Abstract
Purpose
Assessment of sperm morphology has been reconsidered since 2001 with the development of motile sperm organelle morphology examination (MSOME). This observation technique that combines high magnification microscopy and the Nomarski interference contrast makes it possible to select spermatozoa with as few vacuoles as possible before microinjection into the oocyte (intracytoplasmic morphologically selected sperm injection, IMSI). More than 10 years after the development of IMSI, the indications of the IMSI technique and its ability to increase pregnancy and/or birthrates (compared with conventional ICSI) are still subject to debate. We aimed to better define the interest of IMSI in the third attempt.
Methods
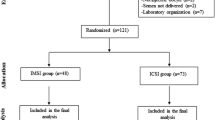
We assessed the benefit of IMSI by carrying out a retrospective comparative study between IMSI and conventional ICSI during a third ART attempt. Two hundred sixteen couples with two previous ICSI failures were studied between February 2010 and June 2014.
Results
IMSI did not significantly improve the clinical outcomes compared with ICSI, either for implantation (12 vs 10 %), clinical pregnancy (23 vs 21 %), or live birth rates (20 vs 19 %).
Conclusion
This study provides supplementary arguments for not achieving IMSI procedure in the third attempt after two previous ICSI failures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decade, higher resolution microscopy techniques have led to improved sperm observation. Since 2001 [1], a new technique called motile sperm organelle morphology examination (MSOME) involves the use of the Nomarski interference contrast microscopy at high magnification (at least ×6000). This technique revealed a new morphological criterion in human spermatozoa: the presence of nuclear vacuoles. Several studies have found increased levels of fragmented DNA in spermatozoa with large vacuoles [2–5], whereas others [6, 7] have shown abnormalities of chromatin condensation in such spermatozoa. Although the origin of these vacuoles raises many questions, some strong correlations have been established between the morphology of the spermatozoon, in particular the presence of large vacuoles, and its nuclear quality (degree of chromatin condensation and/or DNA integrity, chromosomal content) [8]. However, the high frequency of sperm head vacuoles in fertile men highlights the physiological nature of some of these vacuoles [9, 10]. The observation of sperm head vacuoles is used to select spermatozoa before microinjection: intracytoplasmic morphologically selected sperm injection (IMSI). A decade after the introduction of IMSI, the indications of the technique and its ability to increase pregnancy and/or birthrates (compared with conventional ICSI) are still subject to debate. A recent meta-analysis found no evidence of the value of IMSI in terms of either live birth or miscarriage rates [11], and so these findings did not support the use of IMSI in clinical practice. According to these authors, the use of IMSI for sperm selection was associated with an improvement in the clinical pregnancy rate. However, in view of the risk of bias in the studies included, imprecision and strong suspicion of publication bias, the authors concluded that this evidence was of low quality and therefore the estimated benefit was very uncertain [11]. Initial reports showed that IMSI was associated with higher pregnancy rates in couples with repeated ICSI failures [12, 13]. In the first prospective randomized trial [14], the clinical benefit was more significant in patients with two or more previous failed treatment attempts: IMSI resulted in significantly higher clinical pregnancy rates (29.9 vs 12.9 %; P < 0.05). If large vacuoles are linked to chromatin condensation defects, as has been demonstrated by many authors [6, 7, 15], more rigorous selection of the sperm cell (as few vacuoles as possible) could improve the quality of the sperm injected into the oocyte. In a previous randomized controlled trial, we reported that IMSI yielded no benefit during the first assisted reproductive technique (ART) attempt. However, we postulated that after two ICSI failures, the proportion of cases with a poor prognosis linked to sperm nuclear damage might be higher, and so we aimed to assess the value of IMSI in such cases. This retrospective study of 216 ART cycles aimed to provide supplementary data to better define the true indications of IMSI.
Materials and methods
Study design
This retrospective study included couples making their third ART attempt (after two previous ICSI failures) in the ART center of Hôpital Paule de Viguier, Toulouse University Hospital, France, between February 2010 and June 2014.
The initial inclusion criteria for the first ICSI attempt were the following:
-
1.
Male infertility with use of fresh ejaculated spermatozoa, whatever the sperm morphology and whatever the female status.
-
2.
Total failure to achieve fertilization with conventional IVF.
Only one microscope in our laboratory is equipped with an IMSI system (Nomarski interference contrast, total magnification ×6000) and only two of the five embryologists are trained in the IMSI technique. Patients undergoing a third attempt were therefore included in the IMSI group or in the ICSI group depending on the availability of the IMSI microscope and of a trained operator. The fertilization, implantation, and lysis rates of all the embryologists performing the ICSI technique are regularly assessed in the laboratory (every 2 months for fertilization and lysis rates). We found no significant difference in these rates throughout the duration of our study.
