that in the near future, technology will take another leap by which time the embryonic DNA fraction in the SCM can be targeted for NiPGT.
Conclusions
Cell-free DNA of embryonic origin is present in spent culture media and can produce PGT-A profiles. Slight modification to standard procedures in the IVF and PGT laboratories may be possible to enhance the quality of cfDNA found in SCM while strategies must be developed to reduce or eliminate maternal contamination. Although the well-established PGT-A still has its place, NiPGT using SCM has the potential to reflect the comprehensive chromosomal composition of an embryo as a whole and can gradually gain its ground for clinical application.
References
Ferraretti AP, Nygren K, Andersen AN, de Mouzon J, Kupka M, Calhaz-Jorge C, et al. Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Hum Reprod. 2017;2017(2):hox012. Available from. https://doi.org/10.1093/hropen/hox012.
Human Fertilisation & Embryology Authority. Fertility treatment 2014–2016 trends and figures. HFEA, March 2018. Available from, https://www.hfea.gov.uk/about-us/publications/research-and-data/
Division of Reproductive Health, Centers for Disease Control and Prevention. 2015 Assisted reproductive technology fertility clinic success rates report. CDC, October 2017. Available from: https://www.cdc.gov/art/reports/2015/fertility-clinic.html.
Baltaci V, Satiroglu H, Kabukçu C, Unsal E, Aydinuraz B, Uner O, et al. Relationship between embryo quality and aneuploidies. Reprod BioMed Online. 2006;12(1):77–82.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81.
Kuliev A, Cieslak J, Ilkevitch Y, Verlinsky Y. Chromosomal abnormalities in a series of 6,733 human oocytes in preimplantation diagnosis for age-related aneuploidies. Reprod BioMed Online. 2003;6(1):54–9.
Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NGM, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22(4):980–8.
Massie JAM, Shahine LK, Milki AA, Westphal LM, Lathi RB. Ovarian stimulation and the risk of aneuploid conceptions. Fertil Steril. 2011;95(3):970–2.
Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod BioMed Online. 2012;24(6):614–20.
Sekhon L, Shaia K, Santistevan A, Cohn KH, Lee JA, Beim PY, et al. The cumulative dose of gonadotropins used for controlled ovarian stimulation does not influence the odds of embryonic aneuploidy in patients with normal ovarian response. J Assist Reprod Genet. 2017;34(6):749–58.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24.
Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100–7.e1.
Forman EJ, Hong KH, Franasiak JM, Scott J, Richard T. Obstetrical and neonatal outcomes from the BEST Trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol. 2014;210(2):157.e1–6.
Scott RT, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100(3):624–30.
Liu J, Wang W, Sun X, Liu L, Jin H, Li M, et al. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biol Reprod. 2012;87(6):148.
Popovic M, Dheedene A, Christodoulou C, Taelman J, Dhaenens L, Van Nieuwerburgh F, et al. Chromosomal mosaicism in human blastocysts: the ultimate challenge of preimplantation genetic testing? Hum Reprod. 2018 33(7):1342–1354.
Capalbo A, Wright G, Elliott T, Ubaldi FM, Rienzi L, Nagy ZP. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum Reprod. 2013;28(8):2298–307.
Huang J, Yan L, Lu S, Zhao N, Qiao J. Re-analysis of aneuploidy blastocysts with an inner cell mass and different regional trophectoderm cells. J Assist Reprod Genet. 2017;34(4):487–93.
Palini S, Galluzzi L, De Stefani S, Bianchi M, Wells D, Magnani M, et al. Genomic DNA in human blastocoele fluid. Reprod BioMed Online. 2013;26(6):603–10.
Gianaroli L, Magli MC, Pomante A, Crivello AM, Cafueri G, Valerio M, et al. Blastocentesis: a source of DNA for preimplantation genetic testing. Results from a pilot study. Fertil Steril. 2014;102(6):1692–9 e6.
Tobler KJ, Zhao Y, Ross R, Benner AT, Xu X, Du L, et al. Blastocoel fluid from differentiated blastocysts harbors embryonic genomic material capable of a whole-genome deoxyribonucleic acid amplification and comprehensive chromosome microarray analysis. Fertil Steril. 2015;104(2):418–25.
Zhang Y, Li N, Wang L, Sun H, Ma M, Wang H, et al. Molecular analysis of DNA in blastocoele fluid using next-generation sequencing. J Assist Reprod Genet. 2016;33(5):637–45.
