Abstract
Objective
To evaluate the development potential and clinical significance of day 3 4-cell embryos after extended in vitro culture.
Methods
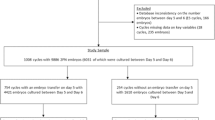
This study was a retrospective cohort study for patients with infertility treatment between January 2011 and July 2013. Patients undergoing blastocyst culture in controlled ovarian hyperstimulation cycles using surplus embryos were analyzed in the study. A total of 764 women undergoing blastocyst culture with 1,522 surplus 4-cell embryos on day 3 were analyzed. An additional 2,391 patients with embryos undergoing blastocyst culture during the same period with embryos having more blastomeres were chosen as control.
Results
After extended culture, 253 embryos from 183 cycles in the study group which developed to blastocysts were frozen, and 118 embryos were warmed in subsequent frozen embryo transfer cycles. Implantation rates, clinical pregnancy rates (PRs) and ongoing PRs were 33.3 %, 38.4 % and 31.4 %, respectively, which were similar to those of the control group. The singleton birth weights of newborns using these blastocysts showed no significant difference to that seen in the control group.
Conclusion
Surplus 4-cell embryos on day 3 displayed lower blastulation rates. However, once a blastocyst is obtained, it has equivalent clinical outcomes. Embryos that are developmentally lagging on day 3 can be observed in extended culture to increase the cumulative chances of a successful pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is generally accepted that embryo quality strongly affects the subsequent pregnancy rate. Embryos are widely evaluated using a scoring system based on cell number, fragmentation, and other morphological criteria [1]. In comparison to other criteria, day 3 cell number has been found to be the most important predictor of pregnancy. Embryos with optimal implantation potential on day 3 are characterized as having seven or more blastomeres, <20 % embryo fragmentation, and absence of multinucleated blastomeres [2]. It has been reported that, in cycles when embryos with more blastomeres are unavailable, with day 3 transfer of 4-cell embryos, the chance of a live birth is dramatically decreased [3].
Developmentally lagging 4-cell embryos on day 3 have been assumed to be a sign of poor quality and deemed to be of limited value for future use. Some laboratories with strict inclusion criteria might be discarding surplus embryos that do not reach 5 blastomeres on day 3 [4]. In recent years, extended embryo culture techniques have been widely used that allow embryos to be observed for more than 3 days after fertilization [5–7]. Based on the development of this culture technique, embryos that are developmentally slower on day 3 can be cultured for additional days to observe whether they have the competency to reach the blastocyst stage. Although hundreds of articles have been published on embryo morphology and blastulation potential, scarce information has been obtained on the blastulation rates from day 3 4-cell embryos and subsequent clinical outcomes in frozen embryo transfer cycles [8–11].
The objective of this study was to assess the clinical outcomes of cryopreserved blastocysts developed from day 3 4-cell embryos, to determine if they may substantially increase the cumulative chances of a successful pregnancy.
Materials and methods
This retrospective cohort study was designed to compare implantation and pregnancy rates in blastocysts originating from day 3 4-cell embryos to those in blastocysts originating from day 3 embryos with more than 4 cells. The study was approved by the Ethics Committee of Peking University Third Hospital. All data were collected from patients treated in a private infertility practice in the Reproductive Medical Center of Peking University Third Hospital. Cycles with surplus embryos undergoing blastocyst culture after day 3 transfer, between January 2011 and December 2012, were analyzed. As embryos are usually cultured in group in our IVF laboratory, patients undergoing blastocyst culture with 4-cell embryos only were selected for inclusion in the study. Patients undergoing blastocyst culture during the same period with embryos having more blastomeres were chosen as control. Women underwent controlled ovarian hyperstimulation (COH) with a GnRH agonist or GnRH antagonist protocol. Embryos that were biopsied for preimplantation genetic diagnosis or screening and embryos resulting from a donor egg were excluded.
