Abstract
Purpose
Polycystic ovary syndrome (PCOS) is a complex multifactorial endocrine disorder affecting approximately 5–10% of women of reproductive age. Affected women have menstrual disturbances due to anovulation, infertility, and hyperandrogenism. Ovarian androgen overproduction is the key physiopathologic feature of PCOS. A number of genes encoding major enzymes of the androgen metabolic pathways, such as HSD17B6, CYP19A1, CYP11A1, CYP17A1, and INSR, have been examined. Very few studies have been done in North India. There is an increasing prevalence of PCOS in women in Punjab and it is the leading cause of female infertility. In view of the strong evidence implicating the importance of CYP19A1 and CYP17A1 in androgen metabolic pathways, we investigated the association of rs700519, rs2414096, and rs743572 (− 34T>C) polymorphisms on susceptibility of developing PCOS, in North India.
Methods
A total of 500 subjects (women of reproductive age) including 250 PCOS cases and 250 healthy age-matched controls were included in the present study. DNA was extracted from venous blood for all samples, and association analysis for rs2414096, rs700519, and rs743572 was done by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. Lipid profile was done using a biochemical analyzer and body mass index (BMI) was measured for all cases. Statistical analysis was performed.
Results
Significant association of − 34T>C polymorphism of CYP17A1 was found with PCOS (p = 0.0005). BMI was statistically different between PCOS cases and controls (p = 0.000). Triglycerides were high in PCOS women. Variations of CYP19A1 were not statistically significant with PCOS.
Conclusions
These data suggest that − 34T>C polymorphism in CYP17A1 is associated with PCOS in North India. No polymorphism of CYP19A1 was found to be associated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is a complex and heterogeneous disorder of endocrine system among women of reproductive age, affecting approximately 5–10% women worldwide [1]. The main characteristics of PCOS are hyperandrogenism, anovulation, polycystic ovaries, hirsutism, obesity, and hyperinsulinemia [2]. In response to increased luteinizing hormone (LH), ovaries induce high secretion of androgens, leading to suppression of follicular growth and maturation [3]. In a study of 460 girls from south India, 9.13% girls satisfied the Rotterdam’s criteria for PCOS [4]. Around 50% of the PCOS women are overweight or obese and mostly having abdominal fat distribution [5]. There is a high prevalence of obesity in Punjab. A study showed that 14.8% of women were overweight and 13.8% were obese. This could be contributory in the increase in the number of cases of PCOS [6].
The exact mode of inheritance of PCOS has not been firmly established yet, although some studies on familial cases of PCOS suggest it to be an autosomal dominant trait [7,8,9]. Both environmental and genetic factors play a role in the pathogenesis of PCOS, and several pathways implicated in its etiology include the metabolic or regulatory pathway of steroid hormone synthesis, regulatory pathways of gonadotropin action, the insulin-signaling pathway, and the regulation of glucose and lipid metabolism pathway [10, 11]. CYP19A1, a key enzyme essential for estrogen biosynthesis, helps in transformation of testosterone to estradiol during steroidogenesis [12, 13]. CYP19A1 encodes aromatase enzyme and is located at 15p21.13. Studies on several genetic polymorphisms of CYP19A1 have indicated that aromatase activity might be decreased in PCOS follicles, resulting in abnormal follicle development [14, 15]. Rare loss-of-function mutations in the CYP19A1 have been identified in women with aromatase deficiency, and these women may develop some features of the PCOS phenotype [16, 17].
CYP17A1 encodes the key enzyme 17-α-hydroxylase/17–20 lyase (P450 17α), which is a qualitative regulator catalyzing the conversion of pregnenolone to 17-hydroxy-pregnenolone and progesterone to 17-hydroxyprogesterone (17-OHP), which are rate-limiting steps in androgen biosynthesis [18]. CYP17 is predominantly expressed in the adrenal gland, testicular Leydig cells, and ovarian theca cells. Increased activity of this enzyme has been hypothesized to enhance androgen biosynthesis and secretion in PCOS [19]. CYP17A1 is located on 10q24-q25. A common polymorphism T>C at − 34 base pairs from the translation initiation point in the promoter region has been hypothesized to upregulate the expression of CYP17A1 by increasing the transcription binding site to a Sp-1 transcription factor, which further results in increased synthesis of androgens and affecting the PCOS phenotype [20].
