Abstract
Polyhydroxybutyrate (PHB) is a potential biopolymer and can replace petroleum-based plastic. PHB accumulated by bacteria needs a supply of carbon source whereas cyanobacteria are capable of synthesizing PHB by carbon fixation using sunlight energy. Since PHB is an intracellular product the polymer recovery cost is about 50 % of production cost. Apart from these, cyanobacterial pigments also affect the purity and recovery yield. In the present study, Chlorogloea fritschii TISTR 8527 was chosen as a PHB cell factory which accumulated 28 % of biopolymer. The hypochlorite-chloroform dispersion method could remove the pigments along with PHB extraction with 95.51 ± 3.164 % of the recovery in a single step. In addition to this, PHB extraction was performed with various halogenated solvents and green non-halogenated solvents with hypochlorite pretreatment. Selected solvents were unable to recover more than 90 % of polymer yield due to unsuitable operating conditions. Besides this, the green solvent dimethyl carbonate (DMC) was employed for PHB recovery with prior pigment removal from biomass using chilled methanol. DMC was capable of 83.34 ± 0.76 % biopolymer recovery with a purity of 75 %. PHB recovered using DMC has improved thermal and material characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global plastic production was 368 million tonnes in 2019 and is expected to double in the next two decades. Plastic is an inexpensive, nonbiodegradable, hydrophobic material that consumers may utilize in their daily routine. Plastic films are strong, impermeable to gas and moisture, and found in packaging applications. Due to a lack of infrastructure and process expenses, only 9 % of plastic packaging products are recycled in the USA despite their durability and intactness (Lebreton and Andrady 2019). Approximately 53 t of plastic waste will be entering the aquatic ecosystem by 2030 which affect the food chain and influence human health (Borrelle et al. 2020; Castro-Castellon et al. 2022). Apart from this, plastic processing generates greenhouse gases (GHGs) which contribute to global warming. The solution to these problems is the usage of bioplastics or biodegradable plastics which are functionally similar to polyethylene.
Bioplastics may be biodegradable or partially degradable and include polymers such as polycaprolactone, polybutylene succinate, polylactic acid (PLA), starch, and cellulose-based plastics. The degradation of bioplastics depends on their composition, degree of crystallinity, and environmental factors (Tokiwa et al. 2009). Polyhydroxyalkanoates (PHAs) are esters that can be synthesized from renewable sources: plants and heterotrophic bacteria. Polyhydroxybutyrate is a short chain length PHA made of hydroxybutyric acid with a molecular weight of 105 - 106 Da. PHB is isotactic and hence biodegradable (Wölfle et al. 2009). PHB has a high solubility in organic solvents such as chloroform, dichloromethane, and dichloroethane. Heterotrophic bacteria such as Alcaligenes eutrophus, recombinant Escherichia coli, and Bacillus megaterium need a sterile source of glucose for PHB synthesis (Ahn et al. 2000; Yang et al. 2010; Mohanrasu et al. 2020). Cyanobacteria can also produce PHB by CO2 fixation using sunlight with minimal nutrients under nitrogen or phosphate limitations (Lee et al. 2022; Yashavanth and Maiti 2024). The thermoplastic, biocompatible, biodegradable, and non-immunogenic nature of PHB eases its use in the form of surgical mesh and surgical sutures, ocular devices, bone plating systems, cardiovascular patches, bone marrow scaffolds, tendon, and cartilage repair devices (Chen and Wu 2005; Patnaik 2007). The antimicrobial nature of PHB means it is used in aquaculture in preventing Vibrio epidemics (Defoirdt et al. 2007). PHB hydrogels play an important role in targeted drug delivery and sustained release of drugs (Fang et al. 2023). In the skin healing process, methyl groups of PHB help in the adhesion of cells by promoting extracellular matrix (Sangsanoh et al. 2017; Castellano et al. 2018). Applications of PHB-based supercapacitors in consumer and industrial electronics, agriculture, and environmental monitoring are numerous (Migliorini et al. 2020). Thus, various fields demand good quality polymer for its end use.
PHB has to be recovered and purified by various methods such as solvent extraction, surfactants, non-PHB cellular material (NPCM) removal using hypochlorite, dispersion of hypochlorite and chloroform, and enzymatic digestion (Yashavanth et al. 2021). Recovering PHB from cytoplasm increases production cost by up to 50 % of the cost of the polymer since PHB has to be purified in multiple steps (Samorì et al. 2015b). The molecular weight of the recovered polymer may decrease because of NPCM digestion with hypochlorite. Hypochlorite and chloroform dispersion can decrease the loss in molecular weight of recovered PHB while raising the yield and purity (Hahn et al. 1994). Solvent recovery is the most used method by industries due to its high recovery efficiency resulting in polymer of high molecular weight along with the removal of endotoxins (Lee et al. 1999).
Cyanobacteria contain pigments that affect the material properties of PHB after extraction. Therefore, pigment removal from cyanobacteria is essential before PHB recovery with a minimum number of solvents and steps involved (Troschl et al. 2017). Methanol can be used for pigment removal at 4 °C before PHB extraction (Gopi et al. 2014; Zavřel et al. 2015). The combination of hypochlorite and chloroform may result in higher recovery with pigment removal, but both are toxic to humans and the environment. Therefore, the selection of solvents based on safety and reusability has significance in the evaluation of the extraction process (Mongili et al. 2021). Dimethyl carbonate (DMC) is a solvent of industrial importance and is used as a reagent in various processes. DMC is considered a green solvent since it is biodegradable and less toxic to humans and the environment (Delledonne et al. 2001) and could be used as an alternative to halogenated solvents (Mongili et al. 2021). Previously, DMC was used for the extraction of homopolymer PHB and copolymer poly-hydroxybutyrate-hydroxyvalerate (PHBHV) from single strains and mixed consortia (Samorì et al. 2015a, 2015b; Elhami et al. 2022).
The present study focuses on the recovery of PHB from the cyanobacterium Chlorogloea fritschii TISTR 8527 using various extraction methods. PHB was extracted with pigment removal using the dispersion method. Dispersion involves a two-phase extraction procedure using hypochlorite (for biomass digestion) and chloroform (solvent for PHB extraction). Various halogenated such as chloroform and dichloroethane (DCE) and non-halogenated solvents namely propylene carbonate, dioxolane, DMC, and acetic acid were selected for PHB recovery based on the capabilities of solvents to dissolve PHB after hypochlorite pretreatment (Terada and Marchessault 1999). A suitable solvent is selected based on performance and environmental concerns. DMC is employed as a green solvent in the current investigation to recover PHB after pigment removal from cyanobacteria using methanol. The selection of a suitable biomass-to-solvent ratio, extraction temperature, and mixing time are critical factors as they affect the recovery yield. Once the PHB is extracted from the biomass, it is recovered by evaporation of the solvent. Comparison of thermal properties of recovered PHB are shown to decide the most suitable method based on polymer characteristics.
