Abstract
Purpose
To assess effects on fertilization rate, embryo quality, pregnancy, and live birth rates of vitrification and warming of oocytes that matured in vitro (vIVM) compared to fresh in vitro maturation (fIVM) cycles.
Methods
A retrospective cohort study conducted at a university hospital-affiliated IVF unit. Fifty-six cycles of vIVM cycles and 263 fIVM in women diagnosed with polycystic ovarian syndrome (PCOS) ovaries were included in the analysis. The study group included PCOS patients who failed ovulation induction with intrauterine insemination and were offered IVM cycle followed by oocyte vitrification and warming. The embryological aspects and clinical outcomes were compared to those of controls undergoing fresh IVM cycles during the same period. The main outcome measure was live birth rate.
Results
One thousand seventy oocytes were collected from 56 patients and underwent vitrification and warming. In the control group, 4781 oocytes were collected from 219 patients who had undergone a fresh IVM cycle. Oocyte maturation rates were similar between the groups (mean ± SD: 0.7 ± 0.2 vs. 0.6 ± 0.2, for vIVM and fIVM, respectively). Survival rate after warming was 59.8%. Fertilization and embryo cleavage rates per oocyte were significantly lower in the vIVM group. Clinical pregnancy (10.7 vs. 36.1%) and live birth rates (8.9 vs. 25.9%) per cycle were significantly lower in the vIVM group than those in the fIVM group (P = 0.005 and P < 0.001, respectively). Five healthy babies were born in the vIVM group.
Conclusions
The reproductive potential of vitrified IVM oocytes is impaired. This injury likely occurs through vitrification and warming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past, in vitro maturation (IVM) of oocytes was applied primarily for women with polycystic ovaries to prevent ovarian hyperstimulation syndrome (OHSS); IVM has also been suggested for women with poor response to ovarian stimulation [1] and for fertility preservation. IVM for fertility preservation allows rapid collection of oocytes when gonadotoxic treatment cannot be delayed and for prepubertal girls [2,3,4]. However, implantation and live birth rates of IVM cycles have not matched those of IVF, and more research is required to improve the clinical outcomes of IVM cycles.
Oocyte cryopreservation by vitrification for mature oocytes has been successfully implemented [5]. Vitrification is based on an ultra-rapid cooling technique with a glass-like solidification of the cell, almost eliminating the creation of cellular ice crystals and thus reducing damage to the oocyte during freezing and warming [6]. However, available data on the outcomes of IVM oocytes undergoing cryopreservation by vitrification and warming is limited [7]. During IVM, the immature oocyte is exposed to multiple mechanisms of damage thermal, mechanical, and chemical that can eventually cause nuclear and cytoplasmic incoordination and damage [8,9,10,11].
Cryopreservation has the potential to further injure the oocytes by causing cytoskeletal damage through the disruption of the actin and the microtubule networks. Spindle formation abnormalities and damage to the plasma membrane and mitochondrial function have also been reported [12, 13]. Minimal data are available on the reproductive potential of cryopreserved and thawed IVM oocytes. In a pilot study, a live birth rate of 20% was reported after vitrification and warming of IVM oocytes. Nevertheless, vitrified in vitro maturation (vIVM) oocytes have lower survival rates after warming and lower fertilization rates and embryo scores than vitrified in vivo matured oocytes [14].
Efficient vitrification of oocytes that matured in vitro for fertility preservation in polycystic ovary syndrome (PCOS) patients can serve as an alternative to ovarian stimulation with gonadotropins, while minimizing the risk of ovarian hyperstimulation syndrome and exposure to high levels of hormones. PCOS patients have many small follicles allowing the collection of more immature oocytes making them good candidates to study the efficacy of vitrification in IVM oocytes. While taking into consideration the limitations, these results could be applied to cancer patients undergoing IVM for fertility preservation.
It is unknown whether the lower reproductive potential of vIVM oocytes is the result of the in vitro maturation process or oocyte damage accumulated during vitrification and warming. The aim of this study was to assess the cycle outcomes of vIVM oocytes in comparison to those of fresh IVM (fIVM) oocytes.