In view of strong evidence implicating the importance of CYP17A1 and CYP19A1 in androgen metabolic pathways, the present study aimed at genotyping rs700519 and rs2414096 polymorphisms of CYP19A1 and − 34T>C (rs743572) of CYP17A1. To best of our knowledge, no study has been conducted to see association of these genes with PCOS in North India; therefore, this study will help in generating baseline data.
Material and methods
Subject selection
After the approval given by the ethics review board of Guru Nanak Dev University, consistent with provisions of the Declaration of Helsinki, the present retrospective case-control study was conducted from the period 2015 to 2017.
The sample size was calculated using the CaTS-Power Calculator to achieve a minimum power of 80% with 95% confidence interval. Assumptions used for calculation were one-stage sample design at 5% significance level (α = 0.05), taking worldwide prevalence of 10% and odds ratio of 1.5. The calculated sample size was 250 PCOS cases and 250 controls for analysis of SNPs in the selected candidate genes (CYP19A1 and CYP17A1) [21].
Criteria for cases and controls
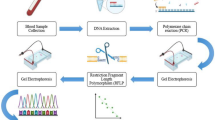
Cases were procured from Hartej Hospital, Amritsar, which fulfilled the Rotterdam 2003 criteria [2]. All the cases having 12 or more follicles measuring 2–9 mm in diameter and/or an increased ovarian volume > 10 cm3 on transvaginal ultrasound scanning, having chronic anovulation, and/or having biochemical or clinical signs of hyperandrogenism were selected. Age-matched healthy women having no signs of PCOS and having normal menstrual cycle formed the control group. Details of their life style and health status were recorded. Voluntary written informed consent was obtained. Detailed information of menstrual and medical history, family history, anthropometric values, and other precipitating factors were recorded on pre-designed proforma from both cases and controls. About 5 ml of venous blood was drawn from cases and controls (3 ml for EDTA vial and 2 ml for serum). The EDTA and serum samples were stored at − 20 °C till further analysis. Genotyping for rs700519, rs2414096 (CYP19A1), and rs743572 (CYP17A1) polymorphisms was done using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique.
Biochemical analysis
Lipid profile including cholesterol, triglycerides, and high-density lipoproteins (HDL) was estimated using specified kits (Erba Mannheim) on biochemical analyzer (Erba Mannheim Chem-7).
Anthropometric measurement
Anthropometric measurements were taken in all cases and controls. Height and weight were recorded. Body mass index was used as a measure of overall adiposity and was defined as weight (kg)/height2 (cm). The category of body weight (lean, normal, obese, or overweight) was defined as given by the World Health Organization criteria [22].
Genotypic analysis using PCR-RFLP method
Genomic DNA was isolated from 1 ml peripheral blood using the phenol-chloroform method by Adeli and Ogbona (1990) by slight modifications [23]. The DNA was suspended in 100 μl of Tris-HCl and EDTA (TE buffer). DNA was quantified using nanodrop (Thermoscientific 2000 c). For CYP19A1 rs700519 (C>T), amplification of 173-bp product size was done using forward and reverse primers as mentioned in Table 1 [24]. PCR product was digested overnight at 37 °C by HpyCH4V (New England Biolabs) restriction enzyme. Digested products were checked on 2.5% agarose gel. CC genotype showed 173-bp band; CT showed 173-, 118-, and 55-bp band; and TT showed 118- and 55-bp band on agarose gel.
PCR amplification for rs2414096 (G>A) was done, using primers as given by Jin et al. [25] CViAII (New England Biolabs) restriction enzyme digested PCR product when treated overnight at 37 °C. After digestion, a band of 189 bp for GG; 189-, 161-, and 28-bp band for GA; and 161- and 28-bp band for AA genotype were seen on 2.5% agarose gel.