Conventional sperm parameter assessment before an ART attempt
Semen evaluation was performed according to standard WHO guidelines [16]. Sperm morphology was examined using the Kruger criteria [17]. Smears were air-dried for 10 min and then stained using the Diff-Quick procedure (Dade, Düdingen, Switzerland). An HTM-IVOS analyzer version 12.3 (Hamilton-Thorne Biosciences, Beverly, MA, USA) was used. The spermatozoon was identified and then analyzed by computerized software according to strict criteria [17, 18].
Semen preparation
Semen samples were collected by masturbation after 2–5 days of sexual abstinence and were processed for IVF after liquefaction for 15 to 60 min.
IMSI procedure
Preparation and selection of sperm for IMSI have previously been described [19]. An aliquot of the sperm preparation was placed in a glass-bottomed dish (WillCo-dish, WillCo Wells BV, Amsterdam, The Netherlands) and examined by the Nomarski interference contrast microscopy with a Leica DFC-280 camera (Leica Microsystems, Nanterre, France) mounted on a Leica DMI 6000 microscope with an immersion objective lens ×100 and camera magnification ×1. Spermatozoa with the smallest relative vacuole area were preferentially selected [6]. If available, spermatozoa without vacuoles or with only small vacuoles were preferentially injected.
Conventional ICSI procedure
Sperm selection for microinjection was performed at a magnification of ×400. Spermatozoa seen to have severe head defects at this magnification were excluded. The procedure of oocyte injection was the same in both the ICSI and the IMSI group and was performed at ×200 magnification using the Hoffman contrast.
Embryo culture
The injected oocytes were transferred to a four-well dish containing 50 μL of culture medium (G1Plus, Vitrolife, Göteborg, Sweden) overlaid with mineral oil (FertiCult Mineral Oil, Fertipro Belgium). The fertilization of the oocytes was checked the next day, 16–20 h after microinjection. Embryo quality was assessed on day 2 according to the Giorgetti classification system [20]. Embryos with a score of 3 or 4 have good morphology.
Statistical analysis
Implantation rate was defined as the ratio of the number of gestational sacs with fetal heart beat to the number of transferred embryos. Statistical analysis was performed using StatView software (Abacus Concepts Inc., Berkeley, CA). Percentages were compared by the chi-square test. Means were compared using the Student’s t test or the Mann–Whitney test according to the normality of data distribution. A P value lower than 0.05 was considered as statistically significant.
Results
Patient characteristics were similar in both groups in terms of female and male age, duration of infertility, and semen parameters (Table 1). Cycle parameters differed, with a greater number of metaphase II oocytes being retrieved in the IMSI group. As shown in Table 2, no statistically significant difference was observed between the two groups with regard to implantation rate (IMSI 12 %, ICSI 10 %, NS), ongoing pregnancy (IMSI 23 %, ICSI 21 %, NS), or delivery rate (IMSI 20 %, ICSI 19 %, NS). However, the fertilization rate was significantly lower in the IMSI group (54 ± 24 vs. 61 ± 26, P < 0.05) with no difference in the number of total embryos obtained, since there was a significantly higher number of mature oocytes in the IMSI group (IMSI 8.1 ± 3.6 %, ICSI 6.9 ± 3.1, P < 0.01). We assumed that there was no operator bias because the fertilization rate between the various operators (data not shown) did not differ significantly (see Materials and methods).
The data of the previous ICSI attempts in the two groups are summarized in Table 3. Except for the total number of mature oocytes, which was significantly higher in the IMSI group (15.1 ± 6.2 vs 13.4 ± 5.0, P < 0.05), we found no other statistically significant differences between the two groups.
Discussion
Although we are well aware of the limitations of our design (non-randomized trial), our study showed that the IMSI technique provided no real benefit over conventional ICSI in the case of a third ART attempt. We found no significant differences regarding live births (19 % with ICSI, 20 % with IMSI). Similarly, no statistical difference was demonstrated for implantation, miscarriage, or clinical pregnancy rate. It should be noted that previous studies evaluating IMSI differed significantly in terms of sperm classification and definition of normal spermatozoa. For some authors, it appeared clear that IMSI was not better than ICSI when used in the first treatment attempt [19, 21, 22]. However, in one randomized study, the benefit of IMSI was especially marked after at least two ICSI failures [14]. This first randomized controlled trial, comparing 227 IMSI attempts with 219 ICSI attempts (at an unspecified magnification) revealed a significantly higher clinical pregnancy rate in the IMSI than in the ICSI group (39 vs 27 %, P = 0.004) and also in the group with two previous ICSI failures (29.9 % in the IMSI vs 12.9 % in the ICSI group, P = 0.017). The advantage of IMSI after repeated IVF-ICSI failure has been investigated in several studies (Table 4). Our results are in agreement with a prospective but non-randomized study which compared IMSI and ICSI outcomes in patients with more than two ICSI failures [25]. These authors found no statistical difference in terms of clinical pregnancy rate and live birth rate per cycle, although it must be borne in mind that the mean number of previous ICSI attempts was significantly greater in the IMSI group than in the ICSI group.