Materials and methods
Patient population
This is a retrospective cohort study conducted at a university hospital reproductive center. We retrospectively analyzed data collected in a clinical trial that was conducted in our center comparing the results of vitrified IVM oocytes to those of in vivo matured oocytes in IVF [14]. The original cohort was extended to include IVM cycles between 2005 and 2010. The study group included women diagnosed with ovulatory dysfunction as a result of PCOS, diagnosed according to the Rotterdam criteria [15]. All participants had normal serum thyroid-stimulating hormone (TSH) and prolactin, according to the assay used. Serum AM fasting 17-hydroxyprogesterone <2 ng/dl, total testosterone, and DHEAS were not in the tumor range. All women had menstrual disturbance, with cycles less frequent than 35 days and serum progesterone levels that ruled out ovulation 7 days before an anticipated menses, based on cycle history. All participants failed to conceive with ovulation induction and intrauterine insemination. These women were offered an IVM cycle followed by oocyte vitrification. The results of vitrified IVM cycles were compared to those of patients undergoing fresh IVM cycles for PCOS, as described above, during the same period.
The study was approved by the institutional review board of McGill University Health Center (IRB:13-409-SDR). Patients with severe stage III or IV endometriosis, large multifibroid uterus, hydrosalpinx, or severe male factor infertility were excluded from this study, as were women of advanced reproductive age (above 40 years of age).
Immature oocyte retrieval and in vitro maturation of immature oocytes
Immature oocyte retrieval was performed according to the McGill reproductive center’s standard protocol: A baseline transvaginal ultrasound was done on days 2 to 4 of menstrual bleeding to ensure that no adnexal finding was present and that the endometrial lining was thin. Patients were scanned again on day 8 of the cycle and every 2 days thereafter. Subcutaneous injection of 10,000 IU of hCG was given when the largest follicle measured 10–12 mm and the endometrial lining reached 6–8 mm in thickness. Oocyte retrieval was performed 36 h later. Oocytes were retrieved transvaginally with a 19-gauge single-lumen needle (K-OPS-7035-RWH-ET; Cook, Australia), using aspiration pressure of 85 mmHg.
Two senior embryologists who have more than 10 years of experience in human IVM and more than 5 years of experience in oocyte vitrification handled the oocytes in this study. Oocyte maturity was assessed as previously described [16]. Mature metaphase II (MII) oocytes were vitrified immediately. Immature (GV [germinal vesicle] and GVBD) oocytes were matured in an organ tissue culture dish (60 × 15 mm; Falcon) containing IVM medium (Cooper Surgical, Trumbull, CT) supplemented with a final concentration of 75 mIU/ml of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) at 37 °C in 6% CO2, 5% O2, and 89% N2. In vitro oocyte maturation was assessed 24 h later after the cumulus cells were denuded, using a fine-drawn glass pipette after 1-min exposure to 0.1% hyaluronidase (Cook, Australia). In vitro matured MII oocytes were vitrified and the remaining immature oocytes were further cultured for another 24 h (total incubation time was 48 h). Any additional MII oocyte that matured was vitrified. To note, only mature metaphase II oocytes were vitrified (vIVM oocytes); immature oocytes at the GV and MI stage after 48 h were not vitrified and were discarded.
Vitrification and warming of oocytes
In the vIVM group, MII oocytes were suspended in equilibration medium (MediCult, Denmark) containing 7.5% (v/v) ethylene glycol + 7.5% (v/v) 1,2-proanediol (PROH) for 5 min at room temperature, and then transferred to vitrification medium (MediCult, Denmark) containing 15% (v/v) ethylene glycol + 15% (v/v) PROH + 0.5 M sucrose at room temperature for 45–60 s. The oocytes were then loaded on a McGill Cryoleaf vitrification device (MediCult, Denmark) and plunged into liquid nitrogen for storage.
Vitrified oocytes were warmed in the subsequent menstrual cycle. The vitrification device was inserted into a warming medium (MediCult, Denmark) containing 1.00 M sucrose for 1 min at 37 °C. The oocytes were then transferred to a diluent medium-I (MediCult, Denmark) containing 0.5 M sucrose followed by a diluent medium-II (MediCult, Denmark) containing 0.25 M sucrose for 3 min each. Oocytes were washed twice for 3 min each time in a washing medium (MediCult, Denmark).
Oocyte fertilization and embryo culture
Oocyte survival after warming was assessed microscopically based on the morphology of the oocyte membrane integrity; degenerated oocytes were removed from the cohort. All mature viable oocytes in the vIVM and fIVM underwent intracytoplasmic sperm injection (ICSI) 2 h after warming. Fertilization was assessed 17–19 h after ICSI for the appearance of two pronuclei (2PN). The zygotes were cultured in an embryo maintenance medium (CooperSurgical) until day 3. Supernumerary good-quality embryos were cryopreserved.
