Abstract
Purpose
The functional role of the FSHR promoter −29G/A polymorphism (rs1394205) in men is not clear. Some studies failed to find a relationship between the FSHR −29G/A and follicle-stimulating hormone (FSH) levels and did not associate the SNP with male infertility. Only one study showed that the FSHR −29 SNP modulates serum FSH levels in Baltic young male cohort. Because the SNP −29G/A has to be shown to have a strong effect on in vitro transcription activity of the FSHR promoter and the activation of FSHR is necessary for a normal FSH function, this study was undertaken to assess whether the FSHR -29G/A SNP modulates the gonadal endocrine function in men.
Methods
A total of 200 men with alteration of conventional sperm parameters or normozoospermia (according to the parameters WHO 2010), were genotyped by TaqMan Assay. Hormone levels were measured by immunoassay, and sperm analysis was performed according to the World Health Organization criteria.
Results
A significant gradient of increasing FSH levels across the FSHR −29G/A genotypes was observed (p < 0.01). Among normozoospermic men (n = 110), those with FSHR −29A-allele carriers (GA + AA and AA) had higher serum FSH (p < 0.01) and LH levels (p < 0.05) and higher body mass index (BMI) (p < 0.01) compared to men with the GG genotype. The carrier status of rs1394205 genotypes did not affect the other endocrine parameters neither in men with altered sperm parameters nor in normozoospermic men.
Conclusions
The FSHR −29G/A polymorphism modulates FSH and, for the first time, LH serum levels and BMI in normozoospermic men. These findings underline the importance to pay close attention to the studies of genetic variations associated with clinical-endocrine parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Follicle-stimulating hormone (FSH) is produced by the anterior pituitary gland and its interaction with FSH receptor (FSHR) is essential for normal oogenenesis and spermatogenesis. In women, FSH regulates the maturation of Graafian follicles and granulosa cell estrogen production and is also essential to prevent apoptosis and to stimulate proliferation of granulosa cells [1]. In men, during the fetal and neonatal development, FSH is fundamental for Sertoli cell proliferation and for spermatogonia mitosis [2]. In adult men, FSH is important to guarantee the metabolic functions of Sertoli cells on the maintenance of quantitatively and qualitatively normal spermatogenesis [3, 4]. FSH is a heterodimeric glycoprotein composed of an α-glycoprotein subunit (αGSU) and a β-subunit (FSHß) that ensures the binding specificity to FSHR [5]. The effectiveness of FSH relates to the intrinsic bioactivity of the hormone, its serum concentration, and the efficacy of its receptor signal transduction in response to hormone stimulation. The FSH receptor (FSHR) belongs to the family of G-protein coupled receptors. It is expressed both in ovarian granulosa and in testicular Sertoli cells, and its activation is necessary for the FSH biological function [6].
The human FSHR gene (MIM 136435, chr.2p21, gene192 kb) consists of ten exons and nine introns, and its activity is regulated by a core promoter spanning 225 bp, which represents a TATA–less promoter [7]. To date, the National Center for Biotechnology Information (NCBI) Single Nucleotide Polymorphism (SNP) database (http://ncbi.nlm.nih.gov/SNP/) indicates that the FSHR gene links to a total of 4874 SNPs, both in the core promoter and in the coding region. In particular, the FSHR −29G/A (rs1394205) is a common SNP located in the core promoter region resulting in a G/A exchange in a potential GGAAA binding domain for a c-E-twenty-six specific (c_ETS) transcription factor [8]. The FSHR SNP has been reported, by an in vitro analysis in CHO cells, to decrease the transcriptional activity of the promoter in the presence of the A-allele [9]. In women, the FSHR −29G/A has not been associated with serum FSH levels [10, 11], but female Indian carriers of the FSHR −29A-allele were less responsive to FSH treatment than other genotypes [12]. The insensitivity to FSH treatment was supported by the finding of reduced levels of mRNA expression and protein in granulosa cells obtained, during assisted reproductive techniques (ART) treatment, from subjects with FSHR AA genotype compared to women with FSHR GG genotype [12].