Amplification of rs743572 (− 34T>C) was done using a primer sequence [26] given in Table 1. Overnight restriction at 37 °C was done using MSpAI (New England Biolabs) restriction enzyme. A single 414-bp product defined the T homozygotes; 414-, 290-, and 124-bp products defined the TC heterozygotes; and 290- and 124-bp products defined the C homozygotes, on 2% agarose gel.
Statistical analysis
Student’s t test and Fisher’s exact test was used to compare the CYP19A1 and CYP17A1 genotype and allele distributions in the case-control study. The relative association between patients and controls for genotype and allele frequencies was assessed by Pearson’s χ2 test. The corresponding odds ratios (ORs) and confidence intervals (95% CIs) were calculated with (SPSS version 17.0, SPSS Chicago, IL, USA). Clinical variables such as cholesterol, triglycerides, HDL, and BMI were compared using one-way analysis of variance (ANOVA). Hardy-Weinberg distribution of genotypes in the PCOS and control groups was assessed. A strong association (significance) was assumed at p < 0.05.
Haploview 4.2 [27] was used to determine haplotypes as well as haplotype blocks, using the solid spine of LD algorithm. SNPs and haplotypes were tested for association with PCOS.
Result
In 500 study subjects, the overall mean ± SD age of cases and controls was 24 ± 5.381 and 26.157 ± 5.443. The mean ± SD BMI calculated was 24.523 ± 5.026 and 22.460 ± 3.471 in cases and controls. BMI was found to be statistically different between cases and controls (p = 0.000). The overall mean of cholesterol, triglycerides, and HDL in PCOS cases was 159.03, 192.21, and 47.35 but no significant difference was observed among different genotypes. The triglyceride levels were found to be high in cases (Table 2).
Association of − 34T>C (rs743572) was found with PCOS. The genotypic and allelic frequencies were statistically different in cases and controls (p = 0.0005) and the genotypic frequencies were in accordance with the Hardy-Weinberg equilibrium (p = 0.369) (Table 2).
The genotypic distributions for rs700519 and rs2414096 in CYP19A1 were not observed to be statistically significant among cases and controls (p = 0.635 and p = 0.614, respectively). For rs700519, no significant deviation from the Hardy-Weinberg equilibrium was observed (p = 0.072); however, for rs2414096, a significant deviation from the Hardy-Weinberg equilibrium was observed (p = 0.000) (Table 3).
Haplotype analysis revealed that the studied SNPs (rs700519 and rs2414096) were not in linkage disequilibrium (LD) with each other among PCOS cases and controls (D′ = 0.112 and r2 = 0.0).
Discussion
The present study is the first to investigate the association of CYP17A1 (rs743572) and CYP19A1 (rs700519 and rs2414096) polymorphisms in North Indian women with PCOS (Table 4).
Polycystic ovary syndrome is considered a multifactorial disorder with various genetic, metabolic, endocrine, and environmental abnormalities. It is well documented that PCOS women are more vulnerable to obesity-related health problems like diabetes, hypertension, cardiovascular disorders, anovulation, infertility, difficulties in conception, and adverse pregnancy outcomes [38, 39]. BMI provides the measure of obesity which throws light on associated problems with it. It has been established that Asian population has higher fat deposition at a lower BMI. The socio-economic development in Punjab has led to changes in dietary pattern and reduced physical activity, which have contributed towards increasing obesity, among women [40]. Our results have shown a statistically significant association of BMI with PCOS (p = 0.000). Our results are in accordance with the study done by Thathapudi et al. in South Indian women which showed BMI to be statistically significant (p < 0.0001) [41]. Similar results were observed by Deepika et al. [42] in South Indian women (p = 0.0001). Our study is analogous with the study done by Chen et al. showing significant differences in BMI among PCOS subjects (p = 0.001) [43]. In the present study, 70% of PCOS patients were overweight (BMI > 25 kg/m2), higher than the reports of Abdulrazak and Al-Tae [44], who found that 63.55, 50, and 35% of women with PCOS were obese or overweighed, respectively. However, Haider et al. [45] and Zhang et al. [46] reported no significant differences in BMI (p = 0.575, p = 0.831, respectively).