Embryo transfer
Cleavage embryos were defined as of good quality (grade 1 or 2) if they had four cells on day 2 and/or seven or eight cells on day 3, contained < 20% fragmentation, and exhibited no apparent morphological abnormalities. Poor-quality embryos included fair-quality (grade 3) embryos, which had only two cells on day 2, three to five cells on day 3, and/or 20–50% fragmentation and grade 4 embryos with < 3 cells by day 3 and > 50% fragmentation.
Endometrial priming for vIVM cycles consisted of 6 mg daily of estradiol (Estrace, Roberts Pharmaceutical, Canada) starting on day 2 of the menstrual cycle for 10 days. When endometrial thickness reached 8 mm, 200 mg of vaginal progesterone (Prometrium, Schering Inc., Canada) was administered three times a day. Women undergoing fIVM cycle received 6 mg of E2 (Estrace; Roberts Pharmaceutical, Canada) starting on the day of oocyte retrieval and 200 mg of vaginal progesterone (Prometrium, Schering Inc., Canada) three times a day starting on the day of ICSI. Embryos were transferred on day 3 under ultrasound guidance. As implantation rates are lower after IVM [17], more embryos were transferred as per the clinic policy. It should be noted that, in spite of transferring more embryos in IVM cycles, multiple pregnancy rates are much lower compared to IVF cycles [18]. Pregnant women continued this luteal support until the 12th week of pregnancy.
Outcome measures
We compared the results of vIVM cycles to those of fIVM cycles. The primary outcome was a live birth rate per cycle start. Live birth was defined as delivery of a live baby > 24 weeks of pregnancy. A clinical pregnancy was defined by ultrasound visualization of intrauterine gestational sac with fetal heartbeat.
Statistical analysis
A Shapiro–Wilk W test was applied to assess the normality of distribution, a parametric test was used for normally distributed data, and a non-parametric test (Wilcoxon test) for skewed data while categorical variables were compared by chi square test or Fisher’s exact test, as appropriate. Moreover, in order to examine which parameters are independently associated with live births, vitrification of IVM oocytes and variables that were found to be significantly different between the groups were included in a stepwise logistic regression model. Descriptive statistics are given as median (interquartile range, IQR) for skewed data or mean (± standard deviation, SD) for normally distributed data. A P value < 0.05 was considered significant. All statistical analysis was performed using JMP 13 for Macintosh.
Results
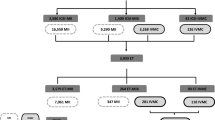
Three hundred nineteen cycles of IVM were included in the final analysis. Cycle outcomes of 1070 oocytes collected from 56 women who underwent a single-IVM cycle followed by oocyte vitrification of mature oocytes (vIVM) were compared to 4781 oocytes collected from 219 women undergoing 263 fIVM cycles. Fifty-six and 24 women underwent two and three IVM cycles, respectively. Patients in the vIVM group were younger and had a higher antral follicle count (AFC). Patient demographic parameters and baseline hormonal tests are presented in Table 1.
In the vIVM group, a total of 68 MII and 1002 GV oocytes were retrieved; 645 oocytes (64.4%) matured to MII oocytes within 48 h in the IVM culture. All the MII oocytes were vitrified. In the fIVM group, 485 MII and 4296 GV oocytes were retrieved; 2630 (61.2%) matured to MII in the culture medium. A similar number of oocytes were retrieved and maturation rates were recorded in the study groups. The survival rate of vitrified MII oocytes was 59.8% (416/713). Fertilization rate, embryo development, and pregnancy outcomes are presented in Table 2. For comparison in the last 2 years, our oocyte survival rate after warming of vitrified in vivo matured oocytes is 91.2% with a live birth rate of 33.3% per cycle. vIVM oocytes performed poorly in every laboratory and clinical aspect, including lower fertilization rate, development of cleavage stage embryos, and number of good-quality embryos. In five vIVM cycles, no embryos were available to transfer (total fertilization failure in 2 cases and no survival of post warming in 3 women). In 28 fIVM cycles, good-quality embryos were cryopreserved, while no good-quality embryos were available for freezing in the vIVM group. Women in the fIVM group had an average endometrial thickness of 8.4 ± 1.9 (mean ± SD), while women in the vIVM received daily estradiol until a minimum endometrial thickness of 8 mm was reached. Pregnancy and live birth rates were significantly lower in the vIVM group.