As in a previous study [19], the fertilization rate obtained using the IMSI technique was significantly lower than with conventional ICSI. Even if we checked that this result was not explained by any difference in skill between the two embryologists who performed IMSI compared with the three other embryologists (data not shown), a limitation of our study is that only two of the five embryologists are trained in the IMSI technique. To explain the difference in the fertilization rate, we had previously hypothesized the importance of the duration of gamete handling. It is important to note that we use two dishes for microinjection: one for sperm selection at ×100 immersion objective and one for oocyte injection at ×20 dry objective. This procedure could thus increase the duration of gamete handling, and the time required for the technique could possibly have been decreased by using a ×63 dry objective or a ×20 immersion objective. Considering the fertilization rates, none of the published data revealed an advantage of IMSI over ICSI.
We found no difference in embryo morphology. Except for two studies [23, 28], IMSI does not improve the morphology of early embryos [14, 19, 21, 22, 29]. However, an increase of development at the blastocyst stage using IMSI has been demonstrated by some authors [28, 30, 31], although randomization at the oocyte level did not confirm this finding [22].
Studies comparing IMSI with ICSI lead to contradictory conclusions. These discrepancies could be explained mainly by two reasons:
-
1.
The way in which conventional ICSI is performed: accuracy of sperm selection and particularly the magnification used: ×200 or ×400 (some abnormalities that are not visible at ×200 might be detected at magnification ×400), and so the lack of superiority of IMSI over ICSI should not discredit the sperm selection.
-
2.
The characteristics of the male population studied: [21, 32] demonstrated a significant increase in implantation rates using IMSI in the male infertility subgroup. Moreover, the benefit of IMSI was enhanced in the case of severe morphological alterations [8, 25, 33]. However, in our previous study we found no benefit of IMSI even in cases of severe teratozoospermia [19].
In conclusion, our results suggest that IMSI does not improve clinical outcomes in couples with two previous ICSI failures. More prospective randomized studies should be performed in order to confirm these results.
References
Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med. 2001;345:1067–8.
Franco Jr JG, Baruffi RL, Mauri AL, Petersen CG, Oliveira JB, Vagnini L. Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod Biomed Online. 2008;17:42–5.
Oliveira JB, Massaro FC, Baruffi RL, Mauri AL, Petersen CG, Silva LF, et al. Correlation between semen analysis by motile sperm organelle morphology examination and sperm DNA damage. Fertil Steril. 2010;94:1937–40.
Boughali H. Intérêt de l’analyse de la morphologie fine et de la qualité nucléaire des spermatozoïdes dans le cadre des techniques d’AMP. Andrologie. 2006;16:38–45.
Garolla A, Fortini D, Menegazzo M, De Toni L, Nicoletti V, Moretti A, et al. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod Biomed Online. 2008;17:610–6.
Perdrix A, Travers A, Chelli MH, Escalier D, Do Rego JL, Milazzo JP, et al. Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod. 2011;26:47–58.
Boitrelle F, Ferfouri F, Petit JM, Segretain D, Tourain C, Bergere M, et al. Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprints linked to failure of chromatin condensation. Hum Reprod. 2011;26:1650–8.
Perdrix A, Rives N. Motile sperm organelle morphology examination (MSOME) and sperm head vacuoles: state of the art in 2013. Hum Reprod Update. 2013;19:527–41.
Tanaka A, Nagayoshi M, Tanaka I, Kusunoki H. Human sperm head vacuoles are physiological structures formed during the sperm development and maturation process. Fertil Steril. 2012;98:315–20.
Gatimel N, Leandri RD, Marino L, Esquerre-Lamare C, Parinaud J. Sperm vacuoles cannot help to differentiate fertile men from infertile men with normal sperm parameter values. Hum Reprod. 2014;29:2359–67.
Teixeira DM, Barbosa MA, Ferriani RA, Navarro PA, Raine-Fenning N, Nastri CO, et al. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database Syst Rev. 2013;7, CD010167.
Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J Androl. 2002;23:1–8.
Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, et al. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 2003;80:1413–9.
Antinori M, Licata E, Dani G, Cerusico F, Versaci C, d’Angelo D, et al. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reprod Biomed Online. 2008;16:835–41.