Cholesterol, triglycerides, and HDL levels were not statistically significant among genotypes of the studied polymorphisms. Overall triglyceride levels were high in PCOS cases. Similar to present findings, Macut et al. also reported elevated triglycerides levels in PCOS women [47]. The characteristic dyslipidemic profile [high triglycerides and low high-density lipoprotein-cholesterol (HDL-C)] associated with insulin resistance is the most common metabolic abnormality in PCOS.
In the present study, − 34C>T polymorphism in CYP17A1 was observed to be associated with PCOS (p = 0.0005). Diamanti-Kandarakis also reported a high association of − 34T>C polymorphism with PCOS in Greek population, which is similar to the present study [26]. It has been indicated that high frequency of CC genotype has a significant role in hyperandrogenism in PCOS women. In the present study, we have confirmed the high frequency of CC genotype in PCOS cases as compared with controls. In a study conducted on Thai women, no positive association was found [48]. Subsequent comprehensive studies have also failed to detect significant relation between CYP17 and PCOS [49, 50]. Our observation of strong association of this polymorphism with PCOS corresponds to the findings of Carey et al. where a strong association of − 34T>C polymorphism with familial PCOS has been reported [28]. However, Gharani et al. reported lesser association of this polymorphism with non-familial PCOS [51]. Studies on CYP17A1 in Iraqi women showed lack of association between CYP17A1 and PCOS [34].
Aromatase activity is reduced in PCOS follicular fluid. No significant association was observed between CYP19 (rs2414096) polymorphism and PCOS in the present investigated study (p = 0.614). The results of present study were not in accordance with the study done by Reddy et al. in South Indian women [35]. But Guo et al. reported significant association of CYP19 with age at menarche (AAM) in Caucasian females [52]. Jin et al. conducted study on Chinese population and investigated that SNP rs2414096 in CYP19 is associated with susceptibility to PCOS (p = 0.001) [26]. A study by Petry et al. conducted on young women from two different populations including Barcelona women and Oxford women showed that variation in aromatase gene is associated with features of hyperandrogenism [53]. Mutib et al. observed positive association of rs2414096 polymorphism with PCOS hyperandrogenism in Iraqi women [36]. Since, rs2414096 is intronic and therefore does not affect the protein sequence of aromatase and this polymorphism may not be a direct causal factor and there may be functional variants that are in strong linkage disequilibrium and play a role in PCOS in our ethnicity. Aromatase activity is reduced in PCOS follicular fluid, which might be due to genes involved or when follicular stimulating hormone (FSH) activity is low as compared to LH, and due to reduced levels of FSH, aromatase activity is lowered and the androstenedione and testosterone are not completely aromatized to estrogens.
rs700519 (CYP19A1) did not show significant association with PCOS in present study (p = 0.635). These results were in contrast with the study on South Indian women, which showed significant difference [35]. Wang et al. [24] showed significance with PCOS in a large case-control study (p = 0.004). Urbanek et al. (1999) used linkage analysis to study 37 candidate genes (including CYP19A1), but this study found evidence for linkage only with follistatin [54]. Soderlund et al. found no evidence of mutations in CYP19A1 in patients with PCOS after examining the distribution of the variant in the ovary promoter in 25 PCOS patients and 50 controls [55]. More recently, Nectaria Xita found that a CYP19A1 polymorphism was associated with serum testosterone concentration [56]. They concluded that CYP19A1 may not be a major genetic determinant of PCOS but a genetic modifier of the phenotype.