Materials and methods
Conditions for PHB production
Chlorogloea fritschii TISTR 8527 was procured from the Thailand Institute of Scientific and Technological Research, Thailand. The inoculum was prepared in a 1 L flask containing 500 mL of modified BG-11 medium under cool light illumination of 100 µmol photons m-2 s-1 (Monshupanee et al. 2016) with a 12 h:12 h light-dark period. The composition of normal BG-11 medium is 40 mg L-1 K2HPO4, 1.5 g L-1 NaNO3, 36 mg L-1 CaCl2.2H2O, 75 mg L-1 MgSO4.7H2O, 6 mg L-1 citric acid, 6 mg L-1 ferric ammonium citrate, 20 mg L-1 Na2CO3, 1 mg L-1 Na2EDTA and 1 × TRACE. The composition of TRACE is as follows (1000×): 2.86 g L-1 H3BO3, 0.222 g L-1 ZnSO4.7H2O, 1.81 g L-1 MnCl2.4H2O, 0.39 g L-1 Na2MoO4.2H2O, 0.049 g L-1 Co(NO3)2.6H2O and 0.079 g L-1 CuSO4.5H2O (Rippka et al. 1979). For modified BG-11 medium citric acid was replaced with 20 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)-NaOH (HEPES–NaOH) and ferric ammonium citrate was avoided by usage of equimolar concentration of ferric chloride hexahydrate with final pH of 7.5 (Monshupanee et al. 2016). The mixing of inoculum was aided by a silicone rubber sparger with 1 % CO2 and 1 vvm aeration. For experimentation, 1.8 L of optimized BG-11 medium with pH 7.5 was used (Yashavanth and Maiti 2024). The optimized BG-11 resulted from multi-objective media optimization of modified BG-11 media (Yashavanth and Maiti 2024). The composition of optimized BG-11 medium used for experimentation was 0.1 g L-1 NaNO3, 26.24 mg L-1 K2HPO4, 750 mg L-1 MgSO4.7H2O, 36 mg L-1 CaCl2.2H2O, 20 mM HEPES, 6 mg L-1 FeCl3.6H2O, 7.11 mg L-1 Na2EDTA, 20 mg L-1 Na2CO3 and 10× TRACE. The modified BG-11 and optimized BG-11 differ in concentrations of five components namely NaNO3, K2HPO4, MgSO4.7H2O, Na2EDTA and TRACE. An inoculum of 5 % (v/v) was added to the production medium to achieve an initial biomass concentration of 0.05 g L-1. The experiment was performed with diurnal simulated sunlight in a flat-panel photobioreactor (PBR) (Labfors, Infors HT, 25.5 × 2 × 50 cm) with 3 % CO2 (v/v) and 0.5 vvm air. The high cell-density biomass pre-grown under autotrophic mode was subjected to biopolymer production under the dark with 0.2 % (w/v) acetate as an inducer at the stationary phase (Yashavanth and Maiti 2024). The sampling was done at regular intervals to measure the dry cell weight (DCW) after centrifugation at 10000 rpm. The biomass harvested by centrifugation was freeze-dried and used for recovery of stored PHB (Farinacci and Laurent 2023).
PHB quantification by gas chromatography
PHB content of the lyophilized biomass was quantified by gas chromatography (GC) using a Varian 450-GC equipped with CP-Sil 8 CB (30 m x 0.25 mm i.d. 0.25 µm film thickness) column with 0.7 mL min-1 helium as carrier gas with a split ratio of 100. The sample was prepared by the propanolysis method (Riis and Mai 1988) and analyzed using the thermal program by Juengert et al. (2018). The percentage of PHB present in the dried biomass was calculated using Eq. (1). Similarly, the recovered PHB was quantified by GC to calculate the percentage recovery and PHB purity using Eqs. (2) and (3).
Extraction of intracellular PHB
PHB recovery by single-step hypochlorite - chloroform dispersion method
The commercially available hypochlorite (4 w/v %) was from Himedia and diluted to different concentrations (10, 20, 30, 50, 70 and 100 % (v/v)). 1 % (w/v) of lyophilized biomass (i.e., 10 mg of biomass per mL of solvent) was subjected to PHB recovery using the dispersion method with equal volumes of hypochlorite and chloroform at 30 °C for 1 h. After the dispersion the mixture was centrifuged to separate the layers i.e., the upper layer contains NPCM, the middle layer contains cell debris, and the bottom chloroform layer contains PHB. The lower bottom phase was collected to concentrate the biopolymer by evaporation of the solvent (Hahn et al. 1995) and the percentage recovery was calculated by using Eq. (2) after quantification by GC.
PHB recovery using two-step method using hypochlorite pretreatment followed by extraction with different solvents
Solvent extraction is a widely used method in the recovery of PHB due to the efficient recovery of PHB with integrity of the polymer characteristics. Various solvents used for our study were chloroform (boiling point: 61.2 °C), dichloroethane (84 °C), propylene carbonate (242 °C), acetic acid (118 °C), dioxolane (74 °C) and dimethyl carbonate (90 °C). Previous studies showed that the above solvents resulted in higher PHB recovery (Terada and Marchessault 1999). In the present study, 200 mg of lyophilized biomass was pretreated with 20 mL of 4 % (w/v) hypochlorite (Himedia) for 15 min at room temperature (Kanzariya et al. 2023). The pellet was collected by centrifugation after discarding the supernatant. PHB was recovered from the pellet at 100 °C for 1 h with the biomass-to-solvent ratio of 1 % (w/v) for the above-mentioned solvents. After extraction, the suspension was centrifuged to collect the solvent phase. The polymer was recovered by evaporation of solvents and the percentage recovery and purity of the polymer were calculated using Eqs. (2) and (3) after quantification by GC.
PHB recovery by two-step method using pigment removal by methanol followed by extraction with green solvent dimethyl carbonate (DMC)
Effect of biomass loading on PHB recovery by DMC
Lyophilized biomass was used for the extraction of PHB using a green solvent DMC. First the freeze-dried biomass was subjected to pigment removal using chilled methanol at 4 °C overnight in 50 mL Falcons tubes. The biomass used for extraction varied at different concentrations. Lyophilized biomass of 50, 100, 150, and 200 mg were suspended in 4 mL of DMC to get biomass concentrations of 12.5, 25, 37.5, and 50 g L-1. The extraction was performed at 90 °C while mixing at regular intervals till 4 h. After extraction, the mixture was centrifuged at 4696 ×g for 10 min to collect the DMC containing PHB. The solvent evaporated to recover the polymer (Samorì et al. 2015b; Mongili et al. 2021). The recovery percentage was calculated using Eq, (2).