Five healthy singleton babies were born in the vIVM group, and 80 babies including 18 twins and 1 triplet were born following fIVM cycle. The miscarriage rate was 16% (1/6) and 28.4% (27/95) for vitrified and fresh IVM cycles, respectively (P = 1.0). The results of cycle outcomes calculated per oocyte collected are presented in Table 3.
In our IVF lab, mature oocytes collected in IVM cycle are co-cultured with immature ones undergoing IVM. Concerning the effect of vitrification and warning specifically on oocytes that were collected as immature ones and undergone IVM, a subgroup analysis of clinical pregnancy and live birth rated for cycles with only immature oocytes collected and for those with at least one MII oocyte collected was conducted. Significantly lower clinical pregnancy was found with vIVM compared to fIVM, irrespective of whether MII oocytes were retrieved during ovum pickup (Table 4). The differences in live birth rates, although in line with the results of all cycles pooled together, did not reach statistical significance, due to a smaller sample size.
The association between live birth and vitrification, age, number of in vitro matured oocytes, AFC, and number of embryo transferred was analyzed using a stepwise logistic regression analysis. Vitrification of IVM oocytes was associated with a lower likelihood of a live birth (adjusted odds ratio, 0.3; 95% confidence interval, 0.1 to 0.7).
Discussion
Oocyte maturation is a complex event involving both nuclear and cytoplasmic functions. It is unknown to what extent the addition of freezing and thawing may further impair the efficacy of IVM. This study assessed the effect of cryopreservation, per se, on in vitro matured oocytes by comparing cycle outcome to fresh IVM; suboptimal IVM results are further compromised by vitrification. The deleterious effects of vitrification on in vitro matured oocytes are evident from their significantly lower fertilization rate, embryo development, embryo quality, and ultimately lower pregnancy and live birth rates. The lower efficacy of vitrification of IVM oocytes in PCOS patients is evident from the pregnancy and live birth rates calculated per oocyte. Furthermore, after controlling for possible confounding factors, vitrification of IVM oocytes was the only factor associated with live birth. It should be noted the vIVM lowered the likelihood of live birth.
Previous studies have shown that pregnancy and live birth rates after vitrification of IVM oocytes are lower compared to vitrified oocytes collected from IVF cycles. Although the differences were not statistically significant, it was underpowered, containing only 20 patients in the vitrified IVM group [14]. Our data are inconsistent with a previous study that explored the effect of vitrification on IVM oocytes. Cao et al. [19] found a post-thaw survival rate of 86%, which is higher than the survival rate in our study. However, the fertilization rate and the number of embryos reaching cleavage stage were both lower in their study. Pregnancy and live birth were not reported; smaller sample size, different patient selection, and IVM technique could explain these differences. In their study, oocytes were collected only from patients who had more than 20 retrieved immature oocytes, and IVM cycles were primed with clomiphene citrate and human menopausal gonadotropins.
Other studies have explored the results of different cryopreservation methods after IVM of immature oocytes collected from an IVF cycle. These studies found post-thaw survival rates of 87% with vitrification and 70% with slow freezing; fertilization and embryo cleavage rates were 52 and 27%, respectively [20, 21]. The outcomes of the fIVM group in the present study are in accordance with those of previous reports [17]. A high miscarriage rate in PCOS patients undergoing IVM has also been reported by others [22].
In the present study, the differences in cycle outcomes between the groups could not be explained by patient baseline characteristics. Differences in lower ovarian reserve and oocyte quality can be excluded, as women in the vIVM group were younger and had higher antral follicle counts than the women undergoing fIVM. Body mass indices and day 3 FSH and LH were comparable between the groups. Poor pregnancy outcome of vIVM cycles cannot be explained by differences in endometrial thickness, since women undergoing vIVM cycles received daily titrated doses of estradiol until a minimal endometrium thickness of 8 mm was reached. Nevertheless, the median number of good-quality embryos available for transfer was higher in the fIVM group, which could explain the higher clinical pregnancy and live birth rates in this group.
Mature oocytes collected during ovum pickup can be affected differently by cryoinjury than oocytes that have matured in vitro. In a subgroup analysis of 134 cycles with only immature oocytes collected, we found significantly lower clinical pregnancy rate in vIVM oocytes. The differences in live birth rates did not reach statistical significance but were in line with the results of all cycles analyzed together; lesser statistical power due to a smaller sample size could explain this result. Clinical pregnancy and live birth rates were comparable to cycles with at least one MII oocyte retrieved. Therefore, differences in pregnancy outcome between vIVM and fIVM cycles cannot be explained by differences in the number of mature oocytes at the time of collection.