Conclusion
This is the first association study from North India that has reported the association of CYP17A1 − 34T>C (rs743572) polymorphism with polycystic ovary syndrome. This SNP may be a useful marker in determining genetic susceptibility to the pathogenesis of polycystic ovary syndrome, while rs7005195 and rs2414096 did not show any significant association. However, differences in various populations observed can be attributed to diverse ethnic and geographic differences. Identification of genetic markers through case-control association and further functional studies is needed to analyze potential factors for complex disorders such as polycystic ovary syndrome.
References
Akgul S, Derman O, Alikaşifoglu M, Aktaş D. CYP1A1 polymorphism in adolescents with polycystic ovary syndrome. Int J Gynecol Obstet. 2011;112:8–10.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome society. Fertil Steril. 2004;81:19–25.
Dumitrescu R, Mehedintu C, Briceag I, Purcarea V, Hudita D. The polycystic ovary syndrome: an update on metabolic and hormonal mechanisms. J Med Life. 2015;8(2):142–5.
Nidhi R, Padmalatha V, Nagarathna R, Ram A. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011;24:223–7.
Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002 Jul;26(7):883–96.
Kaur A. Survey of obesity among various age group girls of Punjab. Int J Phys Educ Sports Health. 2016;3(2):296–9.
Bentley-Lewis R, Seely E, Dunaif A. Ovarian hypertension: polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2011;40(2):433–x.
Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84(1):165–9.
Govind A, Obhrai MS, Clayton RN. Polycystic ovaries are inherited as an autosomal dominant trait: analysis of 29 polycystic ovary syndrome and 10 control families. J Clin Endocrinol Metab. 1999;84(1):38–43.
Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18(3):598–603.
Diao FY, Xu M, Hu Y, Li J, Xu Z, Lin M, et al. The molecular characteristics of polycystic ovary syndrome (PCOS) ovary defined by human ovary cDNA microarray. J Mol Endocrinol. 2004;33:59–72.
Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking: thematic review series: genetics of human lipid diseases. J Lipid Res. 2011;52(12):2111–35.
Manna PR, Stetson CL, Slominski AT, Pruitt K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine. 2016;51(1):7–21.
Harada N, Ogawa H, Shozu M, Yamada K. Genetic studies to characterize the origin of the mutation in placental aromatase deficiency. Am J Hum G E N. 1992;51:666–72.
Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. Molecular basis of aromatase deficiency in an adult female with sexual infantilism and polycystic ovaries. Proc Natl Acad Sci U S A. 1993;90:11673–7.
Naessen T, Kushnir MM, Chaika A, Nosenko J, Mogilevkina I, Rockwood AL, et al. Steroid profiles in ovarian follicular fluid in women with and without polycystic ovary syndrome, analyzed by liquid chromatography-tandem mass spectrometry. Fertil Steril. 2010;94(6):2228–33.
Magoffin DA. Ovarian enzyme activities in women with polycystic ovary syndrome. Fertil Steril. 2006;86:S9–S11.
Gilep AA, Sushko TA, Usanov SA. At the crossroads of steroid hormone biosynthesis: the role, substrate specificity and evolutionary development of CYP17. Biochim Biophys Acta. 2011;1814:200–9.
Chua AK, Azziz R, Goodarzi MO. Association study of CYP17 and HSD11B1 in polycystic ovary syndrome utilizing comprehensive gene coverage. Mol Hum Reprod. 2012;18(6):320–4.
Carey AH, Chan KL, Short F, White D, Williamson R, Franks S. Evidence for a single gene effect causing polycystic ovaries and male pattern baldness. Clin Endocrinol. 1993;38(6):653–8.
Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–13.
World Health Organisation (WHO). Appropriate body-mass index for Asian populations and its implications for policy and intervention for strategies. Lancet. 2004;363:157–63.
Adeli K, Ogbonna G. Rapid purification of human DNA from whole blood for potential application in clinical chemistry laboratories. Clin Chem. 1990;36(2):261–4.