Effect of pigment removal on PHB recovery by DMC
The biomass-to-methanol ratio played an important role as pigments interfere with the recovery of biopolymer. The biomass in methanol used for pigment removal was 0.1, 0.25, 0.5, and 1 % (w/v). Biomass of 100 mg was suspended in chilled methanol of different volumes (10, 20, 40, and 100 mL) to get the above concentrations. After the incubation at 4 °C, biomass was separated by centrifugation at 8000 rpm for 10 min (Ansari and Fatma 2016). Then the pellet was suspended in 4 mL of DMC for PHB extraction at 90 °C with mixing at regular intervals up to 4 h. Then the mixture was centrifuged at 4696 ×g for 10 min to collect the DMC containing PHB. The solvent was evaporated to recover the polymer (Samorì et al. 2015b; Mongili et al. 2021). The recovery percentage was calculated as before using Eq. (2).
Effect of incubation time on PHB recovery
To optimize the time required for extraction of PHB, 100 mg of biomass per 4 mL of DMC was selected after pigment removal with 0.5 % (w/v) and 0.25 % (w/v) biomass in methanol. The experiment was performed at different time points from 2, 4, 6, and 8 h (Samorì et al. 2015b; Mongili et al. 2021). The polymer was recovered by evaporation of DMC and the percentage recovery and purity of the polymer were calculated using Eqs. (2) and (3) after quantification by GC.
Fourier transformed infrared spectroscopy
A potassium bromide pellet was prepared for 2 mg of standard PHB (Sigma-Aldrich) and recovered PHB. Infrared spectra (IR) were recorded using a Perkin Elmer Fourier Transformed Infrared (FTIR) spectrometer with a spectral range of 3600 – 800 cm-1 at 25 °C (Ansari and Fatma 2016).
Thermogravimetric analysis
The thermal stability of the extracted polymer of 10 mg was studied by thermogravimetric analysis (TGA) from 25 - 700 °C under the argon atmosphere (20 mL min-1) at a heating rate of 10 °C min-1 using NETZSCH STA 449 F3 Jupiter. The decomposition temperature such as T5 (5 % weight loss) and T10 (10 % weight loss) was determined using the TGA curve and maximum decomposition temperature (Tmax) was determined by DTG analysis (Ansari and Fatma 2016).
Differential scanning calorimetry
Differential Scanning Calorimetry (DSC) analysis was done using the NETZSCH instrument with 5 mg of sample in an aluminium pan under a nitrogen environment. The sample was heated to a temperature of -30 to 200 °C, held isothermally at 200 °C for 3 min, and cooled to -30 °C at a rate of 10 °C min-1. Similarly, a second cycle was performed (Ansari and Fatma 2016).
The degree of crystallinity \({X}_{c} (\%)\) was calculated from the Eq. (4).
where \({\Delta H}_{f}\) is the melting enthalpy of the recovered PHB (J g-1), and \({\Delta H}_{{f}_{0}}\) is the theoretical melting enthalpy of the 100 % crystalline PHB which is assumed to be 146.6 J g-1.
Powder X-Ray diffraction
X-ray diffractograms (XRD) of the standard and sample were obtained using Smartlab 9KW Powder X-Ray Diffraction System (Rigaku Technologies, Japan) for 2θ in the range of 10 – 80 °C using Cu-Kα radiation (λ = 1.54 A°). The peaks were analyzed for the crystalline nature of the recovered biopolymer (Devi et al. 2015).
Gel permeation chromatography
The molecular weight of recovered PHB was determined using gel permeation chromatography (GPC) (PL-GPC 220 High-Temperature Chromatograph, Agilent Technologies) at 40 °C with a refractive index detector. Tetrahydrofuran, HPLC grade (stabilized) was used as the mobile phase with a flow rate of 1 mL min-1. PHB was dissolved in chloroform at a concentration of 1 mg mL-1 and a 200 µL sample was injected using an autosampler to PLgel Mixed B column (10 µm, 300 × 7.5 mm) with a runtime of 25 min. Mass standards of Polystyrene in the range of 1.6 x 102 – 6.5 × 106 g mol-1 were used to calibrate the system before sample injection (Lackner et al. 2019).
Mechanical properties
The solvent casting method was used to prepare PHB films to study the tensile strength and elongation at break using a 5kN Universal Testing Machine (UTM) (Zwick Roell, Z005TN). The recovered polymer was dissolved in chloroform while stirring at 60 °C for 20 min. The solution was poured into the glass Petri dish. The films resulting from the evaporation of solvent were cut into rectangular shapes (Supplementary file) and subjected to load at a rate of 1 mm min-1 (Ansari and Fatma 2016). The tensile strength (MPa) and elongation at break (%) were calculated by the stress-strain curve.
Statistical analysis
All experiments were conducted in duplicates with triplicate sampling measurements and average results were reported with standard deviation. One-way ANOVA was performed followed by Tukey’s post hoc tests using the OriginPro 2021 software (OriginLab). A p-value of < 0.05 was considered statistically significant.
Results
PHB production from C. fritschii TISTR 8527
Chlorogloea fritschii was cultivated in flat-panel PBR using an optimized BG-11 medium with 3 % CO2 under diurnal light. The initial cell density of C. fritschii was 0.05 g L-1. After 7 days of cultivation, the culture reached the stationary phase with a DCW of 1.084 g L-1. PHB was induced in the dark with 0.2 % acetate (w/v) after 7 days. PHB of 28 ± 0.08 % was obtained with DCW of 1.3 ± 0.02 g L-1 after 4 days of induction under dark. The biomass with a polymer content of 28 % was collected by centrifugation, freeze-dried, and used for recovery of intracellular PHB by various methods. Previously C. fritschii TISTR 8527 has been reported to produce 31.78 % of polymer using optimized BG-11 medium with 1 % CO2 under diurnal light (Yashavanth and Maiti 2024). An increase in CO2 may reduce the carbon stress required for PHB accumulation.