The available data concerning the FSHR −29G/A SNP in men are less consistent. Some studies and a meta-analysis showed no association between the −29 G/A SNP haplotypes and male infertility in different populations [13,14,15,16,17]. A study reported reduced testis volume and slightly lower sperm counts in FSHR −29 A-allele carriers [18]. Very recently, only one study showed that FSHR −29 SNP was associated with different FSH levels in Baltic young male cohort [19]. This background prompted us to investigate the influence of the FSHR −29G/A polymorphism on reproductive hormonal levels, sperm parameters, and testicular volume in men from Southern Italy.
Subjects and methods
Subjects
We recruited 200 men belonging to the Italian (Caucasian) population from Southern Italy, referred to the Division of Andrology and Endocrinology, University of Catania. The exclusion criteria were male infertility by genetic causes (karyotype anomalies, Yq chromosome microdeletions, and CFTR gene mutations), cryptorchidism, testicular tumors, or pituitary adenomas.
Genotyping
Genomic DNA was purified from total peripheral blood with the High Pure polymerase chain reaction template preparation kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions. DNA concentration was quantified by Nanodrop1000 spectrophotometer V 3.7 (Thermo Fisher Scientific, Wilmington, DE, USA). Genotyping for the −29G/A SNP (rs1394205) was performed with StepOne Real-Time PCR System (Applied Biosystems, USA) for real-time PCR and TaqMan Genotyping Master Mix (Life Technologies, Pleasanton, CA, USA) and a TaqMan SNP Assay (Life Technologies, Austin, Texas, USA) customized for the studied SNP (C_27829553_10). The default thermal cycling conditions (10 min at 95 °C followed by 50 cycles of 15 s at 92 °C plus 1 min and 30 s at 60 °C) were applied. After each amplification, an allelic discrimination was made to determine the genotype of each subject. To find positive control DNAs representing the three genotypes (GG, GA, and AA) into each genotyping reaction plate, we have previously performed direct automated DNA sequencing on AbiPrism 310 Genetic Analizer (Applied Biosystems) of PCR products containing the specific polymorphism.
Reproductive hormone and sperm analyses
All reproductive hormone assays were performed in the central laboratory of the “Policlinico G. Rodolico” teaching Hospital. Serum concentrations of FSH, luteinizing hormone (LH), and testosterone levels were measured by commercial chemiluminescence immunoassay methods (Roche Diagnostic, Mannheim, Germany). Testicular volume was evaluated by Prader’s orchidometer by the same operators (AEC and SLV), and the mean of the two evaluations was calculated and used for further analysis.
Semen samples were collected by masturbation from each patient after 4 days of sexual abstinence on two different occasions about 2 weeks apart. Sperm analysis was performed according to the World Health Organization (WHO) criteria. Patients were classified into two groups according to their sperm count: 90 patients had alteration of conventional sperm parameters, and the remaining 110 men were normozoospermics according to the WHO 2010 criteria (WHO, 2010). Men with one or more conventional sperm parameters (density, total, and progressive motility and normal forms) below the fifth percentile were considered as men with alteration of conventional sperm parameters.
Statistical analysis
Results are reported as mean ± SD and median, as appropriate throughout the text. The sperm parameters used for statistical analysis were the mean of the two sperm collections obtained from each men enrolled. The results of the repeated sperm before statistical analysis, all data, were analyzed for normal distribution using Kolmogorov-Smirnov test and for equal variance using the Bartlett’s test. Among all variables, testosterone, body mass index (BMI), and testicular volume had normal distribution and equal variance and were compared among different genotypes by one-way analysis of variance (ANOVA). The remaining variables were analyzed using the Kruskall-Wallis test. To assess statistical differences in the variables between the carriers (GA + AA or AA) and non-carriers (GG) of the A-allele, we used one-way analysis of variance (ANOVA) or non-parametric Mann-Whitney two tailed U test, as appropriate. Chi-squared analysis was used to determine whether the genotype distribution conformed to Hardy-Weinberg equilibrium and to compare differences in variant tract frequencies between the group of men with alteration of conventional sperm parameters and normozoospermia. The analysis was performed using SPSS software (version 22) (SPSS Inc., Chicago, IL). Statistical significance was set at p ≤ 0.05.