Ovarian follicle growth was monitored with the use of transvaginal ultrasonography and measurements of serum estradiol (E2) levels. When at least one follicle reached a mean diameter of 18 mm, with an estradiol concentration exceeding 500 pg/ml, a dose of 10,000 IU of human chorionic gonadotropin (hCG; Serono, Aubonne, Switzerland) was administered intramuscularly. Ultrasound-guided oocyte retrieval was performed 36 h after hCG injection. Luteal progesterone supplementation was initiated on the day after oocyte retrieval using 60 mg progesterone (Xianju Pharmacy, Zhejiang, China).
All frozen embryo transfer cycles conducted between February 2011 and July 2013 were examined. Endometrial preparation in frozen embryo transfer cycles were achieved with either natural monitored cycles or programmed artificial cycles [12]. Natural cycles were performed for patients with regular ovulatory menstrual cycles. Thawed blastocyst transfers were scheduled for 5 days after ovulation. Luteal support was provided with intramuscular injections of progesterone (Shanghai General Pharmaceutical Company, China). In cycles treated with hormone replacement therapy, endometrial development was achieved with oral E2. When serum E2 concentrations and endometrial thickness were suitable, administration of progesterone in oil was initiated. The blastocyst transfers were performed on day 6 after the initiation of the progesterone treatment. Hormone replacement therapy with estrogen and progesterone was continued until the pregnancy test. The serum hCG levels were measured 12 days after the transfer.
Laboratory protocols
The following three commercially available culture media were used equally: G5™ (Vitrolife, Gottenburg, Sweden); Global (IVF Online, Toronto, Canada) and Quinn’s advantage medium (SAGE, CA, USA). The corresponding sera supplemented the media: HSA solution™ (Vitrolife); HSA solution (IVF Online) and Quinn’s advantage SPS (SAGE). Mineral oil (Sigma, St. Louis, MO, USA) was used after washing and sterile filtration.
IVF and ICSI were performed according to the laboratory’s routine insemination procedures 2–4 h after oocyte retrieval. Fertilization assessment was performed 16–18 h after insemination or injection. The zygotes were then cultured in 25 μl of pre-equilibrated cleavage medium droplets. The embryos were cultured in incubators at 37 °C with 5 or 6 % CO2 [13]. The morphology of embryos was evaluated on day 3 after insemination with respect to cell number, fragmentation and symmetry. The observations were performed at 8:30 AM to 9:30 AM on day 3.
The best embryos were identified using standard grading criteria and used for transfer. The number of embryos transferred was determined based on the age of the patient, and the number of prior failed IVF cycles. After day 3 transfer, embryos were cryopreserved if the patient had >5 usable cleavage stage embryos. If the surplus embryo number was <5 or the embryos were deemed to be unsuitable for freezing on that day (with over 20 % fragmentation), they were cultured further. Embryos that were considered completely fragmented (with over 50 % fragmentation) or terminally arrested (with less than 4 cells on day 3 or at morula stage on day 6) were discarded.
Embryos undergoing blastocyst culture were placed into blastocyst media on day 3. Morphological assessment was carried out on days 5, 6 and 7. Embryos that developed to blastocyst stage were scored according to the criteria used by Gardner and Schoolcraft [14]. Blastocysts that had reached grade 3BB or better quality were vitrified for future use and defined as usable blastocysts.
Vitrified blastocysts were exposed to 7.5 % ethylene glycol (EG) + 7.5 % dimethylsulfoxide (DMSO), then to 15 % EG + 15 % DMSO + 0.65 M sucrose. Quinn’s advantage medium with HEPES was used as the base vitrification medium. Blastocysts were warmed in a stepwise manner (0.33 M and 0.2 M sucrose and Quinn’s advantage medium with HEPES supplemented with 20 % HSA). Vitrified and warmed blastocysts were transferred in either natural or hormonally supplemented cycles. Clinical pregnancy was defined as the detection of a gestational sac on ultrasound examination on day 35 after transfer. Ongoing pregnancy was defined as a pregnancy that progressed beyond the first trimester. Implantation rate was calculated as the number of gestational sacs per number of embryos transferred.