PHB extraction by single-step hypochlorite-chloroform dispersion method
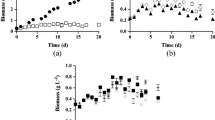
Firstly, the lyophilized biomass was subjected to the hypochlorite-chloroform dispersion method. It was observed that the percentage recovery of biopolymer increased with an increase in the hypochlorite percentage used for cell lysis. Use of 10 % (v/v) hypochlorite was inefficient in cell lysis and resulted in lower PHB recovery of 4.44 ± 0.574 % (w/w). Gradual increase in hypochlorite concentrations from 20 to 70 % (v/v) led to the improvement in PHB recovery from 42.88 ± 4.09 to 90.50 ± 3.26 % (w/w). Direct use of commercial hypochlorite from Himedia (100 % v/v or 4 % w/v) with an equal volume of chloroform resulted in the recovery of 95.51 ± 3.164 % PHB (Fig. 1a). The PHB recovery was found to be significant with increase in hypochlorite concentrations (p < 0.05).
PHB recovery with hypochlorite for cell lysis. (a) PHB recovery (%) from C. fritschii using hypochlorite-chloroform dispersion method with an increase in hypochlorite concentrations (v/v %), and (b) PHB recovery (%) from C. fritschii using solvent extraction with different halogenated and non-halogenated solvents after hypochlorite pretreatment. Different letters indicate significant difference between the groups with p ≤ 0.05
PHB recovery by two-step method using hypochlorite pretreatment followed by extraction with different solvents
Various organic solvents used for our study were chloroform, dichloroethane (DCE), propylene carbonate, acetic acid, dioxolane, and dimethyl carbonate (DMC). These solvents were screened for recovery of PHB at 100 °C for 1 h from C. fritschii after pretreatment of 200 mg of lyophilized biomass with 4 % (w/v) hypochlorite for 15 min at room temperature. The recovered polymer content varied with different solvents employed. is shown in Fig. 1b. The maximum recovery of 74.79 % was achieved with green solvent DMC after digestion of biomass with hypochlorite which aided in cell lysis along with pigment removal. Apart from DMC, chloroform also resulted in PHB recovery of 73.1 % but at high temperatures chloroform is highly volatile and continuous exposure to the fumes by is carcinogenic and chloroform is not eco-friendly (Sekar et al. 2023). Hence, DMC was selected for further experiments based on its capability to solubilize PHB along with health and environmental concerns (Mongili et al. 2021).
PHB recovery by two-step method using pigment removal by methanol followed by extraction with green solvent DMC
Being an industrially important solvent, DMC recovered 19.62 % PHB directly from C. fritschii biomass without the removal of pigments (data not shown). Pigments were also extracted with PHB when the biomass was heated with DMC. This resulted in a lower recovery of polymer. Hence the biomass was subjected to pigment removal using chilled methanol followed by PHB extraction with DMC. The % recovery is shown in Fig. 2a. It was found that 100 mg biomass per 4 mL DMC i.e. 25 g L-1 biomass resulted in maximum recovery of 53.52 % polymer in biomass variation experiment with an incubation period of 4 h. The decrease in the % recovery with an increase in the biomass was due to the limitation of solvent. Therefore 25 g L-1 of biomass in DMC was selected for further studies (Fig. 2a).
PHB recovery (%) from C. fritschii using DMC with pigment removal with chilled methanol. (a) Effect of biomass amount for PHB recovery using DMC, (b) Effect of pigment removal by varying biomass to methanol ratio on PHB recovery by DMC, (c) Effect of contact time on PHB recovery with 0.5 % (w/v) biomass to methanol ratio for pigment removal, and (d) Effect of contact time on PHB recovery with 0.25 % (w/v) biomass to methanol ratio for pigment removal. Different letters indicate significant difference between the groups with p ≤ 0.05
The biomass-to-methanol ratio was varied from 1, 0.5, 0.25, and 0.1 % (w/v) to study the effect of pigment removal on PHB recovery. After pigment removal, the pelleted biomass was subjected to PHB recovery with 100 mg of biomass per 4 mL of DMC (i.e. 25 g L-1) for 4 h at 90 ºC. Percentage recoveries of PHB was 9.98 ± 0.83, 53.63 ± 1.38, 81.88 ± 0.91, and 82.93 ± 0.37 %, respectively (Fig. 2b). Still there was scope to enhance the percentage recovery by increasing the contact time of biomass with DMC. Hence 0.5 % w/v biomass in methanol was selected for pigment removal followed by extraction with DMC resulted in the polymer recoveries of 10.77 ± 0.28, 54.42 ± 1.19, 68.87 ± 0.125, and 42.28 ± 1.11 % with an incubation times of 2, 4, 6, and 8 h respectively (Fig. 2c). Using 0.25 % w/v biomass in methanol for pigment removal and PHB recovery using DMC with 2, 4, 6, and 8 h contact time gave recovery yields of 22.97 ± 0.18, 83.34 ± 0.76, 62.2 ± 1.59 and 57.17 ± 0.54 %, respectively (Fig. 2d). PHB recovery using DMC with considering biomass variation, effect of biomass to methanol for pigment removal on PHB recovery, and contact time studies on PHB recovery with 0.25 % and 0.5 % biomass to methanol were statistically significant with p < 0.05 (Fig. 2c and d).
Characterization of biopolymer
Fourier Transformed Infrared Spectroscopy
PHBs from both methods were subjected to FTIR analysis by the pellet method. The FTIR spectra of PHB from both methods were similar. The -CH group was represented by 2930 to 2975 cm-1. The characteristic peak at 1728 cm-1 represents the -C=O group. The bands from 1058 -1281 cm-1 represented the C-O-C stretching vibration. The FTIR peaks of the standard and samples (Supplementary Fig. S1 and Table S1) were similar. These results were in agreement with previous studies (Ansari and Fatma 2016; Pradhan et al. 2018).
Thermogravimetric analysis
PHB derived from the hypochlorite-chloroform dispersion method and DMC method were subjected to thermal degradation under an inert atmosphere. Initial mass loss corresponds to the loss of solvents adsorbed to the film (i.e. chloroform and DMC). The standard PHB was degraded in a single step since it is highly pure. PHB derived from both methods was degraded in two steps. The loss is due to the random chain scission by β-elimination reaction and conversion of cleaved chains to crotonic acid or oligomers of crotonic acid (Ariffin et al. 2008). The former showed 49.6 % degradation in the first phase, followed by 37 % degradation in the second phase with a residual mass of 12.5 %. Later one showed a mass loss of 33.8 % in the first step and 56.2 % in the second step with a residual of 10.8 % (Fig. 3a). The characteristic decomposition temperatures i.e., temperature at 5 % (T5) and temperature at 10 % weight loss (T10) were determined using TGA curve (Fig. 3a). The maximum decomposition temperature (Tmax) was determined by the derivative of thermogravimetry (DTG) curve (Fig. 3b). The T5, T10, and Tmax of PHB recovered using the hypochlorite-chloroform dispersion method were 232.7, 238, and 250 °C, respectively, whereas T5, T10, and Tmax of PHB recovered using DMC are 261.6, 274.2, and 289 °C, respectively (Fig. 3a and b). The standard PHB from Sigma-Aldrich showed T5, T10, and Tmax of 270, 274, and 285 °C, respectively (Table 1). The characteristic decomposition temperatures of PHB recovered using DMC matched that of standard PHB.