Results
The clinical and endocrine characteristics of all men stratified according to the three FSHR genotypes are shown in Table 1. All the end points measured in this study were preliminary analyzed for the FSHR −29G/A polymorphism in the whole group of men. Overall, serum FSH, LH, and testosterone levels were 6 ± 5.7 IU/L, 4.7 ± 2.3 IU/L, and 5.1 ± 1.8 IU/L, respectively. We found that serum FSH levels were significantly different among the three −29G/A genotypes (GG, GA, and AA). In particular, a significant gradient of increasing FSH level across the FSHR genotypes (GG<GA<AA) was observed (p = 0.003) (Table 1). When we compared the distribution of FSH values between men with GG genotype and the group of men including AA homozygotes alone or with GA heterozygotes, we found a significant difference (p = 0.002 and p = 0.009, respectively) (Table 1). The same upward trend of a suggestive, but not significant, difference was found in LH levels (p = 0.097 and p = 0.1, respectively) and in BMI levels (this latter was observed only when GG genotype was compared with AA homozygotes, p = 0.06) (Table 1). No significant association between the FSHR −29G/A SNP and testicular volume and testosterone levels was found. We analyzed the effect of the SNP on four main seminal parameters (sperm concentration, total sperm count, progressive motility, morphology) in all men. No significant differences were found in any of these sperm parameters among the three genotypes (Table 1).
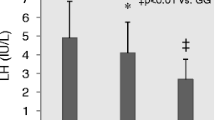
Subsequently, when the clinical-endocrine characteristics among the FSHR genotypes were compared separately in the patients with or without abnormal sperm parameters, we found that the FSHR genotypes resulted in different serum FSH and LH levels and BMI only in men with normozoospermia (Table 2). In particular, the −29 A-allele carrier groups (GA + AA group and AA group) had higher serum FSH and LH levels (p = 0.004 and p = 0.024, respectively) and BMI (p = 0.007) than those observed in the −29 A-allele non-carriers group (GG) (Table 2 and Fig. 1). No significant effect of the −29G/A SNP was detected on testicular volume and testosterone levels in both groups (Table 2). Likewise, no statistically significant association of the three FSHR genotypes with sperm parameters in either group was observed (Table 2).
Serum FSH (upper panels) and LH levels (lower panels) in men from group 2 stratified according to the FSHR −29A-allele non-carrier group (GG) and the A-allele carrier groups (GA + AA and AA). Medians are marked by thick black lines; boxes represent 25th and 75th percentiles; whiskers are lines extending from each box covering the extent of the data on 1.5 × interquartile range. Circles represent the outlier values
The frequency distribution of the FSHR SNP at nucleotide position −29 was 54.5% (n = 109) for GG genotype, 39% (n = 78) for GA genotype, and 6.5% (n = 13) for AA genotype. The FSHR −29 A-allele frequency was 26%. No deviation from Hardy Weinberg equilibrium was observed. The analysis for the three FSHR genotypes frequencies did not show any significant difference between men with alteration of conventional sperm parameters (GG 52.2, GA 41.1, and AA 6.7%) and men with normozoospermia (GG 57.5, GA 35.8, and AA 6.7%) (p = 0.649). In men with altered sperm parameters, the FSHR −29A-allele showed a similar frequency (27.3% or 49/180 chromosomes) than that detected in men with normozoospermia (24.53% or 52/212).
Discussion
Here, we report on the clinical implications of the FSHR −29 G/A SNP in 200 men with alteration of conventional sperm parameters or normozoospermia from Southern Italy. In the overall sample, we observed 42% higher serum FSH levels in men homozygous for the A allele compared to men with FSHR GG genotype. Interestingly, when the cohort of men was studied in more detail, a significant gradient of increasing FSH levels across the three FSHR genotypes was observed only in men with normozoospermia. To our knowledge, this is the second study on an European population to show a significant functional consequence of the FSHR −29G/A polymorphism on serum FSH levels in men. Previous studies have evaluated the −29 G/A polymorphism of the FSHR gene on German [13] and Italian [14] men failing to find any significant difference in serum FSH levels between men with different genotypes within each group of fertile men and infertile patients. Similar results were found in Turkish [15] and Chinese men [16]. Reduced testicular volume and slightly lower sperm count have been reported in −29 A-allele carriers from Estonia [18]. Only a very recent study on a Northern European population of Baltic young men and Estonian patients with oligozoospermia, reported an association between the −29 A-allele and increased serum FSH levels only in the first group of men [19]. In women, the clinical implications of −29G/A polymorphism are more clear than those in men. A higher frequency of the FSHR −29 AA genotype has been reported in Indian women with primary or secondary amenorrhea compared to normally cycling fertile women, and patients with primary amenorrhea with AA genotype had increased serum FSH levels [19].