The statistical analyses were performed using SPSS 16.0 (SPSS Inc.). For group differences, the level of statistical significance was set at p < 0.05. Data are presented as the means ± standard deviation (SD). For the comparison of the mean values of quantitative variables, the one-way analysis of variance (ANOVA) was used. Chi-square test analysis was adopted for comparison of the rates between two groups. Due to small number, the Fisher's exact test was used to compare miscarriage rates, preterm birth rates and abnormal birthweight rates.
Results
General information
We retrospectively analyzed 764 IVF-ET cycles during which 1,522 surplus 4-cell embryos underwent extended culture. These were compared to a group of embryos with higher cell numbers (18 % 5-cell embryos, 71 % 6–8 cell embryos and 11 % >8 cell embryos) also undergoing extended culture. In the control group, blastocyst culture were performed from 2391 IVF-ET cycles with 5,934 embryos. The average age of women in the study group at the time of oocyte retrieval was 33.56 years which was statistically significant older than the control group. The mean numbers of oocytes from the two groups were 11.01 and 10.67 per retrieval with no statistical difference (Table 1).
In vitro development
Blastulation rates were significantly lower in the study cycles than in the controls. Usable blastocysts were also significantly reduced in the study group compared with the control group: 253 (16.6 %) of the study group and 1,915 (32.3 %) of the control group. During the course of the study, 183 (24.0 %) and 1,183 (49.5 %) cycles finally achieved cryopreservation in the study and the control groups, respectively (Table 2).
Clinical outcomes
Until July 2013, 118 blastocysts from 91 cycles of the study group and 674 blastocysts from 466 cycles of the control group were thawed with the intention of transfer. There were no significant differences in blastocyst survival rates between the study group and the control group. The clinical pregnancy rate and implantation rate were 38.4 % and 33.3 % in the study group, respectively, which were similar to those of the control group. The ongoing PRs were 31.4 % and 36.2 % for the study group and the control group, respectively. No statistical significance was found between the two groups (Table 3).
Birth outcomes
To evaluate the safety of blastocysts from 4-cell embryos, the birth outcomes of these patients were investigated. There were no significant differences in the mean gestational age of two groups. Among the 25 women who delivered singletons in the study group, the mean birth weight was 3218g. Among the 135 women who delivered singletons in control group, the mean birth weight was 3345g. No adverse outcome was observed in singletons born after transfer of vitrified blastocysts from the two groups. Due to the small number in twins born, data were not compared with the two groups (Table 4).
Discussion
The present study demonstrates that the developmentally lagging 4-cell embryos on day 3 had a blastocyst formation rate of 31.7 %, which was significantly lower than that of embryos with more blastomeres on day 3. However, the pregnancy rate, implantation rates and the ongoing pregnancy rates from cryopreserved blastocysts developed from day 3 4-cell embryos were similar to that of those from other cryopreserved blastocysts. Our data demonstrate that while 4-cell embryos may not blastulate as often as those with more cells on day 3, that once a blastocyst is obtained, it has equivalent clinical outcomes.
Previous studies [15, 16] have demonstrated that embryo-scoring systems at the cleavage stage have a limited ability to predict the developmental potential of a blastocyst. Sjoblom et al. [17] illustrated that when they cultured embryos that were classified as unsuitable for freezing or transfer on day 3, they still obtained a number of good quality blastocysts. Hardarson et al. [18] also reported that good quality blastocysts can develop from embryos classified as suboptimal. Furthermore, Guerif et al. [19] revealed that morphological assessment at days 1 and 2 represents a fairly low prediction of embryo viability. These studies suggest that surplus embryos that might become normal blastocysts are sometimes discarded during IVF treatment. Widely used embryo scoring systems consider three characteristics, including cell number, fragmentation and the general appearance of the blastomeres. Among them, the cleavage rate is believed to be a major determinant of development [8].
Our study focused on the developmental potential of slowly developed 4-cell embryos which were generally believed as suboptimal and even useless on day 3. As embryos with no fragmentation to moderate fragmentation were cultured in group until the blastocyst stage in our IVF laboratory, other parameters including fragmentation and blastomeres size were not estimated in this study.