Thermograms of PHB recovered from C. fritschii under inert atmosphere. (a) Comparison of TGA curve of hypochlorite-chloroform dispersion-based PHB and DMC recovered PHB with standard PHB (Sigma-Aldrich). The TGA curve depicts the step-wise degradation of PHB with a temperature range of 25 – 700 °C. (b) Comparison of DTG curve of hypochlorite-chloroform dispersion-based PHB and DMC recovered PHB with standard PHB (Sigma-Aldrich). The DTG curve depicts the rate of degradation and maximum degradation temperature of the polymer
Differential scanning calorimetry
The thermal and calorimetric properties of PHB recovered from the hypochlorite-chloroform dispersion method and DMC method were studied using Differential Scanning Calorimetry (DSC). The thermograms were used to determine glass transition temperature (Tg), melting temperature (Tm), crystallization temperature (Tc), and degree of crystallinity (Xc). The first heating scan (-30 °C to 200 °C) was used to eliminate the prior thermal history of the polymer. The Tc was calculated from the cooling cycle from 200 °C to -30 °C.
The point of inflection during the second heating cycle is referred to as glass transition temperature (Tg). The Tg for standard, DMC recovered PHB and PHB recovered from the dispersion method was 4, 4.3, and 4.6 °C, respectively (Fig. 4a and Table 2). PHB from Nostoc muscorum has a Tg of 6 °C (Ansari and Fatma 2016). A previous study showed that PHB from C. fritschii TISTR 8527 had a Tg of 3.2 °C (Monshupanee et al. 2016). The melting points (Tm) for dispersion-based PHB and DMC-recovered PHB were 169 and 174 °C, respectively, close to the Tm of the standard (174 °C) (Fig. 4a and Table 2). In an earlier study with the same strain, a Tm of 171.6 °C was reported for PHB recovered by chloroform (Monshupanee et al. 2016). The crystallization temperatures (Tc) of PHB recovered from both methods determined from the cooling curve and were 48.4 °C (DMC recovered PHB) and 48.2 °C (dispersion), respectively, which were less than Tc of the standard (84 °C) (Fig. 4b and Table 2). This was due to the lower degree of crystallinity (Xc) of 38.4 % and 35.9 %, respectively. The crystallinity (Xc) of the standard from Sigma-Aldrich is 60.3 % (Table 2). It was found that PHB recovered with chloroform from the same strain had a reported crystallinity of 45 % in previous findings (Monshupanee et al. 2016).
Differential Scanning Calorimetric (DSC) curve of PHB recovered from C. fritschii by hypochlorite-chloroform dispersion and DMC-based recovery with comparison to standard PHB (Sigma-Aldrich) for analysis of thermal properties such as glass transition temperature (Tg), melting temperature (Tm), and crystallization temperature (Tc). (a) Heating curve and (b) Cooling curve
Powder X-ray diffraction
X-ray diffractograms of standard, PHB recovered from the hypochlorite-chloroform dispersion method and DMC method were obtained using Cu-α X-ray (λ = 1.54 Aº) in the range of 2θ = 10-80 º. Six prominent peaks were observed at 2θ: 13.6, 17, 21.6, 22.7, 25.69, and 27.3°. These peaks correspond to different planes (020, 110, 101, 111, 121, and 040) of orthorhombic unit cells of PHB crystal. Peaks at 2θ = 21.6° and 2θ = 22.7° indicated that the tested polymer is α-PHB crystal (Sun et al. 2011). The peaks at 2θ = 25.69° and 2θ = 27.3° indicated that the PHB from C. fritschii is of partial crystalline nature (Fig. 5). The % crystallinity (Xc) was calculated from analysis of XRD peaks. The Xc of PHB recovered using the hypochlorite-chloroform dispersion method and DMC from C. fritschii were 56.45 ± 1.81 and 56.84 ± 1.56 % , respectively (Table 2). The Xc of standard PHB is 63.33 ± 4.80 % and is in agreement with Anbukarasu et al. (2015). From the above analysis, it can be concluded that PHB from C. fritschii is semi-crystalline in nature.
Determination of molecular weight by gel permeation chromatography (GPC)
The weight average molecular weight (Mw) and number average molecular weight (Mn) was determined using GPC. The hypochlorite-chloroform dispersion-based PHB had a Mw of 319784 Da and Mn of 150268, whereas DMC recovered PHB had Mw of 440067 and Mn of 155963. The Mw and Mn of standard PHB (Sigma-Aldrich) were 570885 and 216638 Da, respectively. The polydispersity index (PDI) of PHB recovered using both methods agreed with that of the standard (Table 2). Monshupanee et al. (2016) reported a Mw of 545000 Da of PHB recovered with chloroform as an extraction solvent from the same strain in previous studies.
Mechanical properties of PHB
Mechanical properties were studied to characterize the biopolymer films (Supplementary Fig. S2). The tensile strength (σ) and elongation at break (ε) of PHB recovered using hypochlorite-chloroform dispersion were 11.11 ± 2.92 MPa and 5.13 ± 2.66 %. Similarly, PHB recovered using DMC resulted in tensile strength and elongation at break of 11.57 ± 0.35 MPa and 6.42 ± 1.04 %, respectively. The standard PHB has a tensile strength of 20.47 ± 1.70 MPa and elongation at break value of 6.39 ± 1.34 % (Fig. 6 and Table 2).