The high FSH levels that we found in men with FSHR −29GA genotype and more consistently in men with FSHR −29AA genotype can be interpreted as a genotype-dependent FSHR gene expression. The receptor encoded from the FSHR AA variant has a lower transcriptional activity, on in vitro analysis, than the GG variant (Nakayama), resulting in significantly reduced expression of the FSHR gene at both mRNA and protein levels on granulosa cells from women undergoing ART [12]. Thus, the findings of the present study indicates the presence of a less active FSH receptor in men with FSHR GA and FSHR AA genotypes.
This is the first study showing serum LH levels to increase concomitantly with the number of A-alleles in men with normozoospermia. This finding suggests that an altered FSH pathway signaling, genetically determined by the presence of FSHR −29A allele, leads to an imbalance in the LH pathway. This may relate to a lower FSH signaling on the hypothalamic GnRH neuron resulting in a higher tone of this neuropeptide and consequently increased LH levels. Likewise, this is the first study showing a significant increasing gradient for higher BMI across the FSHR −29G/A genotypes (GG<GA<AA) in normozoospermic men. Further studies are needed to confirm this finding.
The FSHR −29G/A genotype and allelic distribution did not differ significantly between patients with alteration of conventional sperm parameters and normozoospermic men. These results are comparable with those of previous studies [13,14,15,16,17, 19] and with a meta-analysis, based on studies including 1249 cases and 1572 controls, that showed no significant association between the SNP and the risk of male infertility. All together these findings suggest that the FSHR −29G/A polymorphism alone cannot be considered a genetic risk factor for male infertility. Conversely, if we consider the haplotypes formed with the −29G/A SNP, some studies observed a significantly higher frequency of the A-allele in azoospermic patients compared to controls [12] and in infertile patients compared to proven fathers [14]. However, the evidence is inconsistent since other studies failed to confirm such findings [14, 16].
When the effect of the −29G/A FSHR polymorphism on the clinical-endocrine variables was analyzed separately in men with alteration of conventional sperm parameters and with normozoospermia, the strong serum FSH difference among the three FSHR genotypes was detected only in men with normozoospermia. No significant endocrine implication of the SNP was found in patients with altered sperm parameters suggesting that in men affected by infertility, as multifactorial disease, some mechanisms suppress or mask the influence of this genetic polymorphism on the endocrine variables investigated in this study. The absence of a clinical effect of the FSHR SNP on the patients with alteration of conventional sperm parameters also confirms that this SNP is not associated with male infertility, and since we have studied a thoroughly characterized population of men without known genetic causes of male infertility or endocrine disorders, we conclude that other factors, genetic and/or non-genetic, may be involved in their infertility. However, additional studies are also needed to understand whether in carriers of the FSHR −29A-allele normozoospermic men the reproductive lifespan is or not affected.
The reliable identification of genetic association is fundamental to achieve the results of genomic research to clinical application [20], and the ability to refine diagnostic categories will allow specific treatments [21]. In the light of our results, close attention should be paid to the studies of genetic variations associated with clinical-endocrine parameters that need to distinguish the comparison in the fertile or infertile men, categories, which in turn, should be well defined. Furthermore, the collaboration of researchers in data evaluation and synthesis of results across studies is necessary in the creation of a research network for the recognition of the most credible genetic associations.
In conclusion, this study provides a clear evidence of the endocrine implication of the FSHR −29G/A polymorphism in modulating serum FSH levels and, for the first time, serum LH levels in men. We speculate that this SNP is a genetic variant that contributes to the fine-tuning of the endocrine regulation of the male reproduction.
References
Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137:1447–56.
Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol. 2010;205(2):117–31.
Nieschlag E, Simoni M, Gromoll J, Weinbauer GF. Role of FSH in the regulation of spermatogenesis: clinical aspects. Clin Endocrinol. 1999;51(2):139–46.
Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22(6):764–86.
Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433(7023):269–77.
Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18(6):739–73.