Until now, the reason for the slow cleavage of day 3 4-cell embryos has not been fully explained. Maternal age may influence the cleavage rates. Janny and Menezo [20] demonstrated a clear declination in the quality of human embryos arising from aging oocytes. Our data also indicate an increasing maternal age of patients in the study group. But as both average ages in the study and control groups were considered young, this may not lead to a clinical significant difference. Reports also showed that qualities of either oocytes or sperm may affect the cell divisions in the human preimplantation embryo. Embryo development can be compromised by deficiencies in sperm-derived developmentally relevant cytoplasmic factors, oocyte activating substances and centriole [21, 22].
Furthermore, the significantly lower rate of blastocyst formation in the 4-cell group may be due to an increase in chromosomal anomalies. Many studies have shown that the chromosomal status of embryo may affect the embryo cleavage rate and morphology [18, 23, 24]. Most of the researchers used fluorescence in situ hybridization (FISH) techniques and found a higher incidence of chromosomal abnormality of cleavage-stage embryos with poor morphology. However, FISH has a number of recognized limitations which might influence the interpretations of the findings. Recent years, comparative genomic hybridization (CGH) were widely used to the study of the full karyotype of blastomeres. Dekel-Naftali et al. [25] combined FISH and CGH techniques found that high levels of chromosome abnormalities were observed in embryos at early development stages, while towards the blastocyst stage, the proportion of euploid embryos rises. Fragouli et al. [26] showed that chromosome abnormalities were common even amongst embryos with the best morphology at day 3. However, at the blastocyst stage abnormalities were overrepresented amongst embryos considered to be of poor morphology. Other research based on trophectoderm biopsy to perform chromosomal analysis has shown higher aneuploidy rates in slower developing blastocysts [27]. These studies demonstrate that there is an association between chromosomal abnormality and embryo development and that there is progressive loss of chromosomally abnormal embryos during preimplantation development [25].
Due to the low division rate of day 3 4-cell embryos, embryo selection may be fulfilled by culturing the embryos for a prolonged period. During this process, a sizeable number of unsuitable embryos will arrest and potentially viable embryos will be frozen at the blastocyst stage for further frozen embryo transfer cycles.
In conclusion, our findings indicate that surplus 4-cell embryos on day 3 have clinically important potential. During IVF treatment, these later developing preimplantation embryos can be observed for an extended period of in vitro culture. Transfer of vitrified and warmed blastocysts developed from day 3 4-cell embryos may increase the cumulative chances of a successful pregnancy.
References
Scott L. The biological basis of non-invasive strategies for selection of human oocytes and embryos. Hum Reprod Update. 2003;9(3):237–49.
Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15(10):2190–6.
Ertzeid G, Storeng R, Tanbo T, Dale PO, Bjercke S, Abyholm T. Cycle characteristics of day 3 embryo transfers with 4-cell embryos only. J Assist Reprod Genet. 2003;20(9):352–7.
Westergaard LG, Mao Q, Krogslund M, Sandrini S, Lenz S, Grinsted J. Acupuncture on the day of embryo transfer significantly improves the reproductive outcome in infertile women: a prospective, randomized trial. Fertil Steril. 2006;85(5):1341–6.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–11.
Gardner DK, Vella P, Lane M, Wagley L, Shlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69(1):84–8.
Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7, CD002118.
Bolton VN, Hawes SM, Taylor CT, Parsons JH. Development of spare human preimplantation embryos in vitro: an analysis of the correlations among gross morphology, cleavage rates, and development to the blastocyst. J In Vitro Fert Embryo Transf. 1989;6(1):30–5.
Lan KC, Huang FJ, Lin YC, Kung FT, Hsieh CH, Huang HW, et al. The predictive value of using a combined Z-score and day 3 embryo morphology score in the assessment of embryo survival on day 5. Hum Reprod. 2003;18(6):1299–306.
Muggleton-Harris AL, Glazier AM, Wall M. A retrospective analysis of the in-vitro development of 'spare' human in-vitro fertilization preimplantation embryos using 'in-house' prepared medium and 'Medi-Cult' commercial medium. Hum Reprod. 1995;10(11):2976–84.