Discussion
Chlorogloea fritschii TISTR 8527 could produce 28 % PHB in flat panel PBR under diurnal light with single-stage cultivation using optimized BG-11 medium and 3 % CO2. Previously C. fritschii produced PHB of 31.67 % under the same condition except 1 % CO2 was utilized (Yashavanth and Maiti 2024). The lyophilized biomass containing PHB was subjected to PHB recovery by the single-step hypochlorite-chloroform dispersion method. The dispersion method resulted in a maximum recovery of 95.51 % PHB with direct usage of an equal volume of commercial hypochlorite and chloroform. Hahn et al. (1994) also showed that an increase in the hypochlorite concentration increases the percentage recovery of the polymer up to 91 % with a polymer purity of 97 %. Solvent extraction of PHB with hypochlorite pretreatment was performed with solvents such as chloroform, DCE, propylene carbonate, acetic acid, dioxolane, and DMC. Generally, extraction of PHB using solvent depends on the solubility of PHB and the combination of the boiling point of the solvent and operating temperature. In the current extraction process, the operating temperature was 100 °C. The boiling points of propylene carbonate and acetic acid are 242 °C and 118 °C respectively which were much higher than the operating temperature. The boiling points of chloroform, DCE, dioxolane, and DMC are were lower than the operating temperature. Therefore, the PHB recovery was lesser with propylene carbonate and acetic acid (Aramvash et al. 2018). According to the Hansen solubility parameter the distance of DMC from the center of solubility radius of PHB (RPHB = 8.5) is lowest (5.12) compared to other solvents used in this study (Terada and Marchessault 1999). As the solubility of PHB in DMC is very high and the operating temperature is close to the boiling point, the recovery using DMC is higher. When Cupriavidus necator biomass was subjected to PHB recovery using propylene carbonate at 100 °C for 45 min it resulted in a percentage recovery of 45 % (Fiorese et al. 2009). In the present study, a low operating temperature of 100 °C resulted in PHB recovery of 29.2 % using propylene carbonate in C. fritschii. Aramvash et al. (2018) showed that acetic acid can recover the PHB to 36.71 % with 97 % purity using C. necator. In the current study, acetic acid demonstrated a similar recovery of 34.9 % from C. fritschii with a PHB purity of 90 %. Previously, Samori et al. (2015b) reported PHB recovery of 49 % from mixed microbial culture (MMC) using DMC along with purity of 98 % without biomass pretreatment. Hypochlorite pretreatment of MMC up to 15 min resulted in PHB recovery of 62 % with 98 % polymer purity. In the present study, DMC could recover PHB from C. fritschii with a high recovery yield of 74.79 % after hypochlorite pretreatment with a polymer purity of 80 %. The lower purity of the recovered polymer may be due to polymer recovery using solvent evaporation. Dioxolane was capable of PHB recovery of 60 % from C. necator at 100 °C for 4 h (Yabueng and Napathorn 2018). However, in this work dioxolane resulted in a recovery of 48.62 % of PHB which was due to lower contact time (1 h) of solvent with biomass at 100 °C. Chloroform and DCE recovered PHB of 73.1 % and 51.61 % at 100 °C after hypochlorite pretreatment. Chloroform and DCE are well known for good recovery of the polymer due to the high solubility of PHB (Hahn et al. 1995; Terada and Marchessault 1999; Monshupanee et al. 2016) but are not environment and human-friendly in nature (Sekar et al. 2023). Hence, DMC was selected as a green solvent for PHB recovery due to its high solubility for PHB (Terada and Marchessault 1999).
Chlorogloea fritschii biomass resulted in PHB recovery of 63.87 ± 0.13 % and 83.34 ± 0.76 % with biomass-to-methanol ratios of 0.5 % and 0.25 % (w/v), respectively. Previously C. necator biomass was used and DMC resulted in a recovery yield of 88 % with a purity of 95 % (Samorì et al. 2015b). It is interesting to note that in the current work with C. fritschii, pigment removal employing a biomass-to-methanol ratio of 0.25 % (w/v) achieved a maximum recovery of 83.34 % of PHB and purity of 75 % with the same incubation period of 4 h. DMC, being an industrially important solvent, could be employed for PHB recovery from various cyanobacteria. PHB recovered from C. fritschii using dispersion and DMC was characterized using FTIR, TGA, DSC, and tensile strength studies. The characteristic FTIR peaks of PHB from C. fritschii matched with the PHB recovered from Nostoc muscorum NCCU- 442 (Ansari and Fatma 2016). In a study with Nostoc muscorum, a Tmax of 284 °C was reported (Ansari and Fatma 2016) which is close to the Tmax of PHB recovered from C. fritschii using DMC (289 °C) in the current study. Other fossil-based biopolymers such as PLA have a Tmax of 366 ºC which is higher than the Tmax of PHB (285 ºC) (Arrieta et al. 2014). Previously, PHB recovered from Nostoc muscorum had a Tm of 171 °C (Ansari and Fatma 2016) which is close to the Tm of PHB recovered from C. fritschii using dispersion and DMC in the present study. Chemical synthesis of PHB by ring open polymerization of racemic diolide (rac-DL) resulted in complete isotactic PHB with Tm of 170.7 ºC (Tang and Chen 2018). DSC data of PHB from C. fritschii was also compared with fossil-based polymers such as polypropylene (PP) and polyethylene terephthalate (PET) (Table 2). Among the fossil-based polymers, PET was highly amorphous with a high melting temperature of 260 ºC (McAdam et al. 2020). PLA had a higher Tg of 60.4 °C whereas it was highly amorphous in nature with Xc of 5.1 %. Irrespective of crystallinity the solubility parameter (δ) of PLA (19.5-20.5 MPa1/2) was close to that of PHB (18.5 - 20.1 MPa1/2), which aided the blending of both materials in the manufacturing of food packaging material while enhancing the crystallinity of packing material (Arrieta et al. 2014). Apart from these high concentrations of amorphous PHB has been blended with PAZO to form a photonic material while enhancing the miscibility of materials with different solubility parameters (Sharma et al. 2005). Hence DSC helps in manufacturing materials of interest with enhanced solubility along with improved crystallinity. XRD data of PHB from C. fritschii was similar to the PHB obtained from C. necator, Bacillus megaterium, and Bacillus cereus (Devi et al. 2015; Pradhan et al. 2018). X-ray diffractograms also suggest that PHB from C. fritschii was semi-crystalline in nature. Monshupanee et al. (2016) reported Mw of 545000 in C. fritschii with extraction using a methanol-chloroform combination (Monshupanee et al. 2016). In the current study, PHB from C. fritschii has been reported with Mw of 319784 by hypochlorite-chloroform dispersion method and Mw of 440067 by DMC extraction method. As the extraction reported by Monshupanee et al. (2016) and current PHB extraction conditions are different the Mw can be affected. In a study of PHB recovery from Alcaligenes eutrophus by the hypochlorite-chloroform dispersion method, Mw has reduced drastically due to severe degradation of PHB molecules (Hahn et al. 1994). In the current study, similar phenomena have been observed with PHB recovered by dispersion. But Mw of PHB with DMC solvent has not decreased that much. Hence, with the same species molecular weight of PHB can be different (Mw and Mn) based on recovery conditions. PHB which was isotactic in nature and synthesized by ring opening polymerization of rac-DL had a PDI of 1.01 with Mn of 154000 (Tang and Chen 2018). Even though the chemical synthesis of PHB resulted in an isotactic PHB but had a lower molecular weight than PHB from biological origin (Table 2). In conclusion, the molecular weight of the polymer depends on the selected species and upstream and downstream conditions that affect material properties.