Assou S, Aït-Ahmed O, El Messaoudi S, Thierry AR, Hamamah S. Non-invasive pre-implantation genetic diagnosis of X-linked disorders. Med Hypotheses. 2014;83(4):506–8.
Wu H, Ding C, Shen X, Wang J, Li R, Cai B, et al. Medium-based noninvasive preimplantation genetic diagnosis for human α-thalassemias-SEA. Medicine (Baltimore). 2015;94(12):e669.
Shamonki MI, Jin H, Haimowitz Z, Liu L. Proof of concept: preimplantation genetic screening without embryo biopsy through analysis of cell-free DNA in spent embryo culture media. Fertil Steril. 2016;106(6):1312–8.
Xu J, Fang R, Chen L, Chen D, Xiao JP, Yang W, et al. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci U S A. 2016;113(42):11907–12.
Vera-Rodriguez M, Diez-Juan A, Jimenez-Almazan J, Martinez S, Navarro R, Peinado V, et al. Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Hum Reprod. 2018;33(4):745–56.
Ho JR, Arrach N, Rhodes-Long K, Ahmady A, Ingles S, Chung K, et al. Pushing the limits of detection: investigation of cell-free DNA for aneuploidy screening in embryos. Fertil Steril. 2018;110(3):467–75 e2.
Saravelos SH, Wong AWY, Chan CPS, Kong GWS, Cheung LP, Chung CHS, et al. Assessment of the embryo flash position and migration with 3D ultrasound within 60 min of embryo transfer. Hum Reprod. 2016;31(3):591–6.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–11.
Heitmann RJ, Hill MJ, Richter KS, DeCherney AH, Widra EA. The simplified SART embryo scoring system is highly correlated to implantation and live birth in single blastocyst transfers. J Assist Reprod Genet. 2013;30(4):563–7.
Choy KW, Kwok YK, Cheng YKY, Wong KM, Wong HK, Leung KO, et al. Diagnostic accuracy of the BACs-on-Beads™ assay versus karyotyping for prenatal detection of chromosomal abnormalities: a retrospective consecutive case series. BJOG. 2014;121(10):1245–52.
Scott RTJ, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703.
Munne S, Kaplan B, Frattarelli JL, Gysler M, Child TJ, Nakhuda G, et al. Global multicenter randomized controlled trial comparing single embryo transfer with embryo selected by preimplantation genetic screening using next-generation sequencing versus morphologic assessment. Fertil Steril. 2017;108(3):e19.
Huang L, Ma F, Chapman A, Lu S, Xie XS. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genomics Hum Genet. 2015;16:79–102.
Lane M, Zander-Fox DL, Hamilton H, Jasper MJ, Hodgson BL, Fraser M, et al. Ability to detect aneuploidy from cell free DNA collected from media is dependent on the stage of development of the embryo. Fertil Steril. 2017;108(3):e61.
Hammond ER, Shelling AN, Cree LM. Nuclear and mitochondrial DNA in blastocoele fluid and embryo culture medium: evidence and potential clinical use. Hum Reprod. 2016;31(8):1653–61.
Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373(21):2089–90.
Munné S, Wells D. Detection of mosaicism at blastocyst stage with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;107(5):1085–91.
Munné S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;108(1):62–71.e8.
Harper JC, Coonen E, Handyside AH, Winston RM, Hopman AH, Delhanty JD. Mosaicism of autosomes and sex chromosomes in morphologically normal, monospermic preimplantation human embryos. Prenat Diagn. 1995;15(1):41–9.
Delhanty JD, Harper JC, Ao A, Handyside AH, Winston RM. Multicolour FISH detects frequent chromosomal mosaicism and chaotic division in normal preimplantation embryos from fertile patients. Hum Genet. 1997;99(6):755–60.
Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod. 2000;15(8):1781–6.
Funding
This research was funded by General Research Fund, ref no. 14162417, Research Grant Council (RGC) of Hong Kong, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Yeung, Q.S.Y., Zhang, Y.X., Chung, J.P.W. et al. A prospective study of non-invasive preimplantation genetic testing for aneuploidies (NiPGT-A) using next-generation sequencing (NGS) on spent culture media (SCM). J Assist Reprod Genet 36, 1609–1621 (2019). https://doi.org/10.1007/s10815-019-01517-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01517-7