Neuber E, Rinaudo P, Trimarchi JR, Sakkas D. Sequential assessment of individually cultured human embryos as an indicator of subsequent good quality blastocyst development. Hum Reprod. 2003;18(6):1307–12.
Liu Q, Lian Y, Huang J, Ren X, Li M, Lin S, et al. The safety of long-term cryopreservation on slow-frozen early cleavage human embryos. J Assist Reprod Genet. 2014;31(4):471–5.
Lin S, Li M, Lian Y, Chen L, Liu P. No effect of embryo culture media on birthweight and length of newborns. Hum Reprod. 2013;28(7):1762–7.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8.
Graham J, Han T, Porter R, Levy M, Stillman R, Tucker MJ. Day 3 morphology is a poor predictor of blastocyst quality in extended culture. Fertil Steril. 2000;74(3):495–7.
Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13(10):2869–73.
Sjoblom P, Menezes J, Cummins L, Mathiyalagan B, Costello MF. Prediction of embryo developmental potential and pregnancy based on early stage morphological characteristics. Fertil Steril. 2006;86(4):848–61.
Hardarson T, Caisander G, Sjogren A, Hanson C, Hamberger L, Lundin K. A morphological and chromosomal study of blastocysts developing from morphologically suboptimal human pre-embryos compared with control blastocysts. Hum Reprod. 2003;18(2):399–407.
Guerif F, Le Gouge A, Giraudeau B, Poindron J, Bidault R, Gasnier O, et al. Limited value of morphological assessment at days 1 and 2 to predict blastocyst development potential: a prospective study based on 4042 embryos. Hum Reprod. 2007;22(7):1973–81.
Janny L, Menezo YJ. Maternal age effect on early human embryonic development and blastocyst formation. Mol Reprod Dev. 1996;45(1):31–7.
Tesarik J, Mendoza C, Greco E. Paternal effects acting during the first cell cycle of human preimplantation development after ICSI. Hum Reprod. 2002;17(1):184–9.
Tesarik J. Paternal effects on cell division in the human preimplantation embryo. Reprod Biomed Online. 2005;10(3):370–5.
Kovalevsky G, Carney SM, Morrison LS, Boylan CF, Neithardt AB, Feinberg RF. Should embryos developing to blastocysts on day 7 be cryopreserved and transferred: an analysis of pregnancy and implantation rates. Fertil Steril. 2013;100(4):1008–12.
Almeida PA, Bolton VN. The relationship between chromosomal abnormality in the human preimplantation embryo and development in vitro. Reprod Fertil Dev. 1996;8(2):235–41.
Dekel-Naftali M, Aviram-Goldring A, Litmanovitch T, Shamash J, Yonath H, Hourvitz A, et al. Chromosomal integrity of human preimplantation embryos at different days post fertilization. J Assist Reprod Genet. 2013;30(5):633–48.
Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20(2):117–26.
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft W, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–4.
Acknowledgments
The authors thank Ms. Lixue Chen who is the senior statistician in our department for her kindly help with data analyses.
The authors thank Natural Sciences Foundation of China (Grant Nos. 30900512 and 810705340) for supporting this work.
Conflict of interest
None declared
Authors’ roles
P.L. conceived and designed the study; P.Z., M.L.,Y.L. and X.Z. coordinated data collection; P.Z. and P.L. analyzed and interpreted the data; P.Z. wrote the paper; J.Q. and P.L. revised the manuscript critically. All authors interpreted the data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Surplus 4-cell embryos on day 3 displayed lower blastulation rates. Transfer of cryopreserved blastocysts developed from day 3 4-cell embryos results in equivalent clinical outcomes to that of the other blastocysts.
Rights and permissions
About this article
Cite this article
Zhao, P., Li, M., Lian, Y. et al. The clinical outcomes of day 3 4-cell embryos after extended in vitro culture. J Assist Reprod Genet 32, 55–60 (2015). https://doi.org/10.1007/s10815-014-0361-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0361-6