The mechanical properties of PHB recovered from C. fritschii depend on its molecular weight. In a study of PHB production with Zobellela tiwanensis using banana peel as a feed source, tensile strength of 10.344 MPa was reported with hypochlorite digestion followed by solvent extraction (Maity et al. 2020). The tensile strength of PHB from C. fritschii was well agreed with the polymer of Z. tiwanensis. Even though higher crystallinity was present, the low strength of recovered polymer films may be caused by the loose bonding of polymer chains and eased mobility at lower molecular weights due to weak interactions.
Conclusion
In the current work, PHB recovery from the cyanobacterium C. fritschii was performed with different methods. The dispersion method and solvent extraction were the main focus. The dispersion method achieved 95.51 % PHB recovery along with pigment removal. Various solvents showed lower recovery of PHB due to unsuitable operating conditions for PHB recovery. Among such solvents, the selection of a green solvent was critical. Optimization of the recovery process using DMC resulted in a recovery yield of 83.31 % with pigment removal using methanol. DSC and XRD analysis showed that PHB from C. fritschii was partially crystalline. Characterization of recovered PHB suggested that C. fritschii can accumulate biopolymer similar to commercial PHB. Overall, biopolymer recovery using DMC is an eco-friendly process and can be employed for bioplastic recovery from other cyanobacteria.
Availability of data
The data supporting the findings of this study are available upon request.
References
Ahn WS, Park SJ, Lee SY (2000) Production of poly (3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl Environ Microbiol 66:3624–3627
Anbukarasu P, Sauvageau D, Elias A (2015) Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci Rep 5:17884
Ansari S, Fatma T (2016) Cyanobacterial polyhydroxybutyrate (PHB): screening, optimization and characterization. PLoS One 11:e0158168
Aramvash A, Moazzeni Zavareh F, Gholami Banadkuki N (2018) Comparison of different solvents for extraction of polyhydroxybutyrate from Cupriavidus necator. Eng Life Sci 18:20–28
Ariffin H, Nishida H, Shirai Y, Hassan MA (2008) Determination of multiple thermal degradation mechanisms of poly (3-hydroxybutyrate). Polym Degrad Stab 93:1433–1439
Arrieta MP, Samper MD, López J, Jiménez A (2014) Combined effect of poly (hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. J Polym Environ 22:460–470
Borrelle SB, Ringma J, Law KL, Monnahan CC, Lebreton L, McGivern A, Murphy E, Jambeck J, Leonard GH, Hilleary MA (2020) Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369:1515–1518
Castellano D, Sanchis A, Blanes M, Pérez del Caz MD, Ruiz-Saurí A, Piquer-Gil M, Pelacho B, Marco B, Garcia N, Ontoria-Oviedo I (2018) Electrospun poly(hydroxybutyrate) scaffolds promote engraftment of human skin equivalents via macrophage M2 polarization and angiogenesis. J Tissue Eng Regen Med 12:e983–e994
Castro-Castellon AT, Horton AA, Hughes JM, Rampley C, Jeffers ES, Bussi G, Whitehead P (2022) Ecotoxicity of microplastics to freshwater biota: Considering exposure and hazard across trophic levels. Sci Total Environ 816:151638
Chen G-Q, Wu Q (2005) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578
Defoirdt T, Halet D, Vervaeren H, Boon N, Van de Wiele T, Sorgeloos P, Bossier P, Verstraete W (2007) The bacterial storage compound poly-β-hydroxybutyrate protects Artemia franciscana from pathogenic Vibrio campbellii. Env Microbiol 9:445–452
Delledonne D, Rivetti F, Romano U (2001) Developments in the production and application of dimethylcarbonate. Appl Catal A 221:241–251
Devi AB, Nachiyar CV, Kaviyarasi T, Samrot AV (2015) Characterization of polyhydroxybutyrate synthesized by Bacillus cereus. Int J Pharm Pharm Sci 7:140–144
Elhami V, van de Beek N, Wang LS, Picken SJ, Tamis J, Sousa JAB, Hempenius MA, Schuur B (2022) Extraction of low molecular weight polyhydroxyalkanoates from mixed microbial cultures using bio-based solvents. Sep Purif Technol 299:121773
Fang Z, Chen P, Ji Q, Yan C, Gong A (2023) Stimuli-responsive hydrogel for disease therapy. Polymer Bull 81:1981–2000
Farinacci J, Laurent J (2023) Critical assessment of the sulfo-phospho-vanillin method to quantify lipids in freeze-dried microalgae. J Appl Phycol 35:1–12
Fiorese ML, Freitas F, Pais J, Ramos AM, de Aragão GM, Reis MA (2009) Recovery of polyhydroxybutyrate (PHB) from Cupriavidus necator biomass by solvent extraction with 1,2-propylene carbonate. Eng Life Sci 9:454–461
Gopi K, Balaji S, Muthuvelan B (2014) Isolation purification and screening of biodegradable polymer PHB producing cyanobacteria from marine and fresh water resources. Iranica J Energy Environ 5:94–100
Hahn SK, Chang YK, Kim BS, Chang HN (1994) Optimization of microbial poly(3-hydroxybutyrate) recover using dispersions of sodium hypochlorite solution and chloroform. Biotechnol Bioeng 44:256–261
Hahn SK, Chang YK, Lee SY (1995) Recovery and characterization of poly(3-hydroxybutyric acid) synthesized in Alcaligenes eutrophus and recombinant Escherichia coli. Appl Environ Microbiol 61:34–39
Juengert JR, Bresan S, Jendrossek D (2018) Determination of polyhydroxybutyrate (PHB) content in Ralstonia eutropha using gas chromatography and Nile red staining. Bio-protoc 8:1–15
Kanzariya R, Gautam A, Parikh S, Shah M, Gautam S (2023) Structure analysis and thermal stability of PHB recovered from sugar industry waste. Biotechnol Genet Eng Rev 39:1–23
Lackner M, Kamravamanesh D, Krampl M, Itzinger R, Paulik C, Chodak I, Herwig C (2019) Characterization of photosynthetically synthesized poly(3-hydroxybutyrate) using a randomly mutated strain of Synechocystis sp. PCC 6714. Int J Biobased Plast 1:48–59
Lebreton L, Andrady A (2019) Future scenarios of global plastic waste generation and disposal. Palgrave Commun 5:6
Lee SY, Choi J-i, Han K, Song JY (1999) Removal of endotoxin during purification of poly(3-hydroxybutyrate) from gram-negative bacteria. Appl Environ Microbiol 65:2762–2764
Lee JS, Sung YJ, Kim DH, Lee JY, Sim SJ (2022) Development of a limitless scale-up photobioreactor for highly efficient photosynthesis-based polyhydroxybutyrate (PHB)-producing cyanobacteria. Bioresour Technol 364:128121