Gromoll J, Dankbar B, Gudermann T. Characterization of the 5′ flanking region of the human follicle-stimulating hormone receptor gene. Mol Cell Endocrinol. 1994;102(1–2):93–102.
Simoni M, Nieschlag E, Gromoll J. Isoforms and single nucleotide polymorphisms of the FSH receptor gene: implications for human reproduction. Hum Reprod Update. 2002;8(5):413–21.
Nakayama T, Kuroi N, Sano M, Tabara Y, Katsuya T, Ogihara T, et al. Mutation of the follicle-stimulating hormone receptor gene 5′-untranslated region associated with female hypertension. Hypertension. 2006;48(3):512–8.
Wunsch A, Ahda Y, Banaz-Yasar F, Sonntag B, Nieschlag E, Simoni M, et al. Single-nucleotide polymorphisms in the promoter region influence the expression of the human follicle-stimulating hormone receptor. Fertil Steril. 2005;84(2):446–53.
Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod BioMed Online. 2009;18(4):509–15.
Desai SS, Achrekar SK, Pathak BR, Desai SK, Mangoli VS, Mangoli RV, et al. Follicle-stimulating hormone receptor polymorphism (G-29A) is associated with altered level of receptor expression in Granulosa cells. J Clin Endocrinol Metab. 2011;96(9):2805–12.
Ahda Y, Gromoll J, Wunsch A, Asatiani K, Zitzmann M, Nieschlag E, et al. Follicle-stimulating hormone receptor gene haplotype distribution in normozoospermic and azoospermic men. J Androl. 2005;26(4):494–9.
Pengo M, Ferlin A, Arredi B, Ganz F, Selice R, Garolla A, et al. FSH receptor gene polymorphisms in fertile and infertile Italian men. Reprod BioMed Online. 2006;13(6):795–800.
Balkan M, Gedik A, Akkoc H, Izci Ay O, Erdal ME, Isi H, et al. FSHR single nucleotide polymorphism frequencies in proven fathers and infertile men in Southeast Turkey. J Biomed Biotechnol. 2010;2010:640318.
Li Y, Gu A, Yang H, Ding X, Ji G, Lu C, et al. FSH receptor gene polymorphisms in fertile and infertile Han-Chinese males. Clinica chimica acta; international journal of clinical chemistry. 2011;412(11–12):1048–52.
Wu W, Cai H, Sun H, Lu J, Zhao D, Qin Y, et al. Follicle stimulating hormone receptor G-29A, 919A>G, 2039A>G polymorphism and the risk of male infertility: a meta-analysis. Gene. 2012;505(2):388–92.
Lend AK, Belousova A, Haller-Kikkatalo K, Punab M, Poolamets O, Peters M, et al. Follicle-stimulating hormone receptor gene haplotypes and male infertility in estonian population and meta-analysis. Syst Biol Reprod Med. 2010;56(1):84–90.
Grigorova M, Punab M, Punab AM, Poolamets O, Vihljajev V, Zilaitiene B, et al. Reproductive physiology in young men is cumulatively affected by FSH-action modulating genetic variants: FSHR −29G/A and c.2039 A/G, FSHB -211G/T. PLoS One. 2014;9(4):e94244.
Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in medicine : official journal of the American College of Medical Genetics. 2007;9(10):665–74.
Boccia S, Mc Kee M, Adany R, Boffetta P, Burton H, Cambon-Thomsen A, et al. Beyond public health genomics: proposals from an international working group. Eur J Pub Health. 2014;24(6):877–9.
Acknowledgments
We thank Dr. Luca Lo Nigro, Center of Pediatric Hematology Oncology, “G. Rodolico” Hospital, for allowing us the use of AbiPrism 310 Genetic Analizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving human participants were in accordance with the ethical standard of institutional research committee and with Helsinki declaration as revised in 2013. This is a clinical study that did not involved animal participants.
Informed consent
Informed consent of the present retrospective study was waived.
Disclosure statement
The authors have nothing to disclose
Rights and permissions
About this article
Cite this article
Tamburino, L., La Vignera, S., Tomaselli, V. et al. The −29G/A FSH receptor gene polymorphism is associated with higher FSH and LH levels in normozoospermic men. J Assist Reprod Genet 34, 1289–1294 (2017). https://doi.org/10.1007/s10815-017-0970-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0970-y