Autologous mature oocyte vitrification in women undergoing IVF cycles is highly effective, with an 85% survival rate and an ongoing pregnancy rate of 31% per transfer [5]. Recently, a survival rate of 86% and a live birth rate of 44% per transfer were reported in cancer patients undergoing fertility preservation [23]. The results of our study and those of Cao et al. show that vIVM oocytes that were cryopreserved at the MII stage perform poorly. Can vitrification of GV oocytes overcome these difficulties and improve the success rate? It has been hypothesized that GV-stage oocyte could be more resistant to freezing and thawing injury due to its microstructure [24]. However, current evidence shows that better results are achieved by vitrification of mature oocytes retrieved during an IVM cycle [19]. These results were further supported by studies on oocytes that failed to mature in IVF cycles [20].
Lower embryo implantation is a major concern in fresh IVM cycles, and the lower success rates compared to IVF were attributed to altered endometrial receptivity. Theoretically, endometrial receptivity should be corrected by vIVM cycles followed by frozen embryo transfer, but embryo transfer was attempted in this study only when a minimal thickness of 8 mm was reached. Nevertheless, our results show that cryoinjury is probably the main cause for immature oocyte damage. Frozen oocytes are exposed to several mechanisms of cryoinjury, including meiotic spindle damage [25], disruption and hardening of the zona pellucida [26], and damage to the cortical granules [27]. It is possible that cryopreservation and warming injury is even more detrimental to oocytes which underwent IVM, as is suggested by the lower survival rate found in this study. Immature oocytes that complete the IVM process followed by freezing and thawing are exposed to numerous mechanisms of damage both during IVM and vitrification, including a higher rate of meiotic spindle abnormalities and excessive DNA fragments than in fresh in vivo matured oocytes [28]. Mitochondrial dysfunction in vitrified in vitro matured oocytes was also reported [13].
It is paramount that women undergoing fertility preservation are counseled on different fertility preservation techniques and success rates. Few live births have been reported after using vitrified/warmed IVM embryos produced from immature oocytes retrieved from extracorporeal ovarian tissue [29, 30]. However, the availability of thawed vIVM oocytes for clinical research is very limited, and no studies explored the reproductive potential and success rates of oocytes that matured in vitro and were vitrified for fertility preservation. This study was conducted on women with PCOS. Theoretically, poor results of oocyte vitrification could be the result of pathologic mechanisms that are specific to women with PCOS. Moreover, IVM techniques differ across clinics [31], which might affect the results of vIVM cycles. Further research using different IVM protocols is required to search for any IVM technique-specific effects on the results of vitrification. These results should be interpreted with caution in women undergoing fertility preservation for cancer. Another limitation of this study is its retrospective design.
IVM of immature oocytes combined with vitrification is an option for women who cannot pursue IVF oocyte vitrification for their fertility preservation. However, the results of this study raise a concern about the lower reproductive potential of oocytes vitrified after IVM. Alternative fertility preservation techniques should be considered, and patients should be fully informed as to the prognosis. Further advances in IVM and cryopreservation technology are required to improve its effectiveness for fertility preservation for medical reasons.
References
Liu J, Lu G, Qian Y, Mao Y, Ding W. Pregnancies and births achieved from in vitro matured oocytes retrieved from poor responders undergoing stimulation in in vitro fertilization cycles. Fertil Steril. 2003;80:447–9.
De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–10.
Creux H, Monnier P, Son WY, Tulandi T, Buckett W. Immature oocyte retrieval and in vitro oocyte maturation at different phases of the menstrual cycle in women with cancer who require urgent gonadotoxic treatment. Fertil Steril. 2017;107:198–204.
Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. 2016;31:750–62.
Cobo A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105:755–764e8.
Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod BioMed Online. 2005;11:300–8.
Shalom-Paz E, Almog B, Shehata F, Huang J, Holzer H, Chian RC, et al. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod BioMed Online. 2010;21:566–71.
Ali A, Benkhalifa M, Miron P. In-vitro maturation of oocytes: biological aspects. Reprod BioMed Online. 2006;13:437–46.
Sanfins A, Plancha CE, Overstrom EW, Albertini DF. Meiotic spindle morphogenesis in in vivo and in vitro matured mouse oocytes: insights into the relationship between nuclear and cytoplasmic quality. Hum Reprod. 2004;19:2889–99.
Walls ML, Ryan JP, Keelan JA, Hart R. In vitro maturation is associated with increased early embryo arrest without impairing morphokinetic development of useable embryos progressing to blastocysts. Hum Reprod. 2015;30:1842–9.