Wang H, Li Q, Wang T, Yang G, Wang Y, Zhang X, et al. A common polymorphism in the human aromatase gene alters the risk for polycystic ovary syndrome and modifies aromatase activity in vitro. Mol Hum Reprod. 2011;17(6):386–91.
Jin JL, Sun J, Ge HJ, Cao YX, Wu XK, Liang FJ, et al. Association between CYP19 gene SNP rs2414096 polymorphism and polycystic ovary syndrome in Chinese women. BMC Med Genet. 2009;10:139.
Diamanti-Kandarakis E, Bartzis MI, Zapanti ED, Spina GG, Filandra FA, Tsianateli TC, et al. Polymorphism T→ C (− 34 bp) of gene CYP17 promoter in Greek patients with polycystic ovary syndrome. Fertil Steril. 1999;71(3):431–5.
Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5.
Carey AH, Waterworth D, Patel K, White D, Little J, Novelli P, et al. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3(10):1873–6.
Kahsar-Miller M, Boots L, Bartolucci A, Azziz R. Role of a CYP17 polymorphism in the regulation of circulating dehydroepiandrosterone sulfate levels in women with polycystic ovary syndrome. Fertil Steril. 2004;82:973–5.
Echiburú B, Pérez-Bravo F, Maliqueo M, Sánchez F, Crisosto N, Sir-Petermann T. Polymorphism T –> C (−34 base pairs) of gene CYP17 promoter in women with polycystic ovary syndrome is associated with increased body weight and insulin resistance: a preliminary study. Metabolism. 2008;57:1765–71.
Park J, Lee E, Ramakrishna S, Cha D, Baek K. Association study for single nucleotide polymorphisms in the CYP17A1 gene and polycystic ovary syndrome. Int J Mol Med. 2008;22:249–54.
Unsal T, Konac E, Yesilkaya E, Yilmaz A, Bideci A, Ilke OH, et al. Genetic polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in adolescent girls with polycystic ovary syndrome. J Assist Reprod Genet. 2009;26:205–16.
Pusalkar M, Meherji P, Gokral J, Chinnaraj S, Maitra A. CYP11A1 and CYP17 promoter polymorphisms associate with hyperandrogenemia in polycystic ovary syndrome. Fertil Steril. 2009;92:653–9.
Mohammed MB, AL-Awadi SJ, Omran MA. Association between polycystic ovary syndrome and genetic polymorphisms of CYP 17 gene in Iraqi women. Iraqi J Biotechnol. 2015;14(2):99–110.
Reddy KR, Deepika MLN, Latha KP, Sagurthi SR, Lakshmanarao SS, Rahman PF, et al. Polycystic ovary syndrome: role of aromatase gene variants in south Indian women. Int J Pharm Bio Sci. 2015;6(2):1283–96.
Mutib MT, Hamdam FB, Salihi ARA. Effect of CYP19 gene on polycystic ovary syndrome phenotype in Iraqi women. Iraqi J Med Sci. 2015;13(3):272–8.
Dou Q, Tan L, Ma LY, Sun YP. The relationship between the CYP19 alleles rs727479A/C, rs700518A/G, and rs700519C/T and pregnancy outcome after assisted reproductive technology in patients with polycystic ovary syndrome in a Chinese population: a population-based study. Kaohsiung J Med Sci. 2017;33(11):558–66.
Joshi SR. Metabolic syndrome—emerging clusters of the Indian phenotype. J Assoc Physicians India. 2003;51:445–6.
Dağ ZÖ, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16(2):111–7.
Sidhu S, Tatla HS. Prevalence of overweight and obesity among adult urban females of Punjab: a cross-sectional study. Anthropologist. 2002;4(2):101–3.
Thathapudi S, Kodati V, Erukkambattu J, Katragadda A, Addepally U, Hasan Q. Anthropometric and biochemical characteristics of polycystic ovarian syndrome in South Indian women using AES-2006 criteria. Int J Endocrinol Metab. 2014;12(1):e12470.