Franco Jr JG, Mauri AL, Petersen CG, Massaro FC, Silva LF, Felipe V, et al. Large nuclear vacuoles are indicative of abnormal chromatin packaging in human spermatozoa. Int J Androl. 2012;35:46–51.
WHO. WHO laboratory manual for the examination of human semen and sperm-cervical mucus and interaction. 5th ed. Cambridge: World Health Organisation; 2010.
Kruger TF, du Toit TC, Franken DR, Menkveld R, Lombard CJ. Sperm morphology: assessing the agreement between the manual method (strict criteria) and the sperm morphology analyzer IVOS. Fertil Steril. 1995;63:134–41.
Marnet B, Vieitez G, Milhet P, Richoilley G, Lesourd F, Parinaud J. Computer-assisted assessment of sperm morphology: comparison with conventional techniques. Int J Androl. 2000;23:22–8.
Leandri RD, Gachet A, Pfeffer J, Celebi C, Rives N, Carre-Pigeon F, et al. Is intracytoplasmic morphologically selected sperm injection (IMSI) beneficial in the first ART cycle? A multicentric randomized controlled trial. Andrology. 2013.
Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al. Embryo score to predict implantation after in vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–31.
Balaban B, Yakin K, Alatas C, Oktem O, Isiklar A, Urman B. Clinical outcome of intracytoplasmic injection of spermatozoa morphologically selected under high magnification: a prospective randomized study. Reprod Biomed Online. 2011;22:472–6.
De Vos A, Van de Velde H, Bocken G, Eylenbosch G, Franceus N, Meersdom G, et al. Does intracytoplasmic morphologically selected sperm injection improve embryo development? A randomized sibling-oocyte study. Hum Reprod. 2013;28:617–26.
Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, et al. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online. 2006;12:634–8.
Knez K, Zorn B, Tomazevic T, Vrtacnik-Bokal E, Virant-Klun I. The IMSI procedure improves poor embryo development in the same infertile couples with poor semen quality: a comparative prospective randomized study. Reprod Biol Endocrinol. 2011;9:123.
El Khattabi L, Dupont C, Sermondade N, Hugues JN, Poncelet C, Porcher R, et al. Is intracytoplasmic morphologically selected sperm injection effective in patients with infertility related to teratozoospermia or repeated implantation failure? Fertil Steril. 2013;100:62–8.
Klement AH, Koren-Morag N, Itsykson P, Berkovitz A. Intracytoplasmic morphologically selected sperm injection versus intracytoplasmic sperm injection: a step toward a clinical algorithm. Fertil Steril. 2013;99:1290–3.
Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, et al. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online. 2006;12:634–8.
Setti AS, Braga DP, Figueira RC, Iaconelli Jr A, Borges Jr E. The predictive value of high-magnification sperm morphology examination on ICSI outcomes in the presence of oocyte dysmorphisms. J Assist Reprod Genet. 2012;29:1241–7.
Mauri AL, Petersen CG, Oliveira JB, Massaro FC, Baruffi RL, Franco Jr JG. Comparison of day 2 embryo quality after conventional ICSI versus intracytoplasmic morphologically selected sperm injection (IMSI) using sibling oocytes. Eur J Obstet Gynecol Reprod Biol. 2010;150:42–6.
Cassuto NG, Bouret D, Plouchart JM, Jellad S, Vanderzwalmen P, Balet R, et al. A new real-time morphology classification for human spermatozoa: a link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–25.
Vanderzwalmen P, Hiemer A, Rubner P, Bach M, Neyer A, Stecher A, et al. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod Biomed Online. 2008;17:617–27.
Setti AS, Figueira Rde C, Braga DP, Iaconelli Jr A, Borges Jr E. Intracytoplasmic morphologically selected sperm injection benefits for patients with oligoasthenozoospermia according to the 2010 World Health Organization reference values. Fertil Steril. 2011;95:2711–4.
Knez K, Tomazevic T, Zorn B, Vrtacnik-Bokal E, Virant-Klun I. Intracytoplasmic morphologically selected sperm injection improves development and quality of preimplantation embryos in teratozoospermia patients. Reprod Biomed Online. 2012;25:168–79.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Comité d’Ethique de la Recherche des Hôpitaux de Toulouse. For this type of study formal consent is not required.
Conflict of interest
The authors declare that they have no competing interests..
Additional information
Capsule
This study provides supplementary arguments for not achieving IMSI procedure in the third attempt after two previous ICSI failures.
Rights and permissions
About this article
Cite this article
Gatimel, N., Parinaud, J. & Leandri, R.D. Intracytoplasmic morphologically selected sperm injection (IMSI) does not improve outcome in patients with two successive IVF-ICSI failures. J Assist Reprod Genet 33, 349–355 (2016). https://doi.org/10.1007/s10815-015-0645-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0645-5