Maity S, Das S, Mohapatra S, Tripathi A, Akthar J, Pati S, Pattnaik S, Samantaray D (2020) Growth associated polyhydroxybutyrate production by the novel Zobellellae tiwanensis strain DD5 from banana peels under submerged fermentation. Int J Biol Macromol 153:461–469
McAdam B, Brennan Fournet M, McDonald P, Mojicevic M (2020) Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 12:2908
Migliorini L, Santaniello T, Borghi F, Saettone P, Comes Franchini M, Generali G, Milani P (2020) Eco-friendly supercapacitors based on biodegradable poly(3-hydroxy-butyrate) and ionic liquids. Nanomaterials 10:2062
Mohanrasu K, Rao RGR, Dinesh G, Zhang K, Prakash GS, Song D-P, Muniyasamy S, Pugazhendhi A, Jeyakanthan J, Arun A (2020) Optimization of media components and culture conditions for polyhydroxyalkanoates production by Bacillus megaterium. Fuel 271:117522
Mongili B, Abdel Azim A, Fraterrigo Garofalo S, Batuecas E, Re A, Bocchini S, Fino D (2021) Novel insights in dimethyl carbonate-based extraction of polyhydroxybutyrate (PHB). Biotechnol Biofuels 14:13
Monshupanee T, Nimdach P, Incharoensakdi A (2016) Two-stage (photoautotrophy and heterotrophy) cultivation enables efficient production of bioplastic poly-3-hydroxybutyrate in auto-sedimenting cyanobacterium. Sci Rep 6:37121
Patnaik PR (2007) “Intelligent” descriptions of microbial kinetics in finitely dispersed bioreactors: neural and cybernetic models for PHB biosynthesis by Ralstonia eutropha. Microb Cell Fact 6:23
Pradhan S, Dikshit PK, Moholkar VS (2018) Production, ultrasonic extraction, and characterization of poly(3-hydroxybutyrate) (PHB) using Bacillus megaterium and Cupriavidus necator. Polym Adv Technol 29:2392–2400
Riis V, Mai W (1988) Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J Chromatogr A 445:285–289
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Samorì C, Abbondanzi F, Galletti P, Giorgini L, Mazzocchetti L, Torri C, Tagliavini E (2015a) Extraction of polyhydroxyalkanoates from mixed microbial cultures: impact on polymer quality and recovery. Bioresour Technol 189:195–202
Samorì C, Basaglia M, Casella S, Favaro L, Galletti P, Giorgini L, Marchi D, Mazzocchetti L, Torri C, Tagliavini E (2015b) Dimethyl carbonate and switchable anionic surfactants: two effective tools for the extraction of polyhydroxyalkanoates from microbial biomass. Green Chem 17:1047–1056
Sangsanoh P, Israsena N, Suwantong O, Supaphol P (2017) Effect of the surface topography and chemistry of poly(3-hydroxybutyrate) substrates on cellular behavior of the murine neuroblastoma Neuro2a cell line. Polymer Bull 74:4101–4118
Sekar A, Varghese GK, Varma R (2023) Exposure to volatile organic compounds and associated health risk among workers in lignite mines. Int J Environ Sci Technol 20:4293–4306
Sharma L, Nishida K, Kanaya TJP, Composites P (2005) The effect of solvent on the miscibility of blends of poly 1-[4-(3-carboxy-4-hydroxy-phenylazo)benzene sulphonamido-1,2-ethanediyl, sodium salt)] (PAZO) and polyhydroxybutyrate,(PHB). Polym Polym Compos 13:443–452
Sun X, Guo L, Sato H, Ozaki Y, Yan S, Takahashi I (2011) A study on the crystallization behavior of poly(β-hydroxybutyrate) thin films on Si wafers. Polymer 52:3865–3870
Tang X, Chen EY-X (2018) Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat Commun 9:2345
Terada M, Marchessault R (1999) Determination of solubility parameters for poly(3-hydroxyalkanoates). Int J Biol Macromol 25:207–215
Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (2009) Biodegradability of plastics. Int J Mol Sci 10:3722–3742
Troschl C, Meixner K, Drosg B (2017) Cyanobacterial PHA production—Review of recent advances and a summary of three years’ working experience running a pilot plant. Bioengineering 4:26
Wölfle H, Kopacka H, Wurst K, Preishuber-Pflügl P, Bildstein B (2009) On the way to biodegradable poly(hydroxy butyrate) from propylene oxide and carbon monoxide via β-butyrolactone: Multisite catalysis with newly designed chiral indole-imino chromium(III) complexes. J Organomet Chem 694:2493–2512
Yabueng N, Napathorn SC (2018) Toward non-toxic and simple recovery process of poly(3-hydroxybutyrate) using the green solvent 1,3-dioxolane. Process Biochem 69:197–207
Yang YH, Brigham CJ, Budde CF, Boccazzi P, Willis LB, Hassan MA, Yusof ZA, Rha C, Sinskey AJ (2010) Optimization of growth media components for polyhydroxyalkanoate (PHA) production from organic acids by Ralstonia eutropha. Appl Microbiol Biotechnol 87:2037–2045
Yashavanth P, Maiti SK (2024) A multi-objective optimization approach for the production of polyhydroxybutyrate via Chlorogloea fritschii under diurnal light with single-stage cultivation. Int J Biol Macromol 255:128067
Yashavanth PR, Das M, Maiti SK (2021) Recent progress and challenges in cyanobacterial autotrophic production of polyhydroxybutyrate (PHB), a bioplastic. J Environ Chem Eng 9:105379
Zavřel T, Sinetova MA, Červený J (2015) Measurement of chlorophyll a and carotenoids concentration in cyanobacteria. Bio-protoc 5:e1467–e1467
Acknowledgement
The authors would like to acknowledge the Thailand Institute of Scientific and Technological Research (TISTR), Thailand for providing cyanobacterial strain. The authors acknowledge the access to analytical facilities provided by the Department of Biosciences and Bioengineering and Central Instruments Facility (CIF), Indian Institute of Technology Guwahati. The use of Differential Scanning Calorimetry at the School of Energy Sciences and Engineering, Indian Institute of Technology Guwahati is also acknowledged.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Yashavanth P R: Conceptualization, Experimentation, Analysis, Writing-Original draft preparation
Soumen K Maiti: Conceptualization, Supervision, Writing- Reviewing and Editing
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yashavanth, P.R., Maiti, S.K. Recovery and characterization of polyhydroxybutyrate from Chlorogloea fritschii TISTR 8527 using halogenated and green solvents. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03288-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03288-w