Deepika MLN, Ranjith K, Rani VU, Ishaq M, Jahan P. Familial background of complex diseases in PCOS probands of South Indian population. Asian J Epidemiol. 2012;5(2):50–5.
Chen J, Shen S, Tan Y, Xia D, Xia Y, Cao Y, et al. The correlation of aromatase activity and obesity in women with or without polycystic ovary syndrome. J Ovar Res. 2015;8(11):1–6.
Alnakash Abdulrazak H, Al-Tae e NK. Polycystic ovarian syndrome: the correlation between the LH/FSH ratio and disease manifestations. Middle East Fertil Soc J. 2007;12(1):35.
Haider S, Mannan N, Khan A, Qureshi MA. Influence of anthropometric measurements on abnormal gonadotropin secretion in women with polycystic ovary syndrome. J Coll Physicians Surg Pak. 2014;24(7):463–6.
Zhang XL, Zhang CW, Xu P, Liang FJ, Che YN, Xia YJ, et al. SNP rs2470152 in CYP19 is correlated to aromatase activity in Chinese polycystic ovary syndrome patients. Mol Med Rep. 2012;5:245–9.
Macut D, Panidis D, Glisić B, Spanos N, Petakov M, Bjekić J, et al. Lipid and lipoprotein profile in women with polycystic ovary syndrome. Can J Physiol Pharmacol. 2008;86(4):199–204.
Techatraisak K, Wongmeerit K, Dangrat C, Wongwananuruk T, Indhavivadhana S. Measures of body adiposity and visceral adiposity index as predictors of metabolic syndrome among Thai women with PCOS. Gynecol Endocrinol. 2016;32(4):276–80.
Unluturk U, Harmanci A, Kocaefe C, Yildiz BO. The genetic basis of the polycystic ovary syndrome: a literature review including discussion of PPAR-γ. PPAR Res. 2007;23
Diamanti-Kandarakis E, Piperi C, Spina J, Argyrakopoulou G, Papanastasiou L, Bergiele A, et al. Polycystic ovary syndrome: the influence of environmental and genetic factors. Hormones (Athens). 2006;5(1):17–34.
Gharani N, Waterworth DM, Williamson R, Franks S. 5’ polymorphism of the CYP17 gene is not associated with serum testosterone levels in women with polycystic ovaries. J Clin Endocrinol Metab. 1996;81(11):4174.
Guo Y, Xiong DH, Yang TL, Guo YF, Recker RR, Deng HW. Polymorphisms of estrogen-biosynthesis genes CYP17 and CYP19 may influence age at menarche: a genetic association study in Caucasian females. Hum Mol Genet. 2006;15(16):2401–8.
Petry CJ, Ong KK, Michelmore KF, Artigas S, Wingate DL, Balen AH, et al. Association of aromatase (CYP19) gene variation with features of hyperandrogenism in two populations of young women. Hum Reprod. 2005;20(7):1837–43.
Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, et al. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999;96(15):8573–8.
Söderlund D, Canto P, Carranza-Lira S, Méndez JP. No evidence of mutations in the P450 aromatase gene in patients with polycystic ovary syndrome. Hum Reprod. 2005;20(4):965–9.
Xita N, Lazaros L, Georgiou I, Tsatsoulis A. CYP19 gene: a genetic modifier of polycystic ovary syndrome phenotype. Fertil Steril. 2010;94(1):250–4.
Acknowledgements
We would like to thank patients from Hartej Hospital, Amritsar, for providing us blood samples for our study.
Funding
The study was supported by UGC-UPE (non-NET) fellowship and CPEPA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the ethics review board of Guru Nanak Dev University, consistent with provisions of the Declaration of Helsinki. Voluntary written informed consent was obtained.
Rights and permissions
About this article
Cite this article
Kaur, R., Kaur, T. & Kaur, A. Genetic association study from North India to analyze association of CYP19A1 and CYP17A1 with polycystic ovary syndrome. J Assist Reprod Genet 35, 1123–1129 (2018). https://doi.org/10.1007/s10815-018-1162-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1162-0