Walls ML, Hart R, Keelan JA, Ryan JP. Structural and morphologic differences in human oocytes after in vitro maturation compared with standard in vitro fertilization. Fertil. Steril. 2016;106:1392–1398.e5.
Brambillasca F, Guglielmo MC, Coticchio G, Mignini Renzini M, Dal Canto M, Fadini R. The current challenges to efficient immature oocyte cryopreservation. J Assist Reprod Genet. 2013;30:1531–9.
Lei T, Guo N, Liu J-Q, Tan M-H, Li Y-F. Vitrification of in vitro matured oocytes: effects on meiotic spindle configuration and mitochondrial function. Int J Clin Exp Pathol. 2014;7:1159–65.
Chian RC, Huang JYJ, Gilbert L, Son WY, Holzer H, Cui SJ, et al. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009;91:2391–8.
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7.
Son WY, Chung JT, Demirtas E, Holzer H, Sylvestre C, Buckett W, et al. Comparison of in-vitro maturation cycles with and without in-vivo matured oocytes retrieved. Reprod BioMed Online. 2008;17:59–67.
Son WY, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Hum Reprod Update. 2010;16:675–89.
Shalom-Paz E, Holzer H, Young Son W, Levin I, Tan SL, Almog B. PCOS patients can benefit from in vitro maturation (IVM) of oocytes. Eur J Obstet Gynecol Reprod Biol. 2012;165:53–6.
Cao Y, Xing Q, Zhang Z-G, Wei Z-L, Zhou P, Cong L. Cryopreservation of immature and in-vitro matured human oocytes by vitrification. Reprod BioMed Online. 2009;19:369–73.
Fasano G, Demeestere I, Englert Y. In-vitro maturation of human oocytes: before or after vitrification? J Assist Reprod Genet. 2012;29:507–12.
Wang H, Racowsky C, Combelles CMH. Is it best to cryopreserve human cumulus-free immature oocytes before or after in vitro maturation? Cryobiology. 2012;65:79–87.
Benkhalifa M, Demirol A, Menezo Y, Balashova E, Abduljalil AK, Abbas S, et al. Natural cycle IVF and oocyte in-vitro maturation in polycystic ovary syndrome: a collaborative prospective study. ReprodBiomedOnline. 2009;18:29–36.
Druckenmiller S, Goldman KN, Labella PA, Fino ME, Bazzocchi A, Noyes N. Successful oocyte cryopreservation in reproductive-aged cancer survivors. Obstet Gynecol. 2016;127:474–80.
Cooper A, Paynter SJ, Fuller BJ, Shaw RW. Differential effects of cryopreservation on nuclear or cytoplasmic maturation in vitro in immature mouse oocytes from stimulated ovaries. Hum Reprod. 1998;13:971–8.
Hu W, Marchesi D, Qiao J, Feng HL. Effect of slow freeze versus vitrification on the oocyte: an animal model. Fertil Steril. 2012;98:752–760.e3.
Matson PL, Graefling J, Junk SM, Yovich JL, Edirisinghe WR. Cryopreservation of oocytes and embryos: use of a mouse model to investigate effects upon zona hardness and formulate treatment strategies in an in-vitro fertilization programme. Hum Reprod. 1997;12:1550–3.
Gook DA, Osborn SM, Johnston WI. Cryopreservation of mouse and human oocytes using 1,2-propanediol and the configuration of the meiotic spindle. Hum Reprod. 1993;8:1101–9.
Huang JYJ, Chen HY, Park JYS, Tan SL, Chian RC. Comparison of spindle and chromosome configuration in in vitro- and in vivo-matured mouse oocytes after vitrification. Fertil Steril. 2008;90:1424–32.
Prasath EB, Chan MLH, Wong WHW, Lim CJW, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–8.
Uzelac PS, Delaney AA, Christensen GL, Bohler HCL, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104:1258–60.
Dahan MH, Tan SL, Chung J, Son WY. Clinical definition paper on in vitro maturation of human oocytes. Hum Reprod. 2016;31:1383–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the institutional review board of McGill University Health Center (IRB:13-409-SDR).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cohen, Y., St-Onge-St-Hilaire, A., Tannus, S. et al. Decreased pregnancy and live birth rates after vitrification of in vitro matured oocytes. J Assist Reprod Genet 35, 1683–1689 (2018). https://doi.org/10.1007/s10815-018-1216